Published online May 15, 2019. doi: 10.4251/wjgo.v11.i5.377
Peer-review started: November 20, 2018
First decision: December 7, 2018
Revised: December 17, 2018
Accepted: January 3, 2019
Article in press: January 4, 2019
Published online: May 15, 2019
Processing time: 176 Days and 20.7 Hours
Qingjie Fuzheng granules (QFGs) are part of a traditional Chinese medicine formula, which has been widely used and found to be clinically effective with few side effects in various cancer treatments, including colorectal cancer (CRC). However, the precise mechanisms and molecular signaling pathways involved in the activity of QFGs’ anticancer effect have not been reported in the literature. In this study, we hypothesized that QFGs can inhibit the growth of colorectal cancer cells, and that its mechanism is closely related to one or more intracellular signal transduction pathways.
To better evaluate the mechanism underlying the anti-cancer effect of QFGs on the CRC cell lines HCT-116 and HCT-8.
First, we measured cell viability and cytotoxicity by performing MTT and lactate dehydrogenase (LDH) assays. We evaluated the role of QFGs in cell proliferation and apoptosis by assessing colony formation and analyzing Hoechst 33258 staining. Second, cell cycle and apoptosis rates were measured by fluorescence activated cell sorting, and the expression levels of survivin, cyclin D1, CDK4, p21, Bax, Bcl-2, Fas, FasL, and cleaved-caspase-3/-8/-9 were measured by performing western blots and caspase activity assays. Furthermore, inhibitors of caspase-3/-8/-9 were used to elucidate the specific apoptosis pathway induced by QFGs in cancer cells. Finally, activation of the PI3K/AKT and ERK signaling pathways was examined using the western blot assay to investigate the possible mechanism.
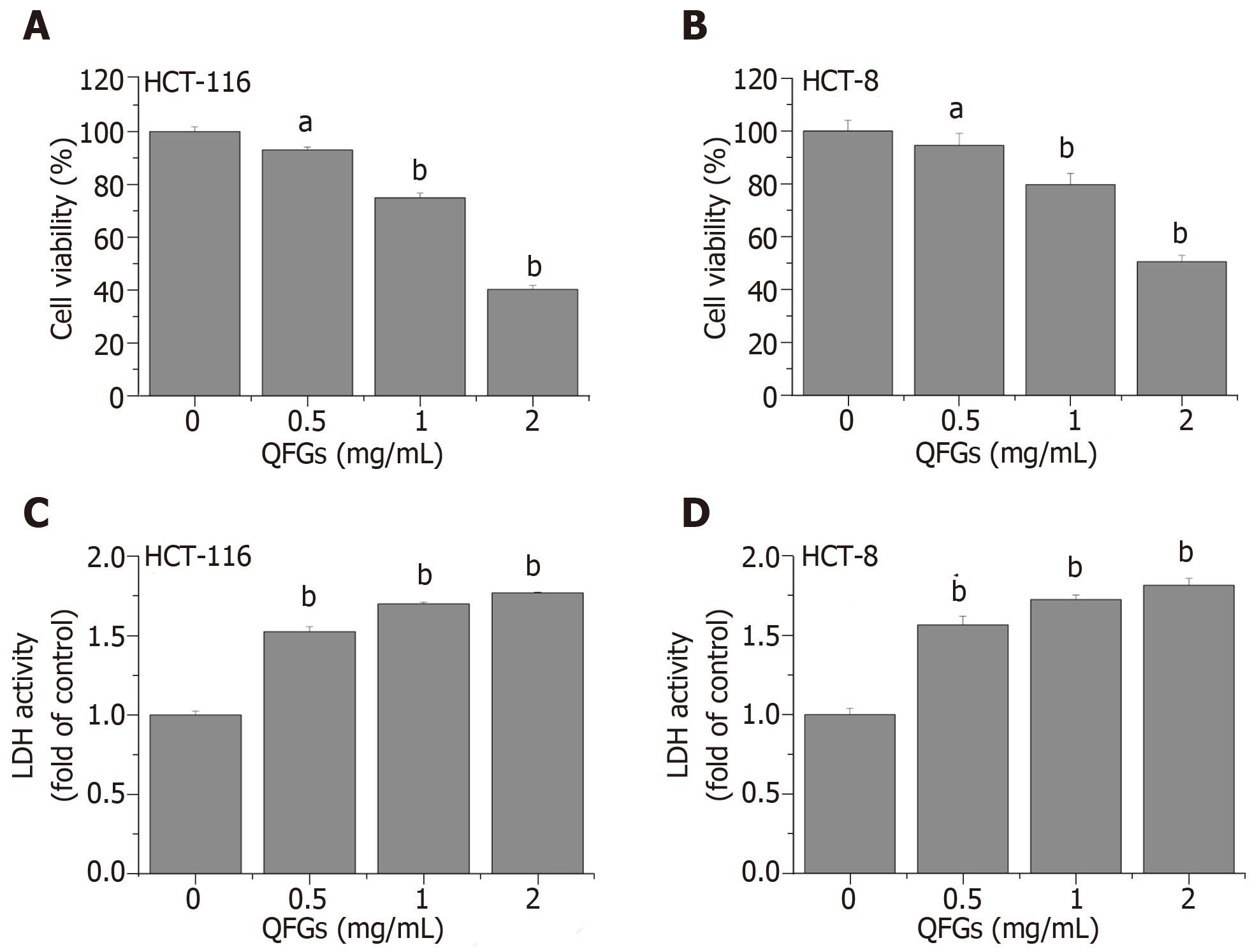
MTT and LDH assays revealed that after 0.5-2.0 mg/mL of QFGs treatment, cell viability was reduced by (6.90% ± 1.03%)–(59.70% ± 1.51%) (HCT-116; P < 0.05) and (5.56% ± 4.52%)–(49.44% ± 2.47%) (HCT-8; P < 0.05), and cytotoxicity was increased from 0.52 ± 0.023 to 0.77 ± 0.002 (HCT-116; P < 0.01) and from 0.56 ± 0.054 to 0.81 ± 0.044 (HCT-8; P < 0.01) compared with the non-QFGs treatment groups. Additionally, colony formation and Hoechst 33258 staining assays showed that QFGs inhibited proliferation and induced apoptosis in CRC cells. QFGs also increased the expression levels of Bax, Fas and FasL, decreased the level of Bcl-2, and stimulated the activation of caspase-3/-8/-9, which were revealed by western blot and caspase activity assays. In contrast, when adding the three caspase inhibitors, the suppression effect of QFGs on cell viability and apoptosis were markedly inhibited. Moreover, QFGs suppressed the phosphorylation levels of PI3K, AKT and ERK.
These results demonstrated that QFGs can inhibit CRC cell proliferation and induce apoptosis by suppressing the PI3K/AKT and ERK signaling pathways.
Core tip: To study the effect and mechanism of Qingjie Fuzheng granules (QFGs) on colorectal cancer (CRC) cells, we measured cell viability and cytotoxicity by performing MTT and LDH assays. We also evaluated the role of QFGs in cell proliferation by conducting colony formation, cell cycle and western blot assays. We evaluated the role of QFGs in cell apoptosis by assessing both Hoechst 33258 and Annexin-V/PI staining and performing western blot assays. Furthermore, the activation of PI3K/AKT and ERK signaling pathways was examined using the western blot assay to investigate the possible mechanism. All results demonstrated that QFGs inhibited CRC cell growth by suppressing the PI3K/AKT and ERK signaling pathways.
- Citation: Yang H, Liu JX, Shang HX, Lin S, Zhao JY, Lin JM. Qingjie Fuzheng granules inhibit colorectal cancer cell growth by the PI3K/AKT and ERK pathways. World J Gastrointest Oncol 2019; 11(5): 377-392
- URL: https://www.wjgnet.com/1948-5204/full/v11/i5/377.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i5.377
As a result of changing lifestyles and aging populations, the prevalence of colorectal cancer (CRC) remains high and accounts for approximately 25% of the world’s cancer-related deaths[1-3]. Although surgical resection and chemoradiotherapy are the most commonly used clinical options, surgery is not an option for all patients, and long-term chemoradiotherapy can cause adverse side effects, such as drug resistance, recurrence and metastasis[4-7]. Therefore, the search for novel therapies has attracted worldwide attention.
Natural products, which often have fewer side effects than synthetic drugs, are important in the treatment of many diseases and have a long history of use in China. Therefore, natural products have been studied by many researchers to find better antitumor drugs[8-11]. Qingjie Fuzheng granules (QFGs) is a traditional Chinese medicine (TCM) formula (Table 1) that consists of a mixture of four herbs (Scutellaria barbata D. Don, malt, Hedyotis diffusa Willd, and Astragalus) that together confer properties of anti-inflammation, antioxidative, antibacterial, immunity enhancement and digestion promotion. QFGs have been widely used and found to be clinically effective in various cancer treatments, including CRC, and have few side effects. However, the precise mechanisms and molecular signaling pathways involved in the activity of QFGs’ anticancer effect have not been reported in the literature.
| Common name | Latin name | Part used | Daily adult dose, g |
| Spreading Hedyotis herb | Hedyotis diffusa Willd | Dried root | 15 |
| Malt | Hordeum vulgare L. | Dried seed | 15 |
| Astragalus | Radix astragali | Dried root | 15 |
| Scutellaria barbata | Scutellaria barbata D. Don | Dried body | 15 |
CRC develops because of a cell growth imbalance caused by excessive proliferation or lack of apoptosis. Eukaryotic cell proliferation is controlled by the cell cycle, which consists of the G0, G1, S, G2 and M phases. In the detection of cell cycle progression, the G1/S transition is one of the main checkpoints[12]. The main regulatory factors in G1/S progression are cyclin D1 and cyclin-dependent kinase 4 (CDK4), which can form complexes to regulate this progress[13-15]. A CDK inhibitor, p21, can change the function of CDK–cyclin complexes by binding to them and then suppressing cell proliferation[16]. Normal cell apoptosis can eliminate surplus, redundant, and aberrant cells in animals, so it is essential for normal tissue maintenance. Disorders in this process trigger many diseases, including CRC[17-19]. The pathways involved in the apoptotic process are the mitochondria-dependent pathway, also called the intrinsic apoptosis pathway, and the death receptor-mediated apoptosis pathway[20]. The former is modulated by the Bax (proapoptotic) and Bcl-2 (anti-apoptotic) family proteins[21], which control the release of apoptotic correlation factors, such as cytochrome C (Cyt C)[22]. When intracellular damage occurs, mitochondria-dependent apoptosis is triggered. Then, Cyt C, together with Apaf-1 and caspase-9, cleaves caspase-3[23]. Receptor-mediated apoptosis originates from outside the cell, with the binding of the Fas ligand (termed FasL or CD95L) to the Fas receptor (termed CD95). Once the death receptor pathway is successfully activated, the Fas-associated death domain and caspase-8 will accumulate, and caspase-8 will be cleaved. Then, caspase-8 cleaves caspase-3, which generates the activated form of caspase-3 that serves as the ultimate activator of apoptosis[24]. Therefore, one of the key approaches in the development of antitumor drugs is to promote apoptosis and inhibit tumor cell proliferation, two processes that typically promote cancer growth. There are multiple signaling pathways that regulate cancer growth, including the PI3K/AKT and ERK signaling pathways, and abnormal activation of these signaling pathways can lead to irregular expression of these factors.
The aim of this study is to better understand the mechanism underlying the potential anticancer effect of QFGs by investigating their biological function using the human CRC cell variants HCT-116 and HCT-8. Our results showed that QFGs inhibit proliferation and increase apoptosis in HCT-116 and HCT-8 cells by inactivating the PI3K/AKT and ERK pathways.
The human colon carcinoma HCT-8 and HCT-116 cell lines were purchased from the American Type Culture Collection. The two cell lines were cultured in Roswell Park Memorial Institute-1640 medium (C11875500BT; Life Technologies Corp. Grand Island, United States) containing 10% fetal bovine serum, 1% penicillin, and 1% streptomycin, and were grown at 37 °C in 5% CO2.
QFGs were obtained and prepared as previously described[11]. Briefly, QFGs powder was dissolved in 1× PBS (store concentration of QFGs is 200 mg/mL) and stored at 20 °C. Inhibitors of caspase-3/-8/-9 (Z-DEVE-FMK, ab120488; Z-IETD-FMK, ab141382; Z-LEHD-FMK, ab142026, Abcam, CA, United States) were dissolved in DMSO to a concentration of 10 mM and stored at -20 °C.
HCT-8 and HCT-116 cells were placed into 96-well plates (1 × 105 cell/well). After 12 h, the cells were treated with different doses of QFGs (0.5, 1 and 2 mg/mL) and grown for 24 h, or the cells were treated with a designated dose of QFGs (2 mg/mL) in combination with inhibitors of caspase-3 (Z-DEVE-FMK), caspase-8 (Z-IETD-FMK), and caspase-9 (Z-LEHD-FMK) at a concentration of 10 μmol/L each and then incubated for 24 h. Then, MTT (0.5 mg/mL) was added to each well (100 μL/well) and incubated for 4 h. Subsequently, all wells were treated with DMSO (100 μL/well). Absorbance at 570 nm in each well was measured by using an ELISA reader (Infinite M200 PRO; Tecan Austria GmbH, Austria).
Cells were seeded into 12-well plates (1 × 105 cell/well), treated with different dose of QFGs (0.5, 1 and 2 mg/mL) and grown for 24 h. Then, a lactate dehydrogenase (LDH) release assay kit (Beyotime, Shanghai, China) was used to determine the LDH activity according to the kit’s manual.
After treatment with different concentrations of QFGs (0.5, 1, and 2 mg/mL) for 24 h, a colony formation assay was performed as described previously[11].
QFGs and 10 μmol/L caspase inhibitors (Z-DEVE-FMK, Z-IETD-FMK, Z-LEHD-FMK) were added to the cells and grown for 24 h. Subsequently, 4% paraformaldehyde was used to fix the cells for 15 min. Then, 4% paraformaldehyde was removed and 1× PBS was used to rinse the cells three times. Then, Hoechst 33258 (c0003; Beyotime, Shanghai, China) (100 μL/well) was added to all wells in the dark for 15 min. The Hoechst 33258 solution was then removed, and 1× PBS was used to rinse the stained cells three times, followed by 1× addition of fresh PBS. An inverted fluorescence microscope (Leica DMI4000B; Leica Camera AG, Solms, Germany) was used to observe and photograph the cells.
Cell cycle was measured in the HCT-8 and HCT-116 cells after treatment with the indicated concentrations of QFGs (0.5, 1 and 2 mg/mL). Cell cycle progression was estimated by using a propidium iodide (PI) kit (KGA512; KeyGen Biotech, Nanjing, China) and fluorescence activated cell sorting according to the manufacturer’s instructions.
Cells were seeded into 6-well plates (1.5 × 105 cell/well), treated with different doses of QFGs (05, 1 and 2 mg/mL) and grown for 24 h. Then, cells were stained by using an Annexin V/PI kit (KGA108; KeyGen BioTech, Nanjing, China) according to the kit’s manual. Annexin V-positivity and PI-negativity (lower-right quadrant) represented early apoptotic cells, whereas Annexin V-positivity and PI-positivity (upper-right quadrant) represented late apoptotic cells.
A caspase activity assay kit (caspase-3, KGA204; caspase-8, KGA304; caspase-9, KGA404; KeyGen BioTech, Nanjing, China) was used to detect the activity of caspases according to the manufacturer’s instructions. In brief, cell lysates were prepared after the addition of the indicated reaction buffer (provided in the kit) at 37 °C for 4 h in the dark. Absorbance at 405 nm was measured by using an ELISA reader.
When CRC cells were treated with different doses of QFGs (05, 1 and 2 mg/mL) for 24 h, a cell lysis buffer containing a cocktail to lyse the cells was added. The bicinchoninic acid assay was used to detect the total protein concentrations, and 50 µg of total protein was used for electroblotting. Five percent skim milk was used to block the NC membranes, and then primary antibodies against Fas, FasL, p-PI3K and p-AKT (ab-110021, ab-15285, ab182651, ab38449; 1:1000, Abcam, CA, United States), p-ERK (sc-16982; 1:1000, Santa Cruz Biotechnology, CA, United States), cleaved-caspase-3, cleaved-caspase-8, cleaved-caspase-9, β-actin, Bcl-2 and Bax (#9662, #4790, #9508, #4967, #4223, #5023; 1:1000, Cell Signaling, Beverly, MA, United States), PI3K, AKT and ERK (13329-1-AP, 10176-2-AP, 16443-1-AP; 1:2000, Proteintech, United States) were added at 4 °C overnight. On the second day, the appropriate HRP-conjugated secondary antibodies (goat anti-mouse IgG secondary antibody, #L3032; goat anti-rabbit IgG secondary, #L3012; 1:5000, Signalway Antibody, PA, United States) were added and the SuperSignal West Pico Chemiluminescent Substrate was used to detect the signal.
One-way ANOVA and SPSS software (version 18.0) were used to analyze all of the data in this study. The data are expressed as the mean ± standard deviation. P < 0.05 indicated statistical significance.
The MTT assays and LDH activity assays were used to evaluate the effect of QFGs on the growth of the two cell types. QFGs inhibited cell viability in a dose-dependent manner (Figure 1A, B), showing cytotoxicity at 0.5-2.0 mg/mL (Figure 1C, D). In Figure 1A and B, cell viability after treatment with QFGs (0.5–2.0 mg/mL, 24 h) decreased by (6.90% ± 1.03%)-(59.70% ± 1.51%) (HCT-116) and (5.56% ± 4.52%)-(49.44% ± 2.47%) (HCT-8) relative to the viability in control cells (P < 0.05). In Figure 1C and D, treatment with QFGs (0.5–2.0 mg/mL, 24 h) increased the LDH activity rate of the cells from 0.52 ± 0.023 to 0.77 ± 0.002 (HCT-116) and from 0.56 ± 0.054 to 0.81 ± 0.044 (HCT-8) relative to that in control cells (P < 0.01). These results proved that QFGs treatment reduced cell viability and increased cytotoxicity in both cell types.
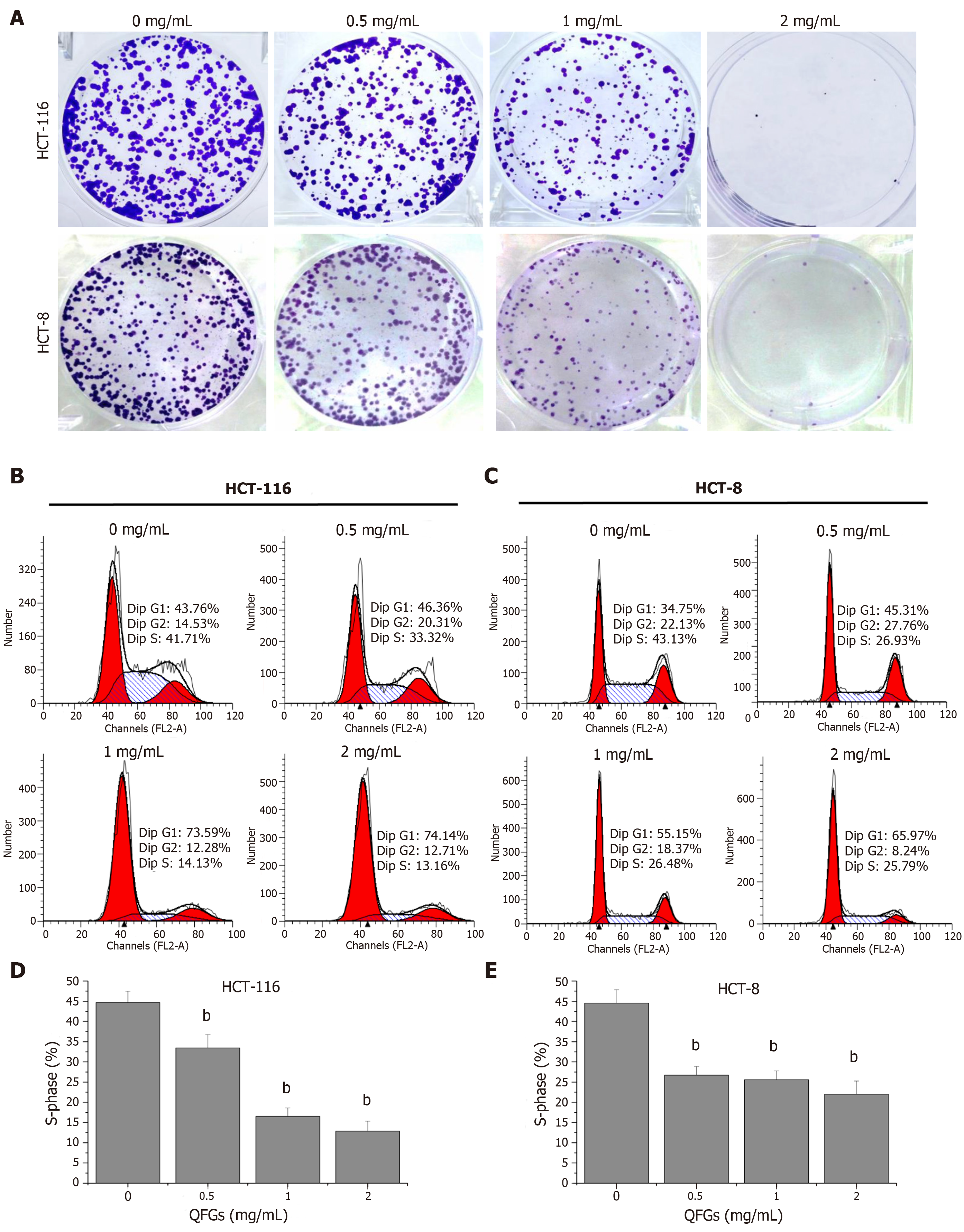
Cell colony formation assays were used to evaluate the changes in cell growth after treatment with QFGs. As shown in Figure 2A, QFGs dose-dependency inhibited colony formation in HCT-116 and HCT-8 cells. Subsequently, cell cycle assays were used to verify the proliferation-inhibiting effects of QFGs. As shown in Figure 2B-E, the percentages of S phase in HCT-116 cells after treatment with 0, 0.5, 1, and 2 mg/mL QFGs were 44.7% ± 2.77%, 33.45% ± 3.30%, 16.50% ± 2.12%, and 12.86% ± 2.51%, respectively (P < 0.01), and the percentages of S phase in HCT-8 cells after treatment with 0, 0.5, 1, and 2 mg/mL QFGs were 44.55% ± 3.32%, 26.71% ± 2.17%, 25.60% ± 2.19%, and 21.99% ± 3.30%, respectively (P < 0.01). These results suggested that QFGs can inhibit proliferation of both cell types by arresting the cell cycle.
Hoechst 33258 staining assays were used to evaluate the changes in cell nuclear morphology after treatment with QFGs (Figure 3A). The degree of staining was low in untreated cells, but it gradually increased in the other three groups, which indicated a gradual increase in apoptosis. Subsequently, Annexin V-FITC/PI assays were used to verify the apoptosis-inducing effect of QFGs. As shown in Figure 3B, apoptosis percentages in HCT-116 cells after treatment with 0, 0.5, 1, and 2 mg/mL QFGs were 4.07% ± 0.48%, 11.87% ± 0.5%, 12.77% ± 0.67%, and 31.13% ± 0.73%, respectively (P < 0.01), and apoptosis percentages in HCT-8 cells after treatment with 0, 0.5, 1, and 2 mg/mL QFGs were 2.23% ± 0.50%, 9.34% ± 0.69%, 17.19% ± 0.55%, and 33.93% ± 0.93%, respectively (P < 0.01). We also found that QFGs upregulated the expression of cleaved-caspase-3/-8/-9 in both cell types (Figure 3C, D; P < 0.01). At the same time, the caspase activity was measured using a commercial caspase activity assay kit. Identical to the western blot results, the activities of caspase-3/-8/-9 were significantly enhanced by QFGs treatment in both cell types (Figure 3E, F; P < 0.01). These results indicated that QFGs induced apoptosis in the two cell types, and suggested that apoptosis occurred via both the mitochondria-dependent and death receptor-mediated pathways.
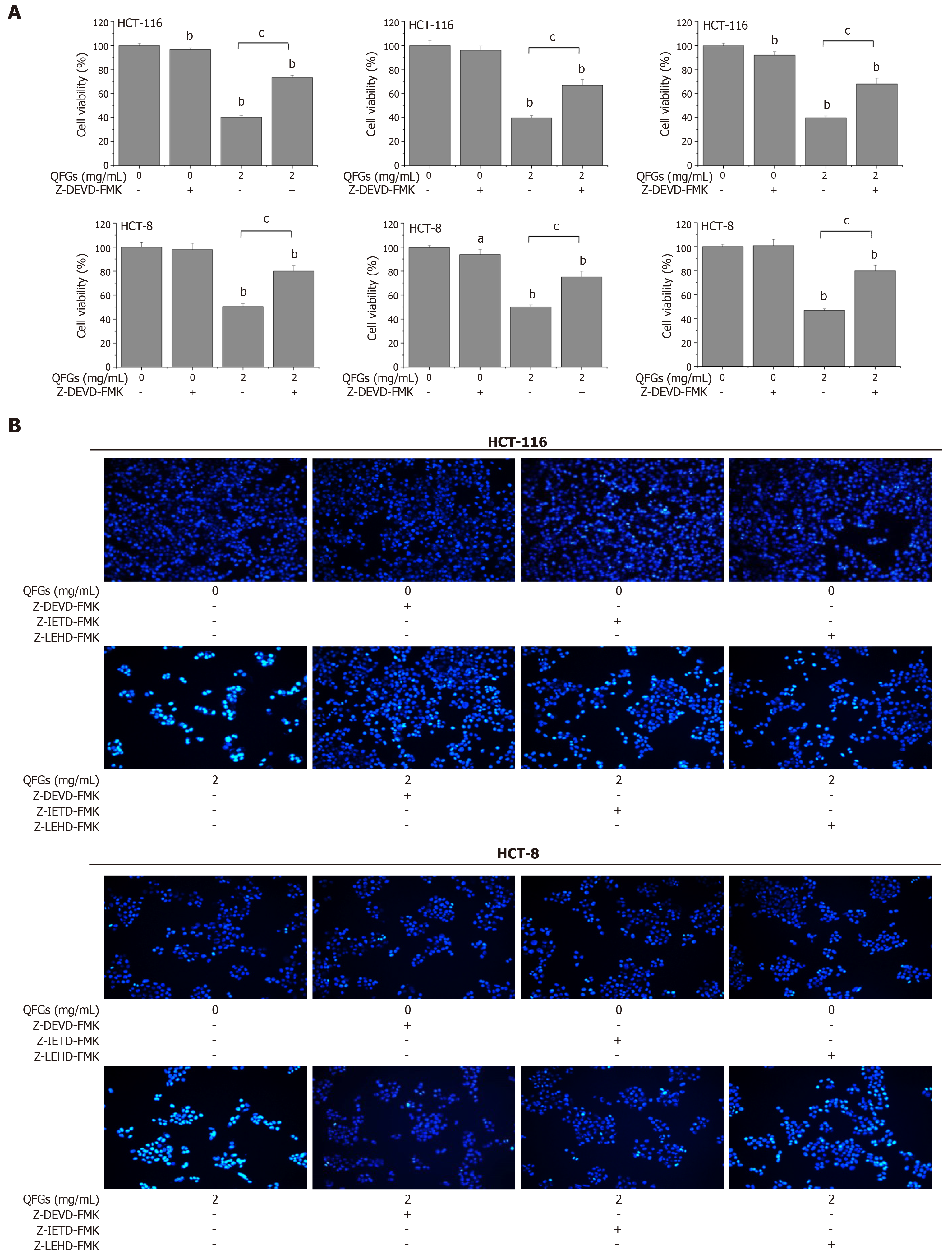
To further confirm these findings, various specific caspase inhibitors were used. As shown in Figure 4A, Z-DEVD-FMK, Z-IETD-FMK, and Z-LEHD-FMK markedly inhibited the inhibitory effect of QFGs on cell viability in both cell types (P < 0.05 and 0.01, respectively). In addition, we used the Hoechst 33258 staining assay to detect nuclear morphological changes, and all three caspase inhibitors clearly inhibited the apoptosis-induced effect of QFGs (2 mg/mL) in both cell types (Figure 4B). These findings proved that QFGs induced apoptosis via the mitochondria-dependent and death receptor-mediated pathways in both HCT-116 and HCT-8 cells.
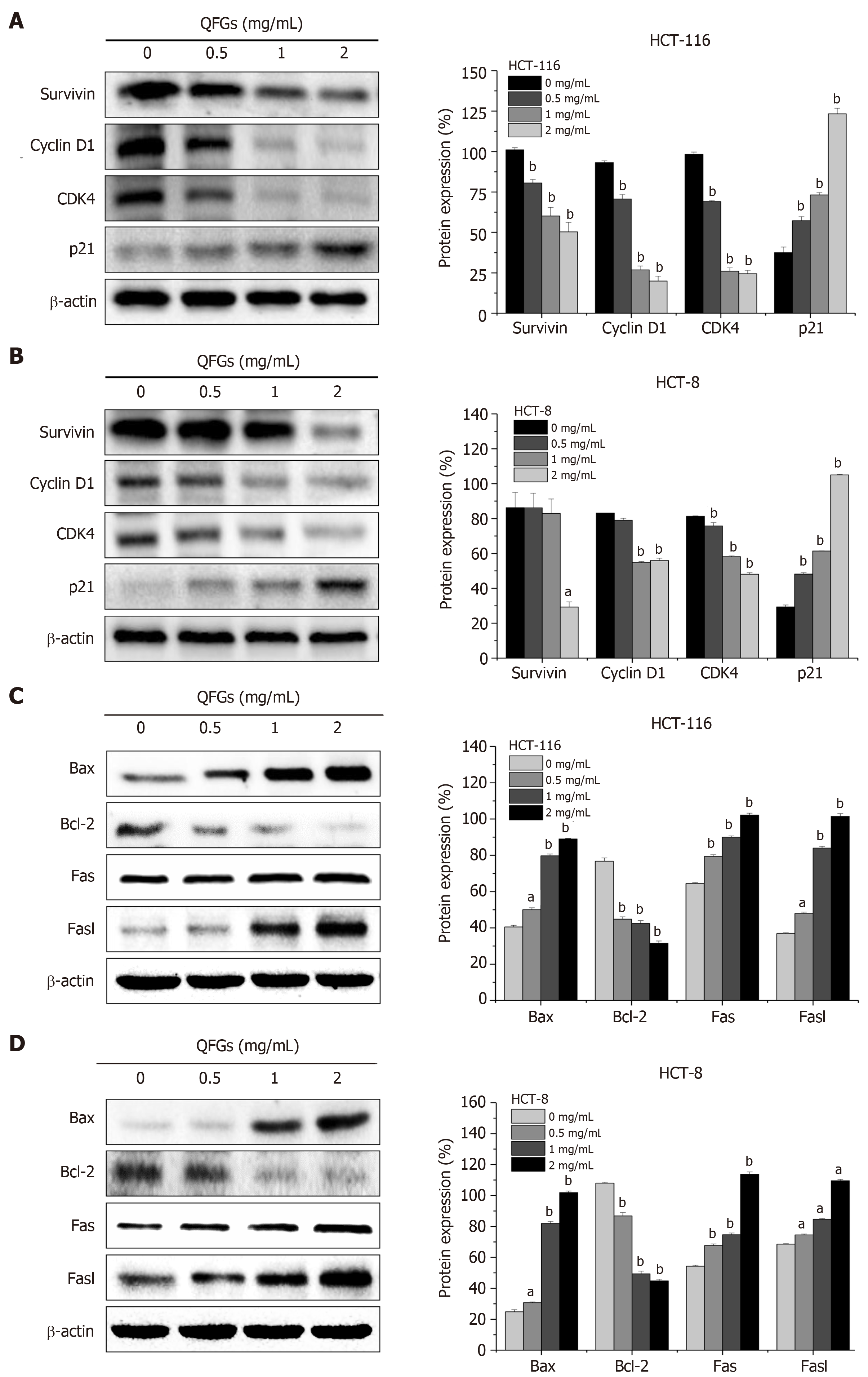
During cell cycle regulation, survivin is a key protein that indicates cell cycle progression, and the complex of cyclin D1 and CDK4 directly regulates cell cycle progression[13]. In a previous study, overexpression of this complex has been found to induce cell proliferation, whereas p21 inhibited the effect of the cyclin D1/CDK4 complex[14]. During the regulation of cell apoptosis, Bax and Bcl-2 regulate the mitochondria-dependent pathway[21], while Fas and FasL activate the death receptor-mediated pathway[24]. We found that QFGs can inhibit proliferation via arrest of the cell cycle in HCT-116 and HCT-8 cells. We used western blotting to test the protein expression levels of survivin, cyclin D1, CDK4, and p21 after the cells were treated with QFGs. As shown in Figure 5A and B, we found that QFGs downregulated the expression of survivin, cyclin D1 and CDK4, but upregulated the level of p21 in both cell types (P < 0.05 and 0.01, respectively). Since we found that QFGs induced apoptosis via the mitochondria-dependent and death receptor-mediated pathways in HCT-116 and HCT-8 cells, we used western blotting to test the protein expression levels of Bcl-2, Bax, Fas, and FasL after the cells were treated with QFGs. As shown in Figure 5C and D, we found that QFGs decreased the expression of Bcl-2 but promoted the expression of Bax, Fas, and FasL in both cell types (P < 0.05 and 0.01, respectively). Briefly, these findings suggest that QFGs inhibit proliferation via cell cycle arrest and induce apoptosis via the mitochondria-dependent and death receptor-mediated pathways in HCT-116 and HCT-8 cells.
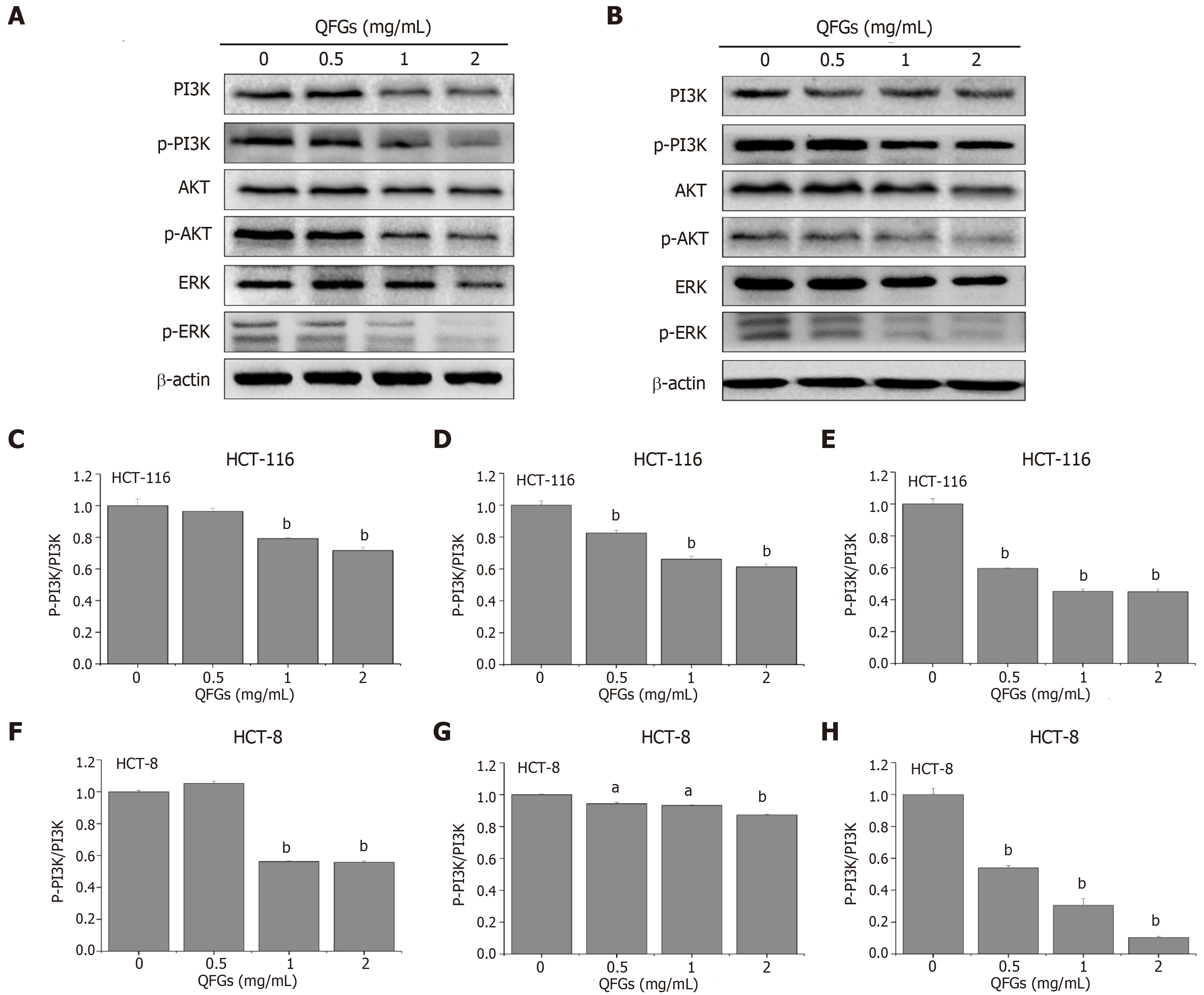
Cancer occurrence and progression are highly associated with the regulation of multiple signaling pathways, including PI3K/AKT and ERK[25,26]. To further explore the potential mechanisms underlying the observed anticancer effects of QFGs, we examined the expression of major regulation factors involved in the PI3K/AKT and ERK signaling pathways. As shown in Figure 6A and B, after treatment with QFGs, the ratios of the phosphorylation expression level to the total expression level were significantly downregulated in PI3K, AKT and ERK in both cell types (P < 0.05 and 0.01, respectively), which suggested that the anticancer effect of QFGs on CRC cells occurs via suppression of the PI3K/AKT and ERK signaling pathways.
CRC is a deadly disease, primarily due to its high rate of metastasis and recurrence in patients. Multidrug combination therapy and surgical treatment are the main therapeutic methods that can significantly improve patients survival. However, there are many patients with cancer that is drug resistant and recurrent after traditional clinical treatment[27,28]. Therefore, there is a need to discover new kinds of anticancer drugs, such as herbal products. In recent years, TCM has attracted increasing attention in the field of oncology because of its relative security and long history of application[29-32]. QFGs are a four-herb TCM formula that consists of Scutellaria barbata D. Don, malt, Hedyotis diffusa Willd, and Astragalus. In the past few years, some studies have proven that Hedyotis diffusa Willd and Scutellaria barbata D. Don are capable of promoting apoptosis and inhibiting growth and angiogenesis in many types of cancer cells, including CRC[33,34]. Malt could boost the movement of Qi and improve food digestion, and Astragalus is a vital component in many TCM formulas that have been used in clinics to cure many cancer patients, reduce the incidence of complications, and improve the quality of life of cancer patients[35]. However, there are no reports on the underlying signaling pathways and mechanisms involved in QFGs anticancer activity.
In this study, we found that QFGs exhibited significant anticancer effects on HCT-116 and HCT-8 cells. The anticancer effect of QFGs is mainly through the inhibition of proliferation and induction of apoptosis, which are mechanisms commonly exploited in tumor therapy. As demonstrated in the current study, QFGs reduced cell viability and increased cytotoxicity in HCT-116 and HCT-8 cells in a dose-dependent manner. Furthermore, we used cell colony formation, nuclear staining, and flow cytometry assays to demonstrate that these effects in HCT-116 and HCT-8 cells resulted from the inhibition of proliferation and induction of apoptosis by QFGs.
Cell proliferation is regulated by the cell cycle, which consists of the G0, G1, S, G2 and M phases. DNA synthesis is completed in S phase, which is responsible for the initiation and completion of DNA replication[20]. Therefore, the G1/S transition is one of the two main checkpoints in the cell cycle. Using a cell cycle assay, we observed that the inhibitory effects of QFGs on HCT-116 and HCT-8 cell proliferation were associated with blocking the G1 to S phase transition. The G1/S process is highly regulated by cyclin D1, which forms complexes with CDK4[21,22]. Overexpression of the cyclin D1/CDK4 complex can enhance cell proliferation, whereas p21 can bind to this complex and inhibit its activity[24]. Therefore, the expression of CDK4, cyclin D1 and p21 indicate the proliferation state of HCT-116 and HCT-8 cells to some extent. This study proved that QFGs administration upregulates p21 protein expression while downregulating cyclin D1 and CDK4 protein expression. These results showed that QFGs inhibit HCT-116 and HCT-8 cell proliferation.
Cell apoptosis or programmed death, which is an essential process in a healthy organism, removes surplus and damaged cells[36-38]. Failure to execute the apoptosis process may lead to various diseases, such as cancer[39] and autoimmune diseases[40], whereas too much apoptosis may lead to neurodegenerative diseases[41]. There are two pathways involved in apoptosis: the mitochondria-dependent and death receptor-mediated pathways. The mitochondria-dependent pathway is initiated by caspase-9, and the death receptor pathway is initiated by caspase-8. Both pathways ultimately rely on the activation of caspase-3[17-19].
In the mitochondria-dependent apoptosis pathway, mitochondrial dysfunction directly leads to the occurrence of apoptosis and is central to the apoptotic pathway[42]. Mitochondrial outer membrane permeabilization (MOMP), an essential event in the mitochondria-mediated apoptosis pathway, causes the transfer of Cyt C and other apoptotic proteins from the mitochondria into the cytosol, which leads to caspase activation and apoptosis[43]. Caspases are the key proteins in the regulation of cell apoptosis. During mitochondria-mediated apoptosis, caspase-3 is an important activator that can be cleaved by its upstream initiators, such as caspase-9. The present study showed that QFGs promoted the activation of both caspase-9 and caspase-3 in HCT-116 and HCT-8 cells. In addition, the process of HCT-116 and HCT-8 cell death induced by QFGs was followed by an increase in the cleavage of caspases-9 and caspase-3, which then promotes the molecular cascade leading to apoptosis. The Bcl-2 protein is the key regulatory protein in mitochondria-dependent apoptosis. Some studies have reported that MOMP occurs when proapoptotic Bax-like proteins form pores in the mitochondria; however, the effect of anti-apoptotic Bcl-2-like members on MOMP is opposite to that of proapoptotic Bax-like proteins on MOMP. Therefore, the ratio of Bax to Bcl-2 is the key to determining cell survival [44,45]. Our study proved that QFGs administration upregulated Bax protein expression and downregulated Bcl-2 protein expression. These results showed that QFGs induce HCT-116 and HCT-8 cell apoptosis via the mitochondria-dependent pathway.
During the death receptor apoptosis pathway, caspase-3 is also the ultimate activator of apoptosis. Caspase-3 is cleaved by its downstream initiators, such as caspase-8. In this study, we discovered that both caspase-8 and caspase-3 can be cleaved by QFGs in HCT-116 and HCT-8 cells. In addition, as noted earlier, HCT-116 and HCT-8 cell death induced by QFGs was followed by increased cleavage of caspase-8 and caspase-3, which accelerates apoptosis. In this pathway, death signals are transmitted via cell surface receptors that communicate with the FasL/Fas signaling pathway, which is part of the death receptor pathway. After binding to FasL, Fas trimerizes and interacts with Fas-associated protein with a death domain, which contributes to the cleavage of caspase-8 and caspase-10 and leads to activation of downstream effector caspases, including caspase-3, caspase-6 and caspase-7, ultimately causing apoptosis[24]. In this study, we demonstrated that QFGs treatment upregulated FasL and Fas protein expression. These results showed that QFGs induce HCT-116 and HCT-8 cell apoptosis through the extrinsic apoptosis pathway.
To determine if the two classic apoptosis pathways were both involved in this study, we added caspase-3/-8/-9 inhibitors and performed the MTT and Hoechst 33258 staining assays to test cell viability and cell apoptosis once again. We found that all three inhibitors markedly inhibited the inhibitory effect of QFGs on cell viability in CRC cells, and inhibited the apoptosis induced by QFGs. This result verified that QFGs induced apoptosis via the mitochondria-dependent and death receptor apoptosis pathways in HCT-116 and HCT-8 cells, which directly revealed the multi-target inhibitory effects of QFGs on CRC cells.
The pathogenic mechanisms underlying the development of cancer, including CRC, are heterogeneous and regulated by multiple signaling pathways, including PI3K/AKT and ERK[25,26]. Previous studies have reported that the PI3K/AKT and ERK signaling pathways regulate cell growth, apoptosis and metastasis[46]. As one of the important intracellular signal transduction pathways, PI3K/AKT signaling has been reported to play important roles in cell survival, apoptosis and metastasis [47]. In previous studies, activated AKT existed in CRC tumors, which has been shown to be a poor prognostic factor for CRC patients[48]. Overexpression of downstream factors of AKT may result in the activation of the PI3K signaling pathway[46]. ERK signaling is also an important pathway that highly regulates cell proliferation and apoptosis. ERK is a mitogen-activated protein kinase (MAPK), which can be activated by MAPK kinase kinase (e.g., Raf), MAPK kinase (e.g., MEK), and MAPK (e.g., ERK)[49]. Activation of the ERK pathway regulates the expression of various genes and proteins that mediate cell proliferation and apoptosis. The present study demonstrated that QFGs suppressed the activations of PI3K, AKT and ERK, which showed that the anticancer effect of QFGs acts on CRC cells via the PI3K/AKT and ERK signaling pathways.
Colorectal cancer (CRC) is a major public health problem, representing the third cause of cancer deaths worldwide. Surgery and adjuvant chemotherapy are the main treatment for CRC. However, 40-50% of patients still die due to recurrence, metastases and drug resistance. In addition, severe side effects caused by chemotherapy agents lead to the deterioration of patient quality-of-life and therapeutic application. Therefore, the search for novel therapies has attracted worldwide attention. Qingjie Fuzheng granules (QFGs) is a traditional Chinese medicine formula with properties of anti-inflammation, antioxidative, antibacterial, immunity enhancement, and digestion promotion. QFGs has been widely used and found to be clinically effective in various cancer treatments, including CRC, and has few side effects. However, the precise mechanisms and molecular signaling pathways involved in the activity of QFGs’ anticancer effects have not been reported in the literature. In this study, we hypothesized that QFGs can inhibit the growth of CRC cells, and that its mechanism is closely related to one or more intracellular signal transduction pathways.
To better understand the mechanism underlying the potential anti-cancer effect of QFGs on the human CRC cell variants HCT-116 and HCT-8.
To elucidate the effect of QFGs on the biological function of CRC cells, and to investigate this biological function to explore the exact mechanism of QFGs effects on CRC cells.
First, cell viability and cytotoxicity were measured by performing MTT and LDH assays. We evaluated the role of QFGs in cell proliferation and apoptosis by assessing colony formation using Hoechst 33258. Second, cell cycle and apoptosis levels were measured by fluorescence-activated cell sorting. The expression levels of survivin, cyclin D1, CDK4, p21, Bax, Bcl-2, Fas, FasL, and cleaved-caspase-3/-8/-9 were measured by performing western blotting and caspase activity assays. Furthermore, inhibitors of caspase-3/-8/-9 were also used to elucidate the exact apoptosis pathway induced by QFGs in cancer cells. Finally, activation of the PI3K/AKT and ERK signaling pathways was examined using the western blot assay to investigate the possible mechanism.
MTT and LDH assays revealed that after 0.5-2.0 mg/mL of QFGs treatment, cell viability was reduced by (6.90% ± 1.03%)–(59.70% ± 1.51%) (HCT-116; P < 0.05) and (5.56% ± 4.52%)–(49.44% ± 2.47%) (HCT-8; P < 0.05). Cytotoxicity was increased from 0.52 ± 0.023 to 0.77±0.002 (HCT-116; P < 0.01) and from 0.56 ± 0.054 to 0.81 ± 0.044 (HCT-8; P < 0.01) compared with non-QFGs treatment groups. Additionally, colony formation and Hoechst 33258 staining assays showed that QFGs inhibited proliferation and induced apoptosis in CRC cells. QFGs also increased the expression levels of Bax, Fas, and FasL, decreased the level of Bcl-2, and stimulated the activation of caspase-3/-8/-9, which were revealed by western blot and caspase activity assays. In contrast, upon adding the three caspase inhibitors, the suppression effect of QFGs on cell viability and apoptosis were markedly inhibited. Moreover, QFGs suppressed the phosphorylation levels of PI3K, AKT and ERK.
These results demonstrated that QFGs inhibit CRC cell proliferation and induce apoptosis by suppressing the PI3K/AKT and ERK signaling pathways. This indicated that QFGs are a potential new therapeutic treatment for CRC and other cancers.
Traditional Chinese Medicine (TCM) is important for the treatment of many cancers and has a long history of clinical use. If the effects and mechanisms of TCM are further elucidated, it may provide a more effective treatment for many cancer types.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Makishima M, Maric I, Sterpetti AV S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55826] [Article Influence: 7975.1] [Reference Citation Analysis (132)] |
| 2. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2912] [Article Influence: 364.0] [Reference Citation Analysis (3)] |
| 3. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21368] [Article Influence: 2136.8] [Reference Citation Analysis (3)] |
| 4. | Son ES, Kim YO, Park CG, Park KH, Jeong SH, Park JW, Kim SH. Coix lacryma-jobi var. ma-yuen Stapf sprout extract has anti-metastatic activity in colon cancer cells in vitro. BMC Complement Altern Med. 2017;17:486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Kim EJ, Kim GT, Kim BM, Lim EG, Kim SY, Kim YM. Apoptosis-induced effects of extract from Artemisia annua Linné by modulating PTEN/p53/PDK1/Akt/ signal pathways through PTEN/p53-independent manner in HCT116 colon cancer cells. BMC Complement Altern Med. 2017;17:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Strickler JH, Wu C, Bekaii-Saab T. Targeting BRAF in metastatic colorectal cancer: Maximizing molecular approaches. Cancer Treat Rev. 2017;60:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | van der Werf A, van Bokhorst QNE, de van der Schueren MAE, Verheul HMW, Langius JAE. Cancer Cachexia: Identification by Clinical Assessment versus International Consensus Criteria in Patients with Metastatic Colorectal Cancer. Nutr Cancer. 2018;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Wang J, Burke A, Tsoh JY, Le G, Wong C, Chow E, Fung LC, Nguyen TT. Exploring a Culturally Relevant Model of Cancer Prevention Involving Traditional Chinese Medicine Providers in a Chinese American Community. Eur J Integr Med. 2014;6:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | He J, Chen X, Li B, Zhou W, Xiao J, He K, Zhang J, Xiang G. Chaetocin induces cell cycle arrest and apoptosis by regulating the ROS-mediated ASK-1/JNK signaling pathways. Oncol Rep. 2017;38:2489-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Chou ST, Hsiang CY, Lo HY, Huang HF, Lai MT, Hsieh CL, Chiang SY, Ho TY. Exploration of anti-cancer effects and mechanisms of Zuo-Jin-Wan and its alkaloid components in vitro and in orthotopic HepG2 xenograft immunocompetent mice. BMC Complement Altern Med. 2017;17:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Zhong P, Yang H, Lin S, Peng J, Lin J. A Traditional Chinese Medicine Herb Mixture Qingjie Fuzheng Granules Inhibits Hepatocellular Carcinoma Cells Growth by Inducing Apoptosis. J Evid Based Integr Med. 2018;23:2515690X18789632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (3)] |
| 12. | Hayashi M, Keyamura K, Hishida T. Cyclin-dependent kinase modulates budding yeast Rad5 stability during cell cycle. PLoS One. 2018;13:e0204680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Ghule PN, Seward DJ, Fritz AJ, Boyd JR, van Wijnen AJ, Lian JB, Stein JL, Stein GS. Higher order genomic organization and regulatory compartmentalization for cell cycle control at the G1/S-phase transition. J Cell Physiol. 2018;233:6406-6413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Hardwick LJA, Azzarelli R, Philpott A. Cell cycle-dependent phosphorylation and regulation of cellular differentiation. Biochem Soc Trans. 2018;46:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Giráldez S, Galindo-Moreno M, Limón-Mortés MC, Rivas AC, Herrero-Ruiz J, Mora-Santos M, Sáez C, Japón MÁ, Tortolero N, Romero F. G<sub>1</sub>/S phase progression is regulated by PLK1 degradation through the CDK1/βTrCP axis. FASEB J. 2017;31:2925-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Magwanga RO, Lu P, Kirungu JN, Cai X, Zhou Z, Wang X, Diouf L, Xu Y, Hou Y, Hu Y, Dong Q, Wang K, Liu F. Whole Genome Analysis of Cyclin Dependent Kinase (CDK) Gene Family in Cotton and Functional Evaluation of the Role of CDKF4 Gene in Drought and Salt Stress Tolerance in Plants. Int J Mol Sci. 2018;19:pii: E2625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Aghdaei HA, Kadijani AA, Sorrentino D, Mirzaei A, Shahrokh S, Balaii H, Geraci M, Zali MR. An increased Bax/Bcl-2 ratio in circulating inflammatory cells predicts primary response to infliximab in inflammatory bowel disease patients. United European Gastroenterol J. 2018;6:1074-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Zhou M, Zhang Q, Zhao J, Liao M, Wen S, Yang M. Phosphorylation of Bcl-2 plays an important role in glycochenodeoxycholate-induced survival and chemoresistance in HCC. Oncol Rep. 2017;38:1742-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Li D, Wang J, Hou J, Fu J, Chang D, Bensoussan A, Liu J. Ginsenoside Rg1 protects starving H9c2 cells by dissociation of Bcl-2-Beclin1 complex. BMC Complement Altern Med. 2016;16:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Guo H, Chen L, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B. Research Advances on Pathways of Nickel-Induced Apoptosis. Int J Mol Sci. 2015;17:pii: E10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Lochmann TL, Bouck YM, Faber AC. BCL-2 inhibition is a promising therapeutic strategy for small cell lung cancer. Oncoscience. 2018;5:218-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Baig S, Seevasant I, Mohamad J, Mukheem A, Huri HZ, Kamarul T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016;7:e2058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 23. | Lin PY, Tsai CT, Chuang WL, Chao YH, Pan IH, Chen YK, Lin CC, Wang BY. Chlorella sorokiniana induces mitochondrial-mediated apoptosis in human non-small cell lung cancer cells and inhibits xenograft tumor growth in vivo. BMC Complement Altern Med. 2017;17:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Jing ZT, Liu W, Wu SX, He Y, Lin YT, Chen WN, Lin XJ, Lin X. Hepatitis B Virus Surface Antigen Enhances the Sensitivity of Hepatocytes to Fas-Mediated Apoptosis via Suppression of AKT Phosphorylation. J Immunol. 2018;201:2303-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Song Y, Zhao Y, Ding X, Wang X. microRNA-532 suppresses the PI3K/Akt signaling pathway to inhibit colorectal cancer progression by directly targeting IGF-1R. Am J Cancer Res. 2018;8:435-449. [PubMed] |
| 26. | He Y, Ge Y, Jiang M, Zhou J, Luo D, Fan H, Shi L, Lin L, Yang L. MiR-592 Promotes Gastric Cancer Proliferation, Migration, and Invasion Through the PI3K/AKT and MAPK/ERK Signaling Pathways by Targeting Spry2. Cell Physiol Biochem. 2018;47:1465-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Rödel C, Hofheinz R, Liersch T. Rectal cancer: state of the art in 2012. Curr Opin Oncol. 2012;24:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2292] [Article Influence: 208.4] [Reference Citation Analysis (1)] |
| 29. | Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 433] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 30. | Zhao J, Jiang P, Zhang W. Molecular networks for the study of TCM pharmacology. Brief Bioinform. 2010;11:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 31. | Lin J, Chen Y, Wei L, Chen X, Xu W, Hong Z, Sferra TJ, Peng J. Hedyotis Diffusa Willd extract induces apoptosis via activation of the mitochondrion-dependent pathway in human colon carcinoma cells. Int J Oncol. 2010;37:1331-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Ji HF, Li XJ, Zhang HY. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009;10:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 379] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 33. | Sun G, Wei L, Feng J, Lin J, Peng J. Inhibitory effects of Hedyotis diffusa Willd. on colorectal cancer stem cells. Oncol Lett. 2016;11:3875-3881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Jiang Q, Li Q, Chen H, Shen A, Cai Q, Lin J, Peng J. Scutellaria barbata D. Don inhibits growth and induces apoptosis by suppressing IL-6-inducible STAT3 pathway activation in human colorectal cancer cells. Exp Ther Med. 2015;10:1602-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Xie W, Ge M, Li G, Zhang L, Tang Z, Li R, Zhang R. Astragalus Polysaccharide Protect against Cadmium-Induced Cytotoxicity through the MDA5/NF-κB Pathway in Chicken Peripheral Blood Lymphocytes. Molecules. 2017;22:pii: E1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Wyllie AH. "Where, O death, is thy sting?" A brief review of apoptosis biology. Mol Neurobiol. 2010;42:4-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 713] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 38. | Mason EF, Rathmell JC. Cell metabolism: an essential link between cell growth and apoptosis. Biochim Biophys Acta. 2011;1813:645-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 39. | Ou L, Lin S, Song B, Liu J, Lai R, Shao L. The mechanisms of graphene-based materials-induced programmed cell death: a review of apoptosis, autophagy, and programmed necrosis. Int J Nanomedicine. 2017;12:6633-6646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 40. | Pawlowska E, Szczepanska J, Szatkowska M, Blasiak J. An Interplay between Senescence, Apoptosis and Autophagy in Glioblastoma Multiforme-Role in Pathogenesis and Therapeutic Perspective. Int J Mol Sci. 2018;19:pii: E889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 41. | Zhu Y, Liu X, Ding X, Wang F, Geng X. Telomere and its role in the aging pathways: telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 42. | Ge X, Wang Y, Li Q, Yu H, Ji G, Miao L. NK4 regulates 5-fluorouracil sensitivity in cholangiocarcinoma cells by modulating the intrinsic apoptosis pathway. Oncol Rep. 2013;30:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | García-Sáez AJ. The secrets of the Bcl-2 family. Cell Death Differ. 2012;19:1733-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 44. | Song Gh, Wang RL, Chen ZY, Zhang B, Wang HL, Liu ML, Gao YP, Yan XY. Toxic effects of sodium fluoride on cell proliferation and apoptosis of Leydig cells from young mice. J Physiol Biochem. 2014;70:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Liu J, Cui H, Peng X, Fang J, Zuo Z, Wang H, Wu B, Deng Y, Wang K. Dietary high fluorine induces apoptosis and alters Bcl-2, Bax, and caspase-3 protein expression in the cecal tonsil lymphocytes of broilers. Biol Trace Elem Res. 2013;152:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Zhou F, Nie L, Feng D, Guo S, Luo R. MicroRNA-379 acts as a tumor suppressor in non-small cell lung cancer by targeting the IGF‑1R-mediated AKT and ERK pathways. Oncol Rep. 2017;38:1857-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Dai X, Wang LJ, Wu J, Shi YX, Li GP, Yang XQ. Src kinase inhibitor PP2 regulates the biological characteristics of A549 cells via the PI3K/Akt signaling pathway. Oncol Lett. 2018;16:5059-5065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Ji B, Feng Y, Sun Y, Ji D, Qian W, Zhang Z, Wang Q, Zhang Y, Zhang C, Sun Y. GPR56 promotes proliferation of colorectal cancer cells and enhances metastasis via epithelial‑mesenchymal transition through PI3K/AKT signaling activation. Oncol Rep. 2018;40:1885-1896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Fang G, Chen S, Huang Q, Chen L, Liao D. Curcumin suppresses cardiac fibroblasts activities by regulating the proliferation and cell cycle via the inhibition of the p38 MAPK/ERK signaling pathway. Mol Med Rep. 2018;18:1433-1438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |