Published online Feb 15, 2019. doi: 10.4251/wjgo.v11.i2.153
Peer-review started: September 30, 2018
First decision: December 30, 2018
Revised: January 7, 2019
Accepted: January 8, 2019
Article in press: January 8, 2019
Published online: February 15, 2019
Processing time: 139 Days and 0.7 Hours
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide, and novel methods for early/rapid diagnosis of HCC are needed. Terahertz (THz) spectroscopy is considered to have the potential to distinguish between normal liver tissue and HCC tissue; however, there are few reports on it. We conduct this observational study to explore the feasibility of THz imaging for the diagnosis of HCC.
To evaluate the feasibility of THz for discriminating between HCC and normal liver tissues using fresh tissue specimens obtained from HCC patients who had undergone surgery.
Normal liver tissue and HCC tissue were cryosectioned into 50 μm-thick slices and placed on cover glass. Two adjacent tissue sections were separated subjected to histopathological examination by hematoxylin and eosin staining or THz transmission examination, and THz images were compared with pathologically mapped images. We determined the typical tumor and normal liver tissue regions by pathological examination; the corresponding areas of adjacent sections were examined by THz transmission.
The transmission rate of HCC tissue was 0.15-0.25, and the transmission rate of typical HCC tissue was about 0.2. THz transmittance in normal liver tissue is slightly higher than 0.4, but there were many influencing factors, including the degree of liver cirrhosis, fat components, ice crystals in frozen sections, and apoptosis.
In conclusion, this study shows that THz imaging can detect HCC tissue. Further research will yield more detailed data of the THz transmission rates of HCC tissue with different degrees of differentiation.
Core tip: Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. Currently, the most accurate diagnostic imaging modality for HCC is magnetic resonance imaging. However, it is often difficult to distinguish cirrhotic lesions. THz might be a novel modality as an early/fast diagnosis. In this study, two adjacent tissue sections, which were obtained from patients who had undergone medical surgery, were separately subjected to histo-pathological examination by either hematoxylin and eosin staining or terahertz (THz) transmission examination, and then THz images were compared with pathologically mapped images. Through transmittance coefficients analysis, it was concluded that THz has the ability to distinguish HCC tissues from normal ones.
- Citation: Duan F, Wang YY, Xu DG, Shi J, Chen LY, Cui L, Bai YH, Xu Y, Yuan J, Chang C. Feasibility of terahertz imaging for discrimination of human hepatocellular carcinoma. World J Gastrointest Oncol 2019; 11(2): 153-160
- URL: https://www.wjgnet.com/1948-5204/full/v11/i2/153.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i2.153
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide; there are an estimated 700,000 new cases and > 1 million deaths each year[1,2]. Early stage HCC has a 5-year survival rate of 40–80%, while late stage disease has a rate of only 5–15%[3], so early diagnosis of HCC is important. Currently, the most accurate diagnostic imaging modality for HCC is magnetic resonance imaging. However, as it is still sometimes difficult to distinguish HCC from cirrhotic lesions[4,5], new methods for early/rapid diagnosis of HCC are needed.
Terahertz (THz) electromagnetic waves range in frequency from 0.1 to 10 THz. The wavelength is between 0.03 and 3 mm, which is between microwave and infrared. Because the energy of the THz spectrum is low, it does not ionize biological tissues. Therefore, THz spectroscopy is considered to hold potential application for clinical examination. THz spectroscopy has been used in many biomedical studies, especially cancer diagnosis[6], including skin cancer[7,8]. Recently, THz studies have been extended to new lesions such as breast, brain and gastrointestinal cancer[9-12], but there are few reports on HCC.
In this study, we evaluated the feasibility of THz imaging for the discrimination of HCC from normal liver tissues using fresh tissue specimens obtained from patients who had undergone surgery.
The study protocol was approved by the Institutional Review Board and Research Ethics Committee of the Chinese PLA General Hospital, Beijing, China. From June to August 2017, 11 HCC patients underwent hepatectomies. Specimens of 1 cm3 were removed from the large surgical specimens obtained at operation. The specimens were cryosectioned into 50 μm-thick slices and placed on cover glass. Two adjacent tissue sections were removed for histopathological examination by hematoxylin and eosin (HE) staining or THz transmission examination.
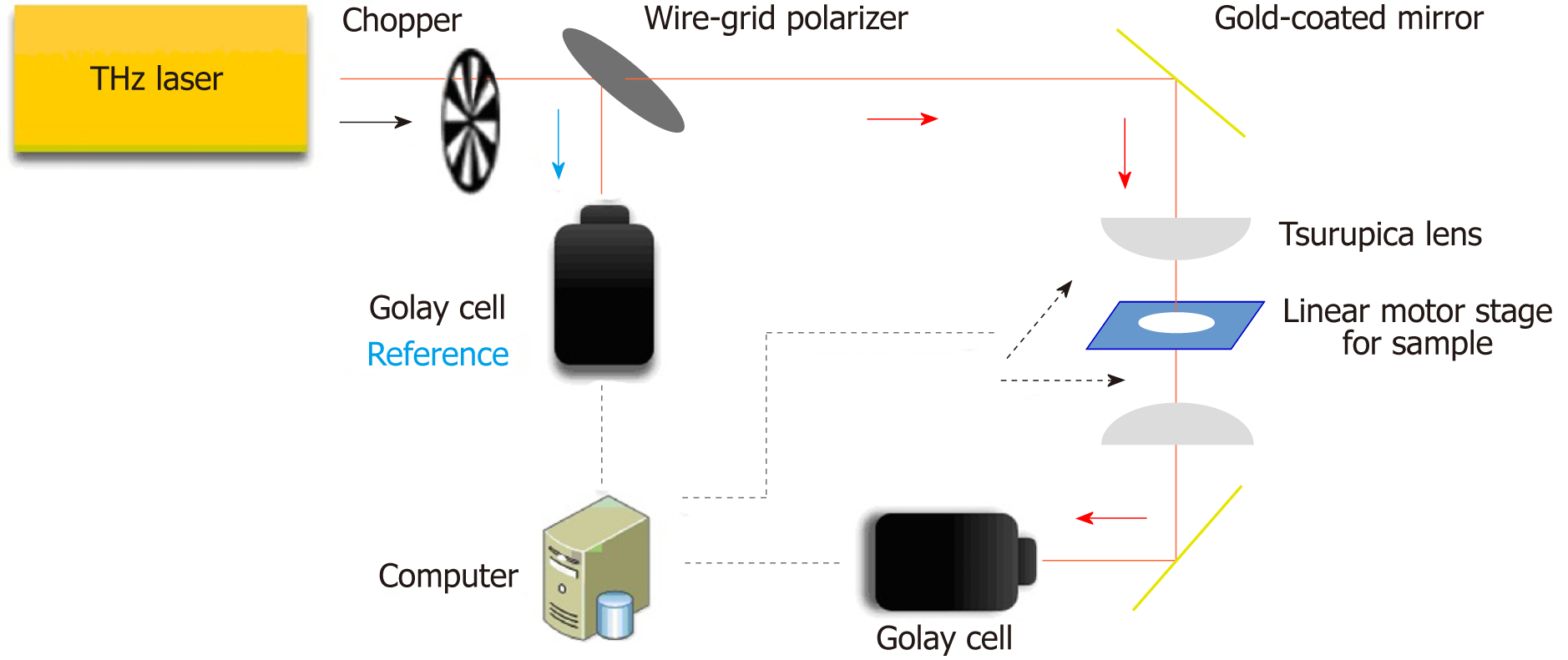
A continuous-wave THz transmission imaging system was used for examination (Figure 1). The THz source was a THz gas laser (FIRL100; Edinburgh Instruments Ltd., UK) operating at 2.52 THz. After chopping, the THz wave was separated into two sub-beams by a wire grid polarizer. One sub-beam was focused on the sample with a Tsurupica lens after several reflections, and the other sub-beam was used as the reference. The tissue section was mounted on a computer controlled x–y linear motor stage that moved through the focused beam in the horizontal plane. The detectors for THz waves were Golay cells (GC-1P; Tydex Ltd. Douhui Road 2338 Lane, Headquarter No. 1, Minhang district, Shanghai, China). The intensity of the acquired pixels in the THz images indicated the tissue transmittance. The imaging resolution was 260 μm × 380 μm, measured using the knife-edge method.
THz images were compared with pathologically mapped images. We determined the typical tumor tissue and normal liver tissue regions by pathological examination; the corresponding areas of adjacent sections were examined by THz transmission.
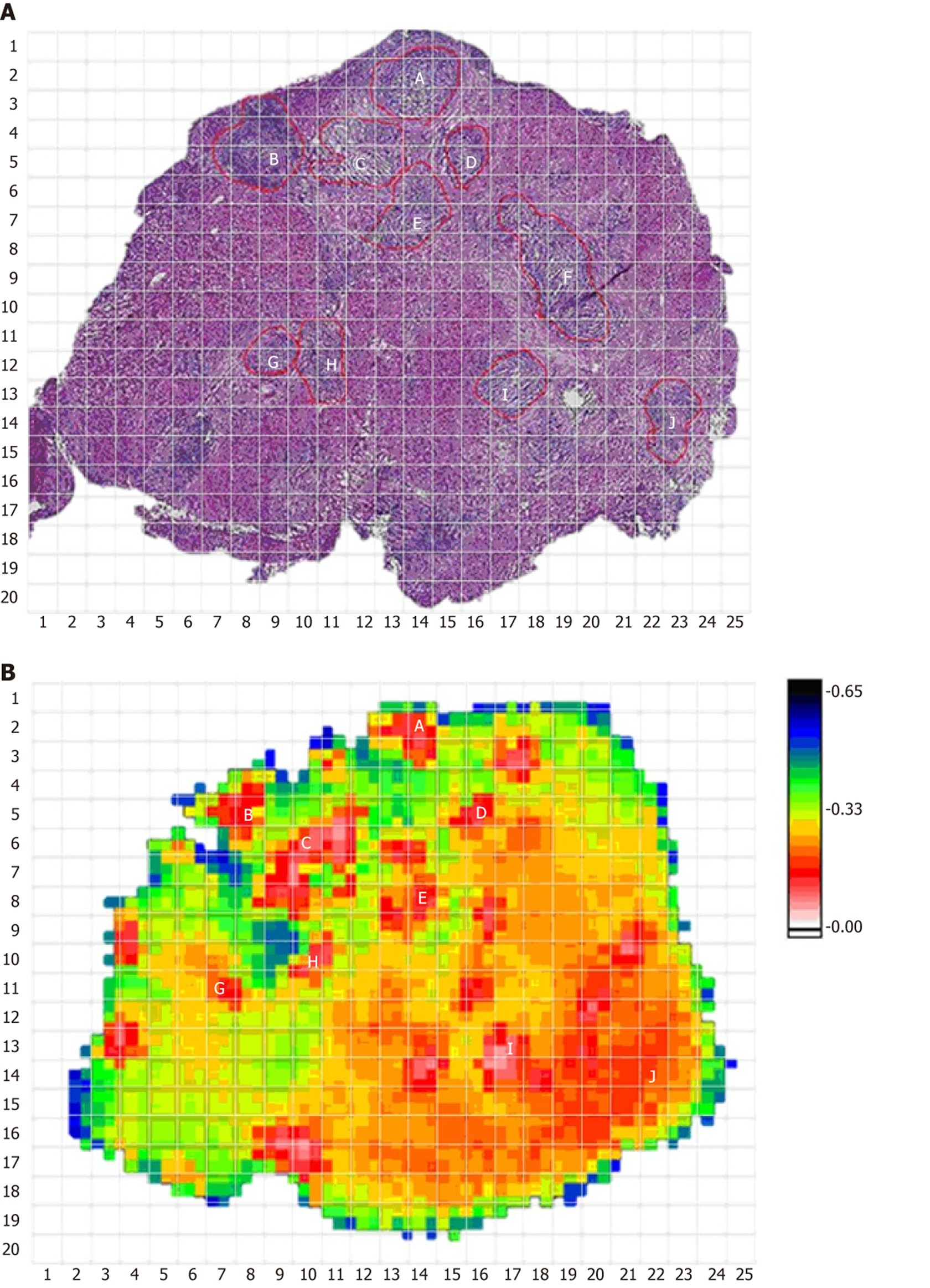
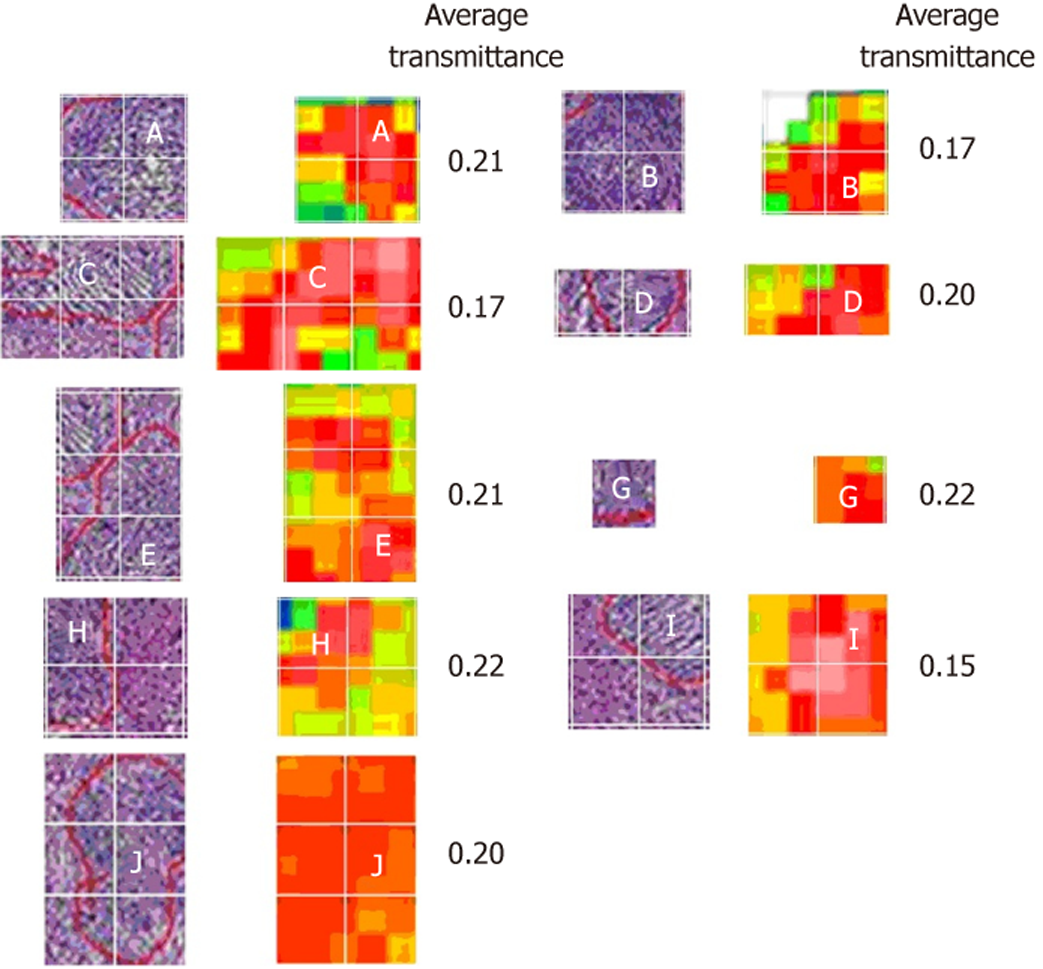
The HE staining image of one sample and its corresponding THz image are shown in Figure 2A and B, respectively. In the THz image, different colors indicate the transmittance of THz waves for tissues. The transmittance of the whole THz image was 0.27. The tumor cells in the HE-stained image and corresponding position in the THz image are marked. Most of the areas with lower transmittance (0.17–0.22) matched well with the position of the tumor cells. Most of the normal tissues had a higher transmittance of about 0.4. To analyze the statistical characteristics, these images were divided as 20 × 25 gridding, and the THz transmittance for tumor cells was calculated. Figure 3 shows the segmentation images of tumor cells in the HE-stained and THz images. The area of tumor cells in the HE-stained image did not completely correspond with the area in the THz image, with a transmittance of 0.17–0.22, which may have been due to tumor heterogeneity.
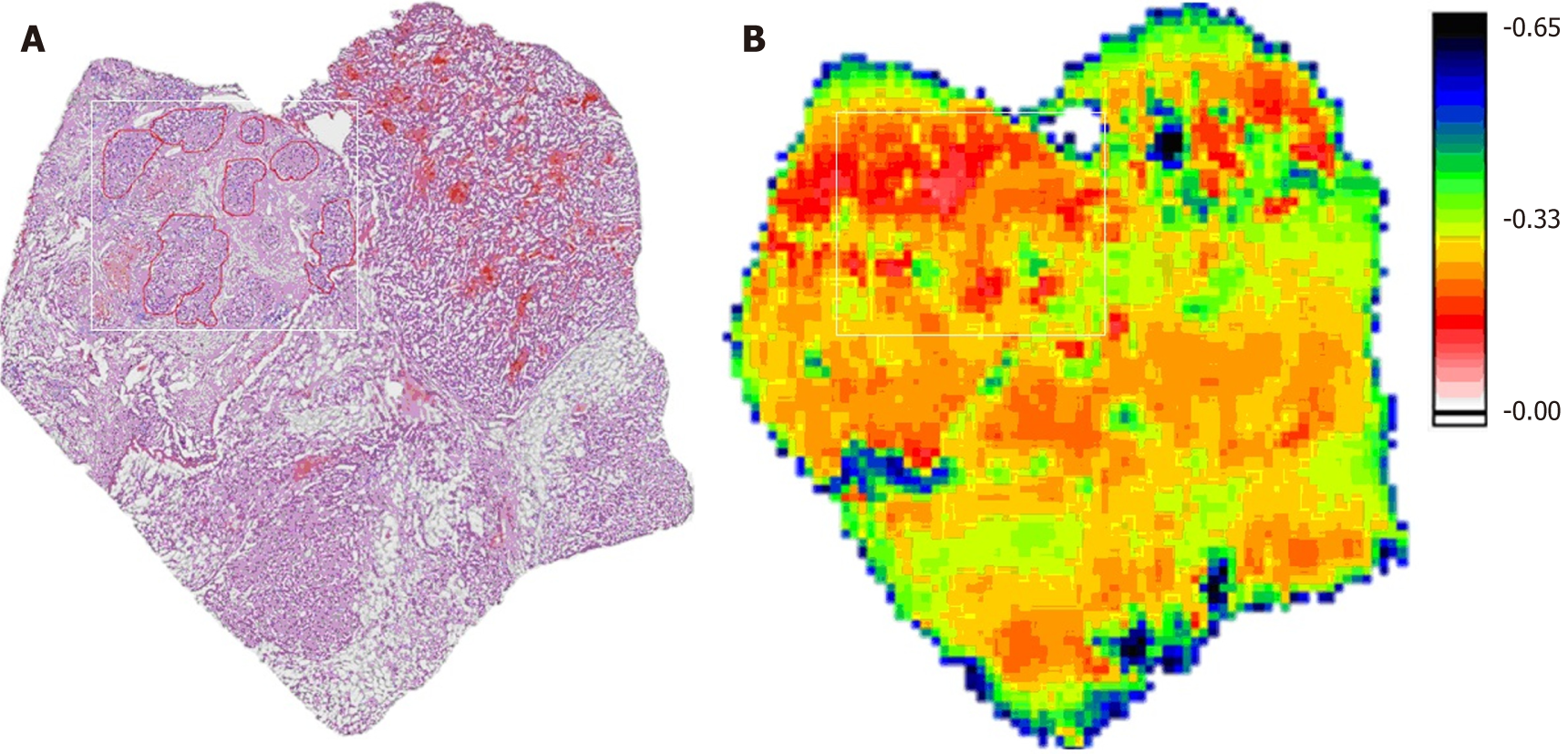
The HE-stained and THz images of a complex sample are shown in Figure 4, which had tumors, ice crystals, tumor hemorrhage and liver cirrhosis. In the THz image of this sample, the tumor area is marked by a white rectangle, and the average transmittance was about 0.2, which agreed with the sample shown in Figure 2. The area of tumor hemorrhage presented with abnormal transmittance, which was higher than that in tumors. Furthermore, the liver cirrhosis and ice crystals had lower transmittance than that of normal tissues.
THz imaging has received increasing attention as an interdisciplinary field. It has been increasingly used in diagnosis because it is fast, lacks ionizing radiation, and has no need for contrast agents.
The histopathological appearance of HCC varies greatly from patient to patient and even within a single patient, and different stages of intratumoral differentiation and growth patterns can be observed. However, progressive HCC has a consistent appearance, and in our study, all patients were in the progressive stage. The pathological components of HCC are complex, including the parenchymal and interstitial parts. The tumor parenchyma is composed of tumor cells, while the tumor interstitium is composed of sinusoids containing blood, and are lined with single endothelial cells[13]. This study mainly analyzed the THz image characteristics of tumor parenchyma.
The parenchymal part of HCC is divided into beam, solid and fibrous scleroses according to the structure of the tumor cells. The tumor cells differ in terms of fatty degeneration, cytoplasmic glass-like variation, and the degree of nuclear differentiation. By those characteristics, the tumor can be divided into high-, medium- and low-differentiated tumor. Cell density, the ratio of the nucleoplasm to cytoplasm, and fat denaturation all affect THz transmittance. In general, the density of tumor cells in HCC is higher than that of hepatocytes in normal liver tissues, and the nucleoplasm to cytoplasm ratio is higher than that of normal liver cells[14-16]. That is, the density of HCC tissues is higher than that of normal liver tissues, and, theoretically, the transmission rate of HCC tissue is lower than that of normal liver tissue, which is consistent with our experimental results[17].
Due to the heterogeneity of HCC, histomorphologic appearance of HCC varies greatly from patient to patient and even in a single patient[18]. Different stages of intratumoral differentiation and growth patterns can be observed, where in some cases HCC cells are mixed with normal cells[19]. This could suggest that the THz transmittance of each section is uneven. In our experiment, we select the corresponding area in the THz image based on the most typical HCC area of the pathological section. The domain is used to determine the THz transmittance of HCC.
One case in our series showed that the transmission rate of HCC tissue was 0.34, which was not consistent with other results, possibly due to the long time period from obtaining the specimen to the final filming. This resulted in the necrosis and disintegration of the tumor cells[20], which might increase the transmission rate of THz. Further studies on the THz transmission rate of apoptotic cells need to be conducted.
In this study, THz images were compared with pathologically mapped images. We determined the typical tumor tissue and normal liver tissue regions by pathological examination, and the characteristics of the THz images in the corresponding regions were analyzed. The HCC THz imaging characteristics did not include the interstitial part of the tumor, nor were they subdivided at each cell level. We therefore need to examine more specimens.
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide, and novel methods for early/rapid diagnosis of HCC are needed. Recently, terahertz (THz) spectroscopy is more and more often used for tumor diagnosis; however, most of these reports focus on superficial tissues, and there are few reports on HCC.
Combined with interventional radiology techniques, THz spectroscopy might be a novel modality as an early/fast diagnostic for HCC. Therefore, we conduct this observational study to explore the feasibility of THz imaging for the diagnosis of HCC.
To evaluate the feasibility of THz for discriminating between HCC and normal liver tissues using fresh tissue specimens obtained from HCC patients who had undergone surgery. The goal was to identify if THz could be a novel modality as an early/fast diagnostic for HCC.
An observational study was conducted. Normal liver tissue and HCC tissue were cryosectioned into 50 μm-thick slices and placed on cover glass. Two adjacent tissue sections were separately subjected to histopathological examination by hematoxylin and eosin staining (HE staining) or THz transmission examination, and THz images were compared with pathologically mapped images. We determined the typical tumor and normal liver tissue regions by pathological examination; the corresponding areas of adjacent sections were examined by THz transmission.
The transmission rate of HCC tissue was 0.15-0.25, and the transmission rate of typical HCC tissue was about 0.2. THz transmittance in normal liver tissue was slightly higher than 0.4, but there were many influencing factors, including the degree of liver cirrhosis, fat components, ice crystals in frozen sections, and apoptosis.
This study shows that THz imaging can identify HCC tissue. Further research will yield more detailed data of THz transmission rates of HCC tissue with different degrees of differentiation. Through our study, we identified that THz could be a novel modality as an early/fast diagnostic for HCC.
In the future, THz combined with interventional radiology techniques might be a novel modality as an early/fast diagnostic for internal body tissue malignancies.
We would like to extend our gratitude to all departmental staff for their support. Tribute should also be paid to the patients who gave signed informed consent.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent):
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Takamatsu S, Charco R, Ward J S- Editor: Wang JL L- Editor: Filipodia E- Editor: Bian YN
| 1. | Ma KW, Cheung TT. Surgical resection of localized hepatocellular carcinoma: patient selection and special consideration. J Hepatocell Carcinoma. 2016;4:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21370] [Article Influence: 2137.0] [Reference Citation Analysis (3)] |
| 3. | Dai CY, Lin CY, Tsai PC, Lin PY, Yeh ML, Huang CF, Chang WT, Huang JF, Yu ML, Chen YL. Impact of tumor size on the prognosis of hepatocellular carcinoma in patients who underwent liver resection. J Chin Med Assoc. 2018;81:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Ayuso C, Rimola J, Vilana R, Burrel M, Darnell A, García-Criado Á, Bianchi L, Belmonte E, Caparroz C, Barrufet M, Bruix J, Brú C. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol. 2018;101:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 5. | Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67:401-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 6. | Oh SJ, Kang J, Maeng I, Suh JS, Huh YM, Haam S, Son JH. Nanoparticle-enabled terahertz imaging for cancer diagnosis. Opt Express. 2009;17:3469-3475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Woodward RM, Wallace VP, Pye RJ, Cole BE, Arnone DD, Linfield EH, Pepper M. Terahertz pulse imaging of ex vivo basal cell carcinoma. J Invest Dermatol. 2003;120:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Wallace VP, Fitzgerald AJ, Shankar S, Flanagan N, Pye R, Cluff J, Arnone DD. Terahertz pulsed imaging of basal cell carcinoma ex vivo and in vivo. Br J Dermatol. 2004;151:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Fitzgerald AJ, Wallace VP, Jimenez-Linan M, Bobrow L, Pye RJ, Purushotham AD, Arnone DD. Terahertz pulsed imaging of human breast tumors. Radiology. 2006;239:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Ashworth PC, Pickwell-MacPherson E, Provenzano E, Pinder SE, Purushotham AD, Pepper M, Wallace VP. Terahertz pulsed spectroscopy of freshly excised human breast cancer. Opt Express. 2009;17:12444-12454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Oh SJ, Kim SH, Ji YB, Jeong K, Park Y, Yang J, Park DW, Noh SK, Kang SG, Huh YM, Son JH, Suh JS. Study of freshly excised brain tissues using terahertz imaging. Biomed Opt Express. 2014;5:2837-2842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Sim YC, Park JY, Ahn KM, Park C, Son JH. Terahertz imaging of excised oral cancer at frozen temperature. Biomed Opt Express. 2013;4:1413-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Schlageter M, Terracciano LM, D'Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol. 2014;20:15955-15964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 138] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (4)] |
| 14. | Schietroma I, Scheri GC, Pinacchio C, Statzu M, Petruzziello A, Vullo V. Hepatitis C Virus and Hepatocellular Carcinoma: Pathogenetic Mechanisms and Impact of Direct-Acting Antivirals. Open Virol J. 2018;12:16-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Miura Y, Kamataki A, Uzuki M, Sasaki T, Nishizawa J, Sawai T. Terahertz-wave spectroscopy for precise histopathological imaging of tumor and non-tumor lesions in paraffin sections. Tohoku J Exp Med. 2011;223:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Minamide H, Tang M, Notake T, Ito H. Study of water concentration measurement in thin tissues with terahertz-wave parametric source. Opt Express. 2010;18:15504-15512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Tewari P, Taylor ZD, Bennett D, Singh RS, Culjat MO, Kealey CP, Hubschman JP, White S, Cochran A, Brown ER, Grundfest WS. Terahertz imaging of biological tissues. Stud Health Technol Inform. 2011;163:653-657. [PubMed] |
| 18. | Jiang K, Al-Diffhala S, Centeno BA. Primary Liver Cancers-Part 1: Histopathology, Differential Diagnoses, and Risk Stratification. Cancer Control. 2018;25:1073274817744625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Geramizadeh B, Asadi N, Tabei SZ. Cytologic comparison between malignant and regenerative nodules in the background of cirrhosis. Hepat Mon. 2012;12:448-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Wang YY, Notake T, Tang M, Nawata K, Ito H, Minamide H. Terahertz-wave water concentration and distribution measurement in thin biotissue based on a novel sample preparation. Phys Med Biol. 2011;56:4517-4527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |