Published online Feb 15, 2019. doi: 10.4251/wjgo.v11.i2.117
Peer-review started: September 19, 2018
First decision: October 16, 2018
Revised: November 20, 2018
Accepted: January 10, 2019
Article in press: January 10, 2019
Published online: February 15, 2019
Processing time: 150 Days and 0.6 Hours
Identifying biomarkers for the risk of developing degenerative processes linked to aging and colorectal cancer (CRC) onset that could improve clinical strategies.
To determine valid targets and a predictive biomarker’s system of chronicization of inflammation for cancer treatment.
A group of 147 CRC patients was studied. Clinical diagnosis was confirmed histopathologically, and patients were sub-typed using the pathological tumor-node-metastasis classification. Thirteen colon adenoma patients and 219 healthy subjects were also studied. A system biology study on Thioredoxin1/CD30 redox-immune systems (Trx1/CD30), T helper cytokines and polymorphisms of killer immunoglobulin-like receptors, FcγRIIa-131H/R and FcγRIIIa-158V/F was carried out. Enzyme-linked immunosorbent assay was performed to analyze sera. Genetic study was executed by polymerase chain reaction sequence-specific primers and sequence-based typing method. Statistical analysis was performed by using the “Statgraphics software systems”.
We found a positive increase between Trx1/RTrx1 levels and sCD30 level and increased age. With respect to the gender relationships, there were distinct differences. Females showed a primary relationship between transforming growth factor beta (TGFβ) with Trx1, whereas males had one with TGFβ and RTrx1. Trx1/CD30 controls the redox immune homeostasis, and an imbalance in the relationship between the Trx1/RTrx1 and sCD30 levels is linked to the onset and progression of tumor. This event happens through different gender-specific cytokine pathways. Our study demonstrated that the serum levels of Trx1/RTrx1, TGFβ/interleukin (IL)6 and TGFβ/IL4 combinations and the sCD30, IFNγ and IL2 combination constitute a predictive gender specific biomarker system. This is relevant for clinical screening to detect the risk of the potential development or progression of a tumor.
Oxidative stress on Trx1/CD30 is a trigger of cancer disease, and the selected oxidation and immune products are a biomarker system for aging and cancer.
Core tip: Identifying biomarkers for the risk of developing degenerative processes linked to aging and colorectal cancer onset could improve clinical strategies. The aim was to establish if Trx1 and CD30 as dual targets, combined with cytokines and polymorphisms of killer immunoglobulin-like receptors, FcγRIIa-131H/R and FcγRIIIa-158V/F, was more effective. Serum measures of Trx1/CD30, RTrx1, multiple cytokine levels and polymorphisms of killer immunoglobulin-like receptors and FcγRIIa were used to estimate the effect of disease, age and gender to describe the variants in the biology of the redox immune system. Through these procedures, the redox immune fingerprint of health, aging and cancer states was determined.
- Citation: Berghella AM, Aureli A, Canossi A, Beato TD, Colanardi A, Pellegrini P. Redox, immune and genetic biomarker system for personalized treatments in colorectal cancer. World J Gastrointest Oncol 2019; 11(2): 117-138
- URL: https://www.wjgnet.com/1948-5204/full/v11/i2/117.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i2.117
We are witnessing a progressive rise in average age and people with chronic inflammation and degenerative pathologies. Thus, for healthy longevity, it is essential to improve the prevention strategies for these pathologies in both the healthy subjects and those who are already ill and need to undergo clinical treatment. However, to obtain this it is necessary to overcome the difficulties in the early diagnosis of diseases and effective treatment. Research[1] indicates that it is necessary to identify valid physiological parameters to select targets and biomarkers with features that make this “utopia” realistic to formulate such solving strategies. Biomarkers are generally selected by system biology studies on biological parameters that affect the pathogenesis and evolution of the disease. We suggest that the best ways to make an early diagnosis of cancer and support clinical and therapeutic monitoring is to employ serum, tissue and micro-environmental biomarkers.
There is a growing consensus that aging is related to a low-grade inflammatory state that could cause the loss of physiological homeostasis and compromise the functioning of physiological regulatory systems and their mutual communication[2]. This could cause the development of a chronic-inflammatory state, which could consequently lead to degenerative processes and to the onset of chronic-degenerative pathologies related to aging.
To identify valid parameters to select targets and biomarkers, the system biology study should shed light on the physio and pathological pathways, which regulate the homeostasis that control inflammation. This clarification is necessary in the transition from the state of healthy (characterized by homeostasis in the control of inflammation), to that of temporary acute inflammation (in which the re-establishment of this equilibrium is yet very probable), and then on to a chronic steady inflammation condition (where this recovery is physiologically very improbable, while the risk of progression toward degenerative pathologies is high). In this way, it will be possible to select the biological molecules, whose variations could quantify our personal future risk of losing control of inflammation, which could allow the tumor to develop and progress. To study the precursor state of colorectal cancer (CRC) tumor it is to be considered that CRC has a stepwise nature, and colon adenoma has been identified as a precursor of this tumor. Based on this, colon adenoma could be evaluated as a pre-stage of CRC stage I. In the latest directives[3] of the European consortium of researchers [European Cooperation in Science and Technology (COST)], the working group on redox biomarkers research networks indicates oxidation products as redox biomarkers. They refer that biological systems are influenced by a variety of endogenous and exogenous oxidants able of altering proteins, which could influence cell-signaling pathways and take part in inflammatory and chronic diseases. Therefore, they indicate the need to increase understanding of redox biology into advances in these human disease therapies. We must take into account that the redox and immune systems are among the most compromised, in the functioning and their mutual communication, by aging[4]. Men and women follow different strategies for a healthy long lasting life[4,5]. In fact, they have different physiological pathways for the regulation of immune response homeostasis. Aging-related alterations of gender pathways predispose men and women differently to diseases and therapeutic response[5,6]. Furthermore, results indicate redox immune homeostasis as a target and biomarker for preventing aging-related immune process and diseases, and T helper (Th) cells are the determinant elements in age-related pathological immunity[4]. Understanding how environmental signals interact with T cell metabolism and redox state to influence Th subset differentiation could clarify how aging compromises immune function. This will allow identifying the target and biomarkers for new resolving treatments[4].
Consequently, we think that the study of Trx1/CD30 interactions could be interesting. This investigation could lead to the discovery of gender-specific changes induced by aging in the control of inflammation and select the above biomarkers for personalized clinical screening. Our hypothesis is based on the following.
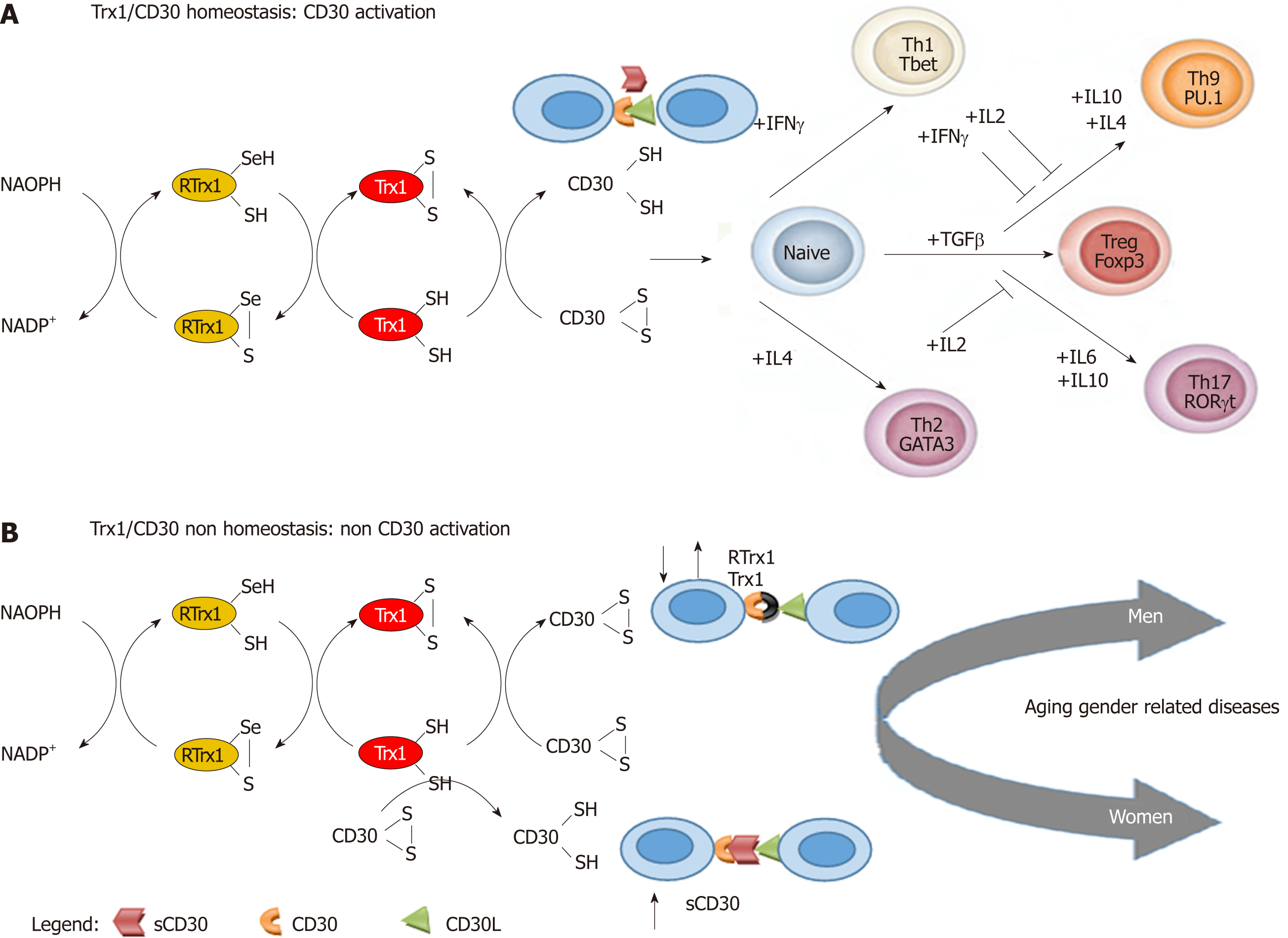
Trx1 is a thermostable redox protein (constituted of 108 amino acids)[7]. It performs its redox immune functions by regulating the cell thiol redox state of the immune cells, which is a crucial mediator of multiple metabolic signaling and transcriptional processes. However, this redox immune regulation can only take place if Trx1 can interact with its specific target, the CD30 receptor, on the cell membrane. It does this by going through the subsequent pathways[8] (Figure 1).
Trx1 contains an S-S bridge, and it has a catalytic domain that is a donor of hydrogen for redox reactions. The Trx1 oxidized form (Trx1-S-S-) is converted into the reduced form (Trx1-SH) by the thioredoxin reductase flavoprotein (RTrx1), with the involvement of nicotinamide adenine dinucleotide phosphate. All these molecules constitute the “thioredoxin1 redox-system” (Figure 1A). The reduced Trx1 form (Trx1-SH) is able to interact with the oxidized CD30 (CD30 S-S) and reduce it (CD30 S-H). It can only interact in this latter form with its ligand (CD30L) on activated natural killer (NK), dendritic (DC), monocytes, T, neutrophils, eosinophils and B cells[1,9]. It has now been established that the normal interaction between these redox-immune molecules regulates the homeostasis of interferon gamma (IFNγ), interleukin (IL)4, transforming growth factor beta (TGFβ), IL6, IL10 and IL2 cytokine and cellular pathways. This homeostasis determines normal balance within Th differentiation in the respective subtypes (Th1, Th2, Treg, Th9 and Th17)[8-10] and, consequently, the homeostasis of Trx1/CD30 (Figure 1A).
The importance of cytokines is explained by the fact that the cytokine type in the cell microenvironment is the most important factor for the above processes. This is because they activate the specific cell transcription factors for the differentiation of the corresponding type of Th subset (Figure 1A). Th1 requires T bet[11], Th2 GATA3[12], Treg cells Forkhead box P3 (Foxp3)[13], Th17 retinoic acid-related orphan receptor gt[14,15] and Th9 PU.1 bet[13-15] (Figure 1A). IFNγ mediated signaling induces the first wave of T-bet expression[11] for the generation of Th1 cells. On the other hand, the expression of GATA-3 that is induced by IL4 is a limited time switch for the differentiation of Th2 cells[12]. Moreover, TGFβ is fundamental for the expression of Foxp3, which generates Treg cells[13]. However, the increase in levels of IL6 can inhibit the TGFβ driven expression of Foxp3[14,15] and activate the ROR-gt transcription factor, which triggers Th17 cells. Nevertheless, IL2 inhibits Th17 induction[16]. A significant IL4 increase also inhibits TGFβ induction of Foxp3 expression, but TGFβ together with IL4 activate the expression of the PU.1 transcription factor, which generates Th9 cells[16]. IFNγ and IL2 inhibit Th9 generation[16]. Therefore, the normal interaction of all these network elements determines redox immune system homeostasis and consequently a healthy state during aging for both men and women (Figure 1A).
Significant changes in the levels of these cytokines in the cellular microenvironment network are polarization biomarkers of inappropriate cell populations that could be the cause of physiological non-homeostasis and a lack of control of inflammation. However, it must be pointed out that these changes could also be caused by a lack of regulation at the level of the interaction between Trx1/CD30[8]. As a consequence, it is interesting to see that the IFNγ pathways are the principal regulators of the male immune system homeostasis, while the IL6 pathways are regulators of the female one[17]. In healthy subjects, the immunological response is produced through the gender-specific pathways, but they usually have no consequences until alterations occur. In both genders, the immune system produces its own responses and retrieves its original equilibrium, careless of the original or the evolution situations. Conversely, if modifications take place in the gender pathways and they become chronic within the physiological network, it could induce a gender specific chronic non-homeostasis state (Figure 1B). The reason should be looked for in the fact that alterations in the IFNγ pathways create an unbalance at the level of inhibiting the effect of these pathways on the Th9 cell generation in men. Likewise, alterations in the IL6 pathways create an unbalance at the level of the activating effect of IL6 on Th17 cells in women. Therefore, we could consider that changes of gender pathways in the cytokine cellular microenvironment network could produce different results. The polarization of Th17 cells plays a key role in autoimmune diseases in women, while the polarization of Th9 cells has a significant role in degenerative diseases in men[18]. Consequently, the chronicization of the increase of both the cytokine couples that induce Th17 and Th9 subtype and the decrease of cytokines, which inhibit these pathways, could be considered as predictive biomarkers in the clinical screening of gender specific susceptibility and clinical course in diseases.
In practice, normal or high levels of both IFNγ and IL2 and a negative relationship between TGFβ-IL6 levels in women and TGFβ-IL4 levels in men could be considered as gender biomarkers of the homeostasis and of non-disease risk. On the contrary, low levels of both IFNγ and IL2 and a positive TGFβ-IL6 relationship in women and TGFβ-IL4 in men could be gender biomarkers of non-homeostasis and of high disease risk.
In normal conditions, Trx1 is able to modify the stoichiometric structure of the CD30 receptor[19] to allow CD30L (CD153) binding (Figure 1A). To do this, it needs similar RTrx1 levels. However, the binding site of CD30L could be blocked by an inappropriate increase in the sCD30 level, with which it has a strong affinity[20]. Thus, regarding the redox molecules (Figure 1B), the unbalance between the Trx1/RTrx1 levels and an increase in the sCD30 level are also non-homeostasis biomarkers in both genders. Hence, they are also able to cause non-homeostasis of the redox immune system through their inhibiting action on the activation of CD30[20]. Therefore, an alteration between Trx1/RTrx1 levels and an increase of the sCD30 level should be biomarkers of the non-homeostasis of the redox immune system (i.e. chronic inflammation) and high disease risk (Figure 1B).
NK cells provide the first-line of defense against malignant cells thanks to their ability to kill tumor cells. They can do this by means of cytokine production and cross talking with the adaptive system. This cooperation is mediated by the interaction between CD30 on the NK cells and CD30L on the immature DC. This binding induces the secretion of cytokines by immature DC via the mitogen-activated protein kinase pathways and causes the differentiation of mature DC cells and the release of TNF/IFNγ by NK cells. The homeostasis of these interactions allows the generation of specific DC for the immune response and then for the preservation of good health[9].
A balance of signal transduction achieved by both activating and inhibitory receptors[21], among which the killer immunoglobulin-like receptors (KIRs) play an important regulatory function, regulates NK cell activity. KIR polymorphisms and their interaction with HLA alleles may influence susceptibility to inflammatory diseases, viral infections and malignancies. It is known that KIR2DL1 and KIR2DS1 bind to HLA-C2, and KIR2DL3, KIR2DS2 and KIR2DL2 bind to HLA-C1. Up to now, specificities of the remaining KIRs are still unknown.
Furthermore, antibody-dependent cell-mediated cytotoxicity (ADCC) is an important mechanism of defense of the immune system in the killing of tumor cells. The receptors for the Fc domain of immunoglobulin G (FcgR), which interact with antibodies linked to their cognate antigens, give a crossing point between specific humoral and cellular immunity. They appear to be the only molecules on human myeloid cells that are able to mediate the ADCC of tumors and may be important in antibody therapy against cancer. FcgRs are expressed in a wide variety of human leukocytes. Several FcR subtypes have been identified and can be distinguished by their affinity for the antibody Fc-fragment and by the signaling pathway they induce. In humans, there are activating FcγRI (CD64), FcγRIIa (CD32A), FcγRIIc (CD32C), FcγRIIIa (CD16A) and FcγRIIIb (CD16B) and inhibitory FcγRIIb (CD32B)[22]. Some polymorphisms of FcgR have been identified and could have significant clinical relevance[23]. FcγRIIIa and FcγRIIa transmit activating signals to NK lymphocytes and myeloid cells, thus assuring the connection between the innate and the adaptive immune responses. FcγRIIIa is expressed in NK lymphocytes and macrophages, while FcγRIIa is widely expressed in myeloid cells[24] . Two functional polymorphisms of human FcgRIIa and FcgRIIIa have been identified in the extracellular regions of these receptors: phenylalanine/valine-158 of FcgRIIIa (FcgRIIIa-158F/V) and histidine/arginine-131 of FcgRIIa (FcgRIIa-131H/R). They both modulate their affinity for certain human immunoglobulin G subclasses[25,26]. Clinical studies have reported that the presence of FcgRIIa-131H/H and FcgRIIIa-158V/V genotypes is associated with a more efficient ADCC antitumor response. Therefore, the FcγRIIa-131H/R and the FcγRIIIa-158V/F polymorphisms are early biomarkers because they are valid as genetic stratification parameters for quantifying the percentage of risk forecast by Trx1/CD30. Consequently, research on Trx1/CD30 must be considered very useful for the formulation of personalized clinical solving strategies to improve healthy aging and tumor treatment. In view of this, in order to validate or refute our hypothesis about the potential benefits of Trx1/CD30 screening in predictive medicine, the interactions between serum levels of Trx1, RTrx1 sCD30 molecules and Treg, Th1, Th9, Th17 cytokines with the polymorphism of KIRs, FcγRIIa-131H/R and FcγRIIIa-158V/F were examined. A redox-immune biological system study was carried out in healthy, adenoma and CRC individuals.
Human study was performed in accordance with the standards of the Ethics Committee (Good clinical practice, No. 18-Prot. n 43/C.E), and all persons gave their written informed consent prior to their inclusion in the study.
We studied a group of 147 patients (90 men and 57 women), distribution of age (66.7 ± 0.1; minimum 44, maximum 90), who were diagnosed for the first time as having CRC and had to undergo colectomy (Supplement Table 1). Distribution of age in male and female groups was the same (respectively: 67.3 ± 1.1 and 68 ± 1.4, P = 0.70).
Clinical diagnosis was confirmed histopathologically and patients were sub-typed using the pathological tumor-node-metastasis classification (according to the diagnostic criteria of the American Joint Committee on Cancer and the Committee of the International Union Against Cancer), as follows: 32 stage I (men 22, women 10), 59 stage II (men 34, women 25), 34 stage III (men 17, women 17), 22 stage IV, (men 17, women 5). Distribution of age in male and female stage groups was the same: stage I (men 67.7 ± 2.4, women 64.4 ± 3.1, P = 0.25), stage II (men 67,3 ± 1.9, women 70.7 ± 1.5, P = 0.40), stage III (men 64,5 ± 2.6, women 65.4 ± 3.3, P = 0.78), stage IV (men 71.7 ± 2.5, women 67.6 ± 5.3, P = 0.55).
A group of 13 colon adenoma patients (five men and eight women) was also studied. Distribution of age in the male and female groups was the same (men: 67.8 ± 3.6; women 69.8 ± 4.3, P = 0.94). Clinical diagnosis was confirmed histopathologically.
A group of 219 healthy subjects was also studied (130 men and 89 women). Distribution of age (51 ± 1.1; minimum 22, maximum 100). None of the subjects was receiving concurrent drug treatment including widely used pharmaceuticals, such as salicylates and sex hormones (contraceptive pill, hormone replacement therapy). Distribution of age in the male and female groups was the same (respectively: 50.0 ± 1.2 and 52.0 ± 1.9, P = 0.70).
A genetic study was carried out in a subgroup of 52 healthy subjects (19 men, 33 women), 34 CRC patients (22 men, 12 women), and five adenoma patients (one man, four women) matched for age (66.6 ± 13.8 in healthy subjects; 71.5 ± 8.6 in CRC patients; 67.0 ± 12.4 in adenomas)
In physiological systems, the different components operate as a network. They vary dynamically and co-vary with each other. Therefore, the comprehension of how the gender-specific physio-pathological pathways of the redox immune systems interfere with aging and lead to diseases can only be achieved through a system biology study. Due to the complexity of network biological systems, this study requires the use of models of the redox immunological system, which provide a framework for determining the outcome and significance of the numerous and simultaneous time-dependent and space-dependent network relationships[27,28].
We designed an experimental approach based on the use of data-driven computational models of the redox immune response of the health and tumor states. The models were performed by using redox immunological molecules, phenotypic antigens cellular expression, genetic polymorphisms and clinical parameters.
To evaluate the obtained results, we worked on the assumption that the balance between the levels of IL2, IFNγ, IL4, IL6, IL10 and the TGFβ cytokine network, in the healthy subject state, is a biomarker of the homeostasis of the Th1, Th2, Treg, Th9 or Th17 cell network and homeostasis of the immune response. The cytokine receptors and cytokines used were sIL2R, sIL6R, IL2, IFNγ, IL4, IL6, IL10 and TGFβ. Levels of Trx1, RTrx1 and sCD30 were determined. Network activation was evaluated by including the serum levels of sIL2R and sIL6R, which were used as markers, in the network[28].
Sera from patients and the healthy controls, collected within 1 h of withdrawal were centrifuged at 250 g and stored frozen in aliquots at -80°C until use. Enzyme-linked immunosorbent assays were employed. This method has been described in detail elsewhere[28]. The sensitivities of these assays were as follows: sIL2R = 0.27 ng/mL, IL2 = 9.1 pg/mL, IL4 = 1.3 pg/mL, IL6 = 0.92 pg/mL, IFNγ = 0.99 pg/mL, sCD30 = 0.33 ng/mL, sIL6R = 0.01 ng/mL, TGFβ = 8.6 pg/mL (Bender Med Systems GmbH, eBioscience, Inc., Vienna, Austria), RTrx1 < 0.135 ng/mL, Trx1 < 13.1 pg/mL (Cloud-Clone Corp., Houston, TX, United States)
The expression of phenotypic antigens on the surface of peripheral blood mononuclear cells was determined using monoclonal antibodies of CD3, CD8, CD16, CD56, CD57 receptors (Becton Dickinson). Peripheral blood mononuclear cells were separated by centrifugation over a Ficoll/Hypaque gradient (20 min, 1000 g). Briefly, the cells were washed twice in phosphate buffered saline (PBS) and resuspended at 5 × 106 cells/mL. One hundred microliters were utilized for each marker. A 20 L sample of fluorescein-isothiocyanate-conjugated antibody was added, vortexed and incubated in ice for 30 min. The sample was then washed twice and resuspended in 0.5 mL cold PBS for subsequent fluorescent-activated flow cytometer analysis. (Cytofluorograf-IIs, OrthoDiagnostic Systems Inc., Westwood, MA, United States)
Genomic DNA was extracted from whole blood of 52 healthy subjects (19 men, 33 women), 34 CRC patients (22 men, 12 women), and five adenoma patients (one man, four women) according to the manufacturer’s protocol (QIAamp DNA blood MiniKit, Qiagen, Courtaboeuf, France) and kept at −20°C until use. DNA concentration and purity were determined using a spectrophotometer (Beckman Instruments, Inc. Fullerton, CA, United States).
KIR genotyping was performed using polymerase chain reaction sequence-specific primers (PCR-SSP) for 17 KIR genes (KIR2DL1, 2DL2, 2DL3, 2DL4, 2DL5A, 2DL5B, 2DS1, 2DS2, 2DS3, 2DS4del and 2DS4ins, 2DS5, 3DL1, 3DL2, 3DL3, 3DS1 and 2DP1, 3DP1 pseudogenes) (KIR Typing Kit, MACS Molecular, Miltenyi Biotec, Bergisch Gladbach, Germany), with a genomic DNA control for contamination, a β-actin positive control and a negative control. PCR products were analyzed on a 2% agarose gel electrophoresis containing ethidium bromide and photo documented on ultraviolet trans-illuminator.
KIR genotyping is utilized for the analysis of gene content and the categorization of A/B haplotypes as well as for prediction of NK cell reactivity in autologous and allo NK cell-based immunotherapy. The A haplotype contains several inhibitory KIR genes and only one activating KIR (KIR2DS4). Conversely, B haplotype displays more activating KIR genes (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5 and KIR3DS1). Based on the linkage disequilibrium between particular alleles of different KIR loci, within B haplotype, two gene clusters were identified. The first group consists of four centromeric KIR genes (C4 group): KIR2DS2-2DL2-2DS3-2DL5B. The second group contains telomeric genes (T4 group) including KIR2DL5A-3DS1-2DS5-2DS1. By the presence or absence of C4 and T4 clusters, four possible subsets could be present: CXT4, C4T4, C4TX and CXTX.
Two different Fcγ receptor polymorphisms, FcγRIIa-H131R (rs1801274) and FcγRIIIa-V158F (rs396991) were performed using sequence-based typing method. DNA segments were amplified by PCR using the following primers set; FcγRIIa-131H/R: forward primer 5’-GGAGAAACCATCATGCTGAG-3’ and reverse primer 5’-CAATTTTGCTGCTATGGGC-3’ and for FcγRIIIa-158V/F: forward primer 5’- TGT AAA ACG ACG GCC AGT TCA TCA TAA TTC TGT CTT CT-3’; reverse primer 5’–CAG GAA ACA GCT ATG ACC CTT GAG TGA TGG TGA TGT TCA-3’.
Briefly, amplifications were performed on 50 ng of genomic DNA per 50 µL reaction. PCR products were then electrophoresed in 1.5% agarose gel containing 0.5 mg/mL ethidium bromide. Amplified products were purified and sequenced using Big Dye Terminator Chemistry (Applied Biosystems, Foster City, CA, United States) on an ABI Prism 3130 Genetic Analyzer (PE Applied Biosystems). Sequencing reactions were purified, and the typing was obtained based on alignment of the processed sequences with the reference sequence retrieved from GenBank.
The first author Anna Maria Berghella, who is an expert in biomedical statistics, performed the statistical study. Statistical techniques were adequate, correct and in accordance with the guidelines of the Baishideng Publishing Group. The methods have been adequately described and only homogeneous data were averaged.
The identification of Trx1/CD30, sCD30 and Treg, Th1, Th9, Th17 gender physiological pathways and biomarkers can only be achieved through procedures that take into account the biological characteristics of the systems. This type of methodology is suitable for multiple component network evaluation in biological systems. Therefore, we firstly determined the serum levels of the parameters (Table 1). Next, we determined the statistical differences by using, where appropriate, the Mann-Whitney U test, the Student's t-test (values are expressed as mean ± SD). The FcγRIIa and FcγRIIIa polymorphism frequencies in Healthy Subject and Tumor Patients, expressed as percentages, were made by using Chi-square (χ2) of goodness-of-fit statistic. Then, through the multivariate statistical procedures of correlation analysis, we evaluated the matrix of correlation of all the redox immune and clinical parameters. Finally, we studied the behavior of the multi component redox immune system as a network, by using “data driven computational models” of the principal component analysis (PCA) multivariate statistical procedures. These mathematical models only show the significant parameters. In this way, through the correlation coefficient matrix of parameters, PCA measures if the variance of each constituent can be explained by its relationships with all the other ones and then provides information about the most meaningful parameters.
| HS | Men | Women | Men vs womendifferences | |
| Trx1 | 107.6 ± 12.2 | 124.3 ± 22.7 | 98.0 ± 14.0 | NS |
| RTrx1 | 2.2 ± 0.2 | 2.7 ± 0.4 | 2.0 ± 0.2 | NS |
| sCD30 | 8.0 ± 0.7 | 6.7 ± 0.9 | 8.9 ± 1.0 | NS |
| TGFβ | 14800.0 ± 498.2 | 15150.2 ± 906.5 | 14598.3 ± 594.8 | NS |
| IL2 | 53.6 ± 4.8 | 42.1 ± 5.7 | 70.0 ± 8.1 | NS |
| IFNγ | 69.4 ± 6.9 | 53.9 ± 9.6 | 92.0 ± 9.5 | NS |
| IL4 | 9.4 ± 0.6 | 8.0 ± 0.8 | 11.4 ± 0.9 | P < 0.00001 |
| IL6 | 4.1 ± 0.8 | 2.7 ± 0.6 | 5.3 ± 1.5 | P = 0.001 |
| IL10 | 5.2 ± 0.7 | 4.8 ± 0.5 | 5.9 ± 1.7 | NS |
| sIL2R | 250.0 ± 10.4 | 238.6 ± 12.5 | 260.6 ± 16.4 | P = 0.00009 |
| sIL6R | 70.7 ± 4.0 | 67.4 ± 6.0 | 73.5 ± 5.4 | NS |
| AS | ||||
| Trx1 | 649.5 ± 60.6 | 637.9 ± 112.7 | 656.7 ± 75.5 | NS |
| RTrx1 | 3.24 ± 0.27 | 3.4 ± 0.4 | 3.1 ± 0.37 | NS |
| sCD30 | 10.7 ± 2.5 | 8.5 ± 3.1 | 12.1 ± 3.7 | NS |
| TGFβ | 24474.3 ± 665.8 | 25050.0 ± 1582.4 | 24114.5 ± 540.8 | NS |
| IL2 | 39.9 ± 23.5 | 19.2 ± 1.4 | 52.8 ± 38.4 | NS |
| IFNγ | 98.3 ± 17.5 | 130.9 ± 35.3 | 78.0 ± 16.1 | NS |
| IL4 | 25.5 ± 4.5 | 15.2 ± 5.4 | 31.9 ± 5.5 | NS |
| IL6 | 6.2 ± 2.1 | 0.5 ± 0.4 | 9.8 ± 2.8 | NS |
| IL10 | 7.1 ± 0.9 | 7.3 ± 2.1 | 6.9 ± 1.0 | NS |
| sIL2R | 295.9 ± 26.7 | 285.6 ± 50.2 | 302.3 ± 32.7 | NS |
| sIL6R | 146.2 ± 11.2 | 154.9 ± 20.2 | 140.7 ± 13.7 | NS |
| TP | ||||
| Trx1 | 1125.0 ± 72.5 | 1083.7 ± 104.3 | 1086.3 ± 108.1 | NS |
| RTrx1 | 3.5 ± 0.5 | 3.4 ± 0.6 | 4.4 ± 1.0 | NS |
| sCD30 | 18.0 ± 2.8 | 21.3 ± 2.9 | 17.8 ± 4.0 | NS |
| TGFβ | 65331.7 ± 6852.0 | 69385.9 ± 7400.6 | 70296.5 ± 16452.3 | NS |
| IL2 | 16.1 ± 3.6 | 11.2 ± 1.9 | 16.1 ± 2.5 | NS |
| IFNγ | 140.0 ± 14.4 | 135.2 ± 7.2 | 140.0 ± 11.4 | NS |
| IL4 | 160.5 ± 20.5 | 186.2 ± 33.8 | 151.6 ± 60.5 | NS |
| IL6 | 140.0 ± 46.0 | 139.5 ± 50.3 | 176.1 ± 92.0 | NS |
| IL10 | 31.4 ± 6.4 | 37.7 ± 9.1 | 22.2 ± 5.7 | NS |
| sIL2R | 532.2 ± 21.8 | 545.2 ± 29.0 | 504.2 ± 44.7 | NS |
The statistical study was performed by using the “Statgraphics software systems” (full system 5.25 version 4.0”- Graphics system by Statistical Graphics Corporation Ed. United States. 1989). Values of P < 0.05 were considered significant. A diagnostic test evaluation calculator/MedCalc-statistical software (version 17.4) was used to compare the outcomes of the patients according to FcγR polymorphisms.
The multivariate statistical procedure analyzed the correlation between the linear associations, producing the matrix of correlation coefficients (which vary from –1 to +1) and the significances (P). This procedure allowed a dynamic analysis. A positive correlation indicates that the parameters vary in the same direction, while a negative one indicates that the parameters vary in the opposite direction. Other changes are considered negative. Statistically independent parameters have an expected correlation of zero.
Using the PCA, we plotted the network of the vectors of the parameters, which were obtained by analyzing the data matrix of correlation coefficients between them. Through these plots, we can clarify the interaction between the redox and immune systems in both healthy aging and tumor progression. The length of each vector is proportional to its contribution to the total parameter variance; the angle between vectors is inversely proportional to the degree of correlation between vectors. Acute angles indicate positive relationships, while obtuse ones indicate negative relationships.
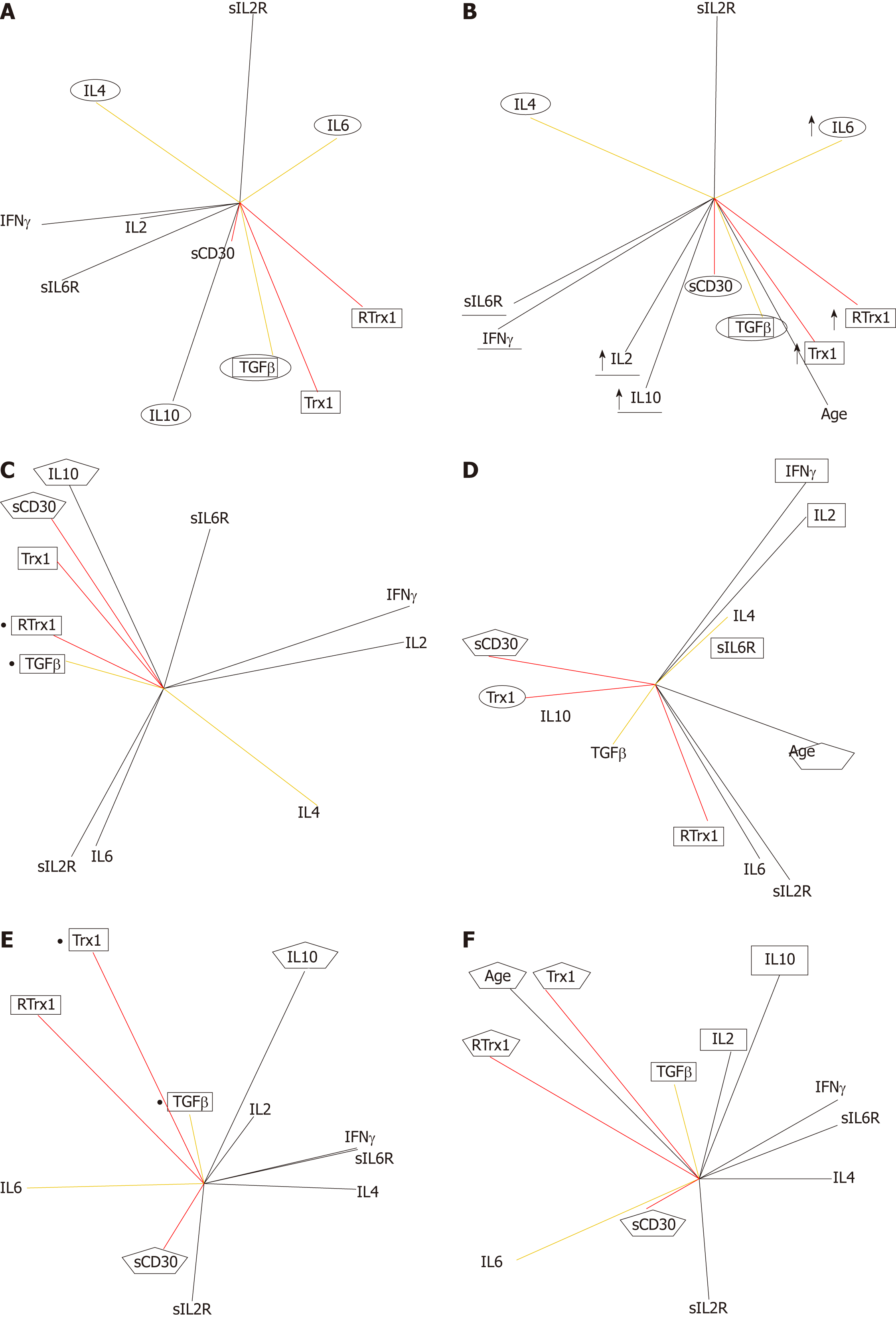
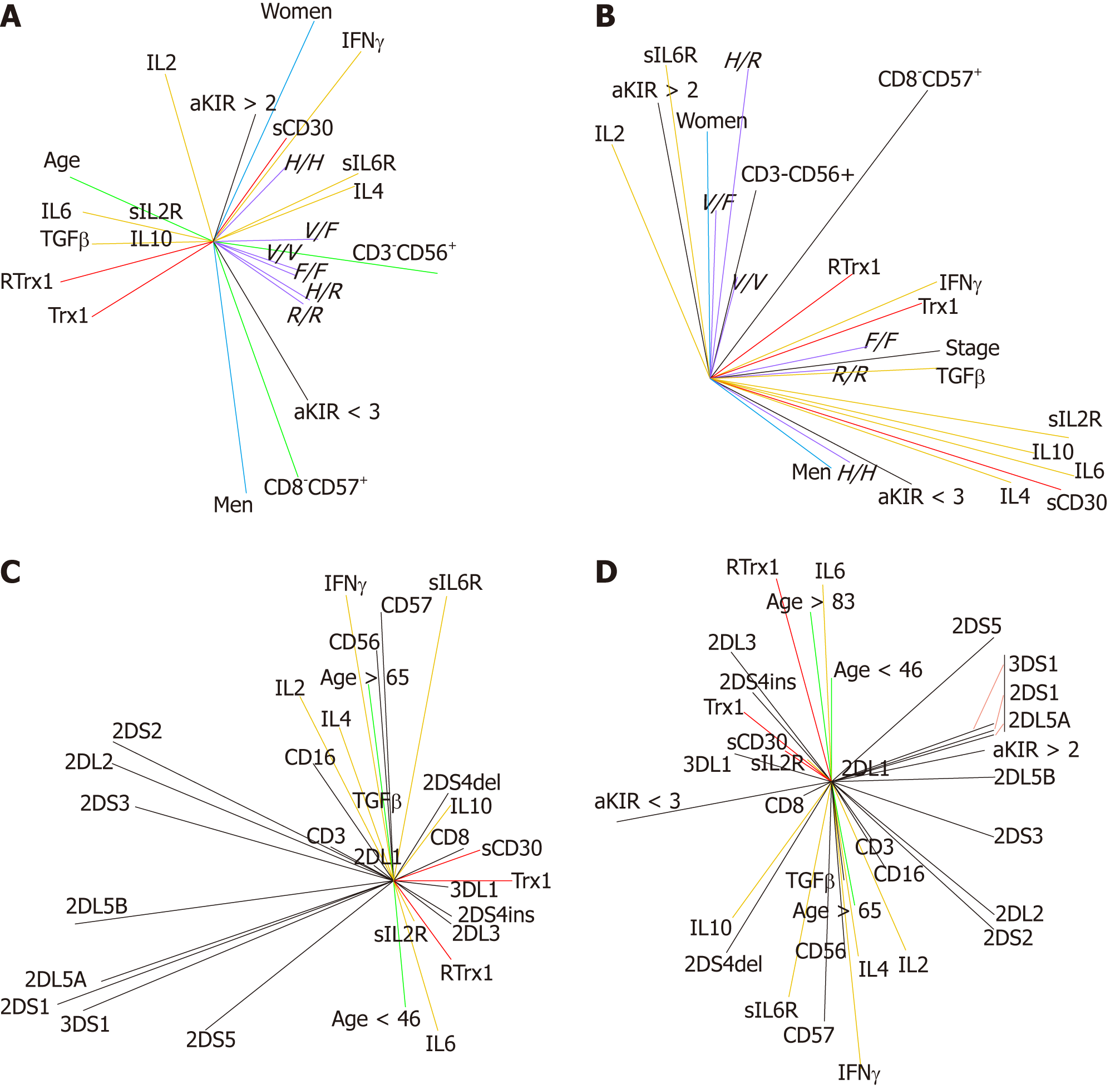
Figures 2-7 describe the physio-pathological network that regulates both the redox immune homeostasis and non-homeostasis states. The PCA plots of serum profile in the healthy state show that the positively related increase between the Trx1/RTrx1 levels and increase in sCD30 level (in red in Figure 2A) produced a positively related increase in the IFNγ and IL2 levels (in black in Figure 2A) and negatively related increases between levels of TGFβ-IL6 and TGFβ-IL4 (in yellow in Figure 2A).
The role of the above-mentioned relationships, as biomarkers of health homeostasis during aging, is showed by their relationships with the age vector (Figure 2B). The data show that the positively related increase between age (in black in Figure 2B) and Trx1/RTrx1 and sCD30 levels (in red in Figure 2B) determine a positively related increase between the IFNγ and IL2 levels (in black in Figure 2B) and negatively related increases between levels of TGFβ-IL6 and TGFβ-IL4 (in yellow in Figure 2B). Furthermore, the results confirm that the most relevant aging relationship is the positive correlation between Trx1/RTrx1. These vectors are very close to each other, go in the same direction and are close to the age vector. This situation indicates that during aging the level variations (their activity) of Trx1 and RTrx1 molecules must necessarily occur in the same phase of the response and in the same direction (increase or decrease).
In the matrix of coefficient correlations, there are the following positive and negative significant values of the above described relationships. The positive ones are Trx1 with RTrx1 (r = 0.65, P = 0.00001), Trx1 and RTrx1 with IL10 (Trx1 r = 0.58, P = 0.0001; RTrx1 r = 0.40, P = 0.0067), RTrx1 with IL6 (r = 0.34, P = 0.024), IL2 with IFNγ (r = 0.56, P = 0.0002) and IFNγ with IL4 (r = 0.52, P = 0.0005). The negative ones are Trx1 e RTrx1 with IL4 (Trx1 r = -0.30 P = 0.040; RTrx1 r= -0.38, P = 0.01) and RTrx1 with both sIL6R (r= -0.39, P = 0.0087) and IFNγ (r= -0.30, P = 0.028).
In addition, it is interesting to see that in order to preserve the homeostasis during aging, if there is an increase in Trx1 (r = 0.46, P = 0.0019), RTrx1(r = 055, P = 0.00001), IL6 (r = 0.36, P = 0.015), IL10 (r = 0.46, P = 0.0022) there must also be one in IL2 (r = 0.39, P = 0.0078).
The results of the evaluation of the effect of the gender parameter, on the interaction of the immune response network, clarify that the Trx1/CD30 controls the homeostasis of this response in both men (Figure 2C and D) and women (Figure 2E and F). Moreover, we can confirm that this regulation follows gender-specific pathways.
We found that for both genders the direct and close relationship between Trx1/RTrx1 and of this one with TGFβ are equally important. However, the female group shows a primary correlation of TGFβ with Trx1 (Figure 2E), while for the male one it is between TGFβ and RTrx1 (Figure 2C). In addition, in both genders the level of sCD30 shows a correlation with the level of IL10, which is a cytokine very important for immune response homeostasis. Our results showed that the correlation is positive in the male group (Figure 2C) and negative in the female one (Figure 2E).
Our data reveal that aging in the female group directly influences the Trx1/RTrx1 relationship (Figure 2F, the respective vectors have the same direction and the age vector is located between the other vectors). In the male group, this effect was indirectly obtained through the sCD30 molecule (Figure 2D, the aging vector has exactly the opposite direction of sCD30). In addition to this, IL2 regulates the interaction between IFNγ and IL4 in the male group (Figure 2D) and TGFβ and IL10 in the female group (Figure 2F). The respective vectors have the same direction, and the IL2 vector is located between the other two.
In the matrix of coefficient correlations, there are the following positive correlations between Trx1 and RTrx1 (r = 0.75, P = 0.00001), Trx1 and RTrx1 with IL10 (r = 0.54, P = 0.0023), Trx1 and RTrx1 with IL6 (r = 0.51, P = 0.0036) in the female group and between Trx1 and IL10 (r = 0.74, P = 0.00001) in the male group.
The age in the male group is negatively related to sCD30 (r = -0.61, P = 0.031) and positively to IFNγ (r = 0.85, P = 0.0002) and sIL2R (r = 0.72, P = 0.0055). In the female group, it is positively associated to sCD30 (r = 0.58, P = 0.00001), Trx1 (r = 0.63, P = 0.0004), RTrx1 (r = 0.53, P = 0.0001), IL6 (r = 0.42, P = 0.018), IL10 (r = 0.64, P = 0.0003) but also to IL2 (r = 0.27, P = 0.0089) and negatively to IL4 (r = -0.58, P = 0.0004), sIL2R (r = -0.39, P = 0.026) and sIL6R (r = -0.40, P = 0.019).
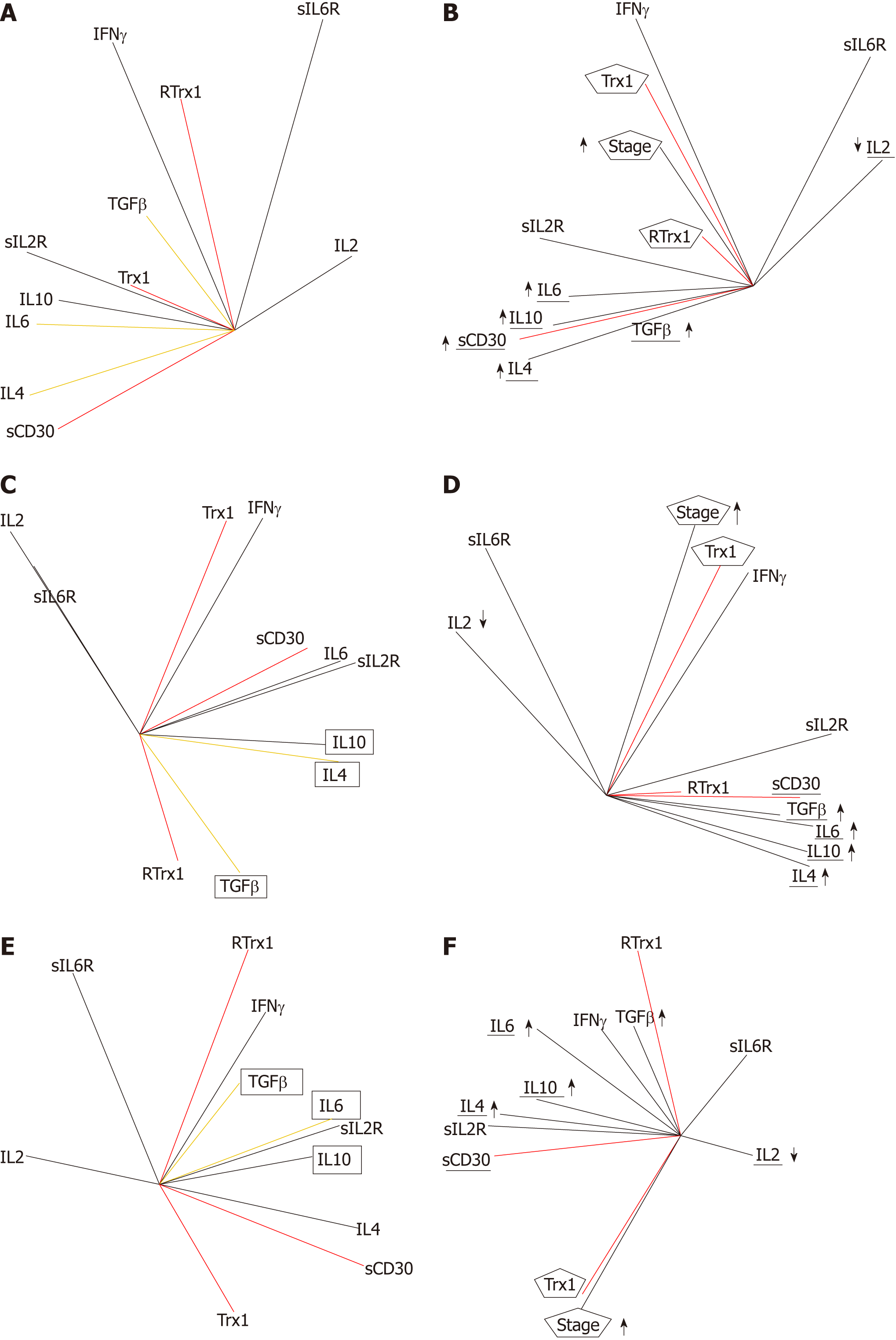
Our results show that the serum levels of Trx1/RTrx1, TGFβ/IL6 and TGFβ/IL4 combinations and of sCD30, IFNγ and IL2 are gender specific biomarkers for clinical screening. Indeed, in the tumor condition, the relationship between the increase of the serum level in Trx1/RTrx1 is no longer positive, while that between TGFβ/IL6 and TGFβ/IL4 is no longer negative. Furthermore, IL2 decreases whereas sCD30 increases and exceeds the ranges of the normal healthy homeostasis state. In fact, the results of the patients' group (Figure 3A) and the ones of the male (Figure 3C) and female (Figure 3E) groups show that these redox immune responses are characterized by the loss of the cytokine network homeostasis and, as a consequence, of cell homeostasis. The relationships between cytokines are not the same as in the healthy state.
Our data show that the tumor stage is related to the increased levels of TGFβ, IL4, IL10 and IL6 cytokines but to the decrease in IL2 (Figure 3B). The changes in Trx1/RTrx1 and sCD30 levels are biomarkers of this unbalance because the progression of the tumor stage in not related to the combined increase (or combined decrease) of both Trx1/RTrx1 levels, but only with the increase in the Trx1 level, both in the male group (Figure 3D) and the female one (Figure 3F). In fact, only the Trx1 vector is positively related to the tumor stage vector in both genders. In addition, the increase in the sCD30 level is positively related to the increase in TGFβ, IL4, IL6, and IL10 and to the decrease in IL2, in both genders (the IL2 vector is opposite to other cytokine vectors, which have the same direction).
The results of the multivariate analysis of correlations show that in the global group of patients, the stage is positively related to IFNγ (r = 0.35, P = 0.043), sIL2R (r = 0.55, P = 0.00001), IL6 (r = 0.37, P = 0.00001), IL10 (r = 0.50, P = 0.00001), sCD30 (r = 0.43, P = 0.013), TGFβ (r = 0.54, P = 0.002), IL4 (r = 0.56, P = 0.0007) and negatively related to IL2 (r = -0.33, P = 0.0003).
Moreover, in the female group, the analysis shows that the tumor stage is positively related to Trx1 (r = 0.065, P = 0.037), IL4 (r = 0.62, P = 0.0001), sIL2R (r = 0.57, P = 0.00001), IL10 (r = 0.31, P = 0.013) and sCD30 (r = 0.73, P = 0.019) and negatively related to RTrx1 (r = -0.57, P= 0.045), IL2 (r = -0.33, P = 0.0003) and sIL6R (r = -0.42, P = 0.0089).
In the male group, the tumor stage is positively related to Trx1 (r = 0.87, P = 0.020), sIL2R (r = 0.54, P = 0.00001), IL6 (r = 0.43, P = 0.0001), IL10 (r = 0.53, P = 0.00001), TGFβ (r = 0.70, P = 0.0005), IL4 (r = 0.48, P = 0.0001); and negatively related to IL2 (r = -0.27, P = 0.023).
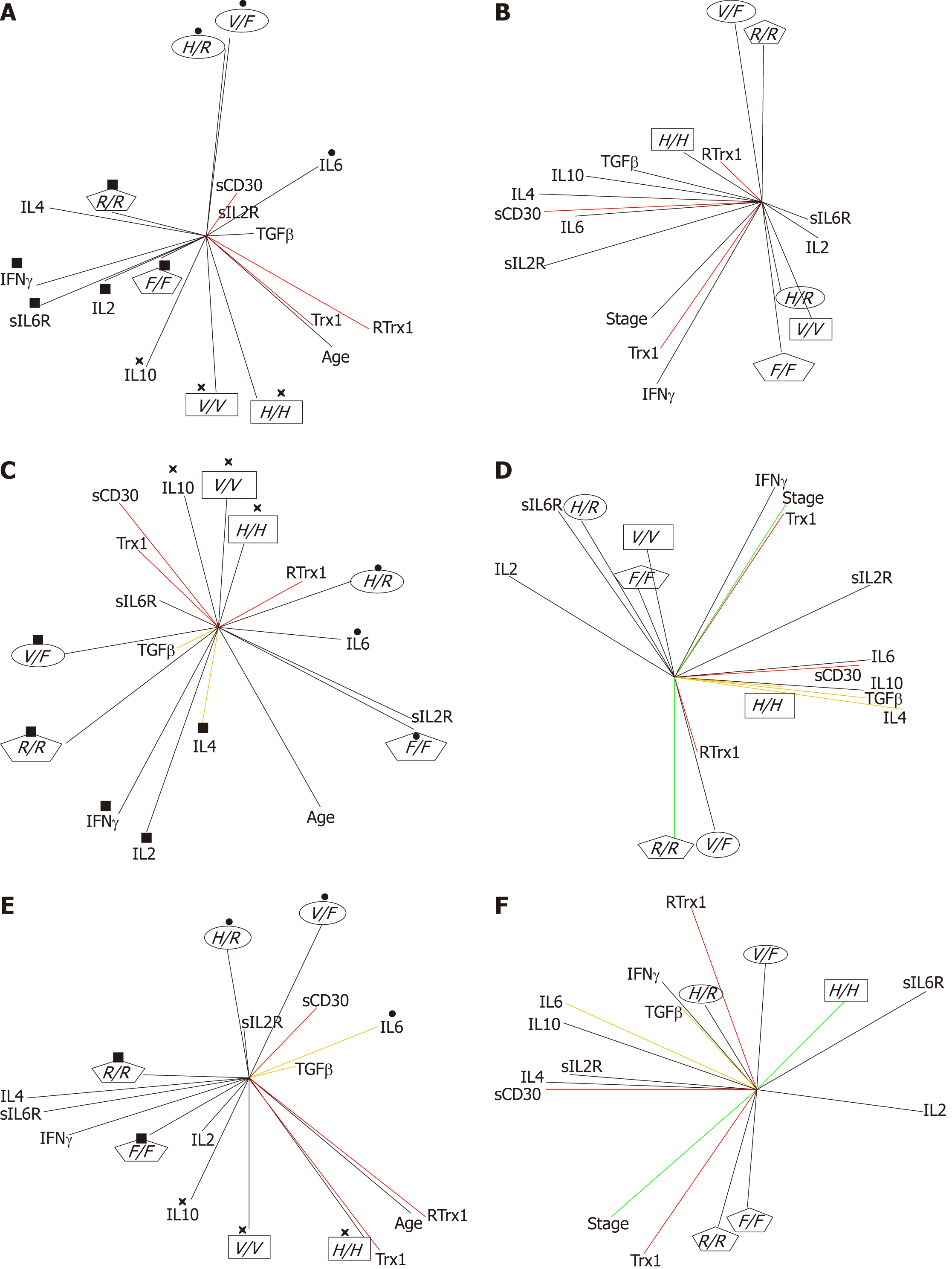
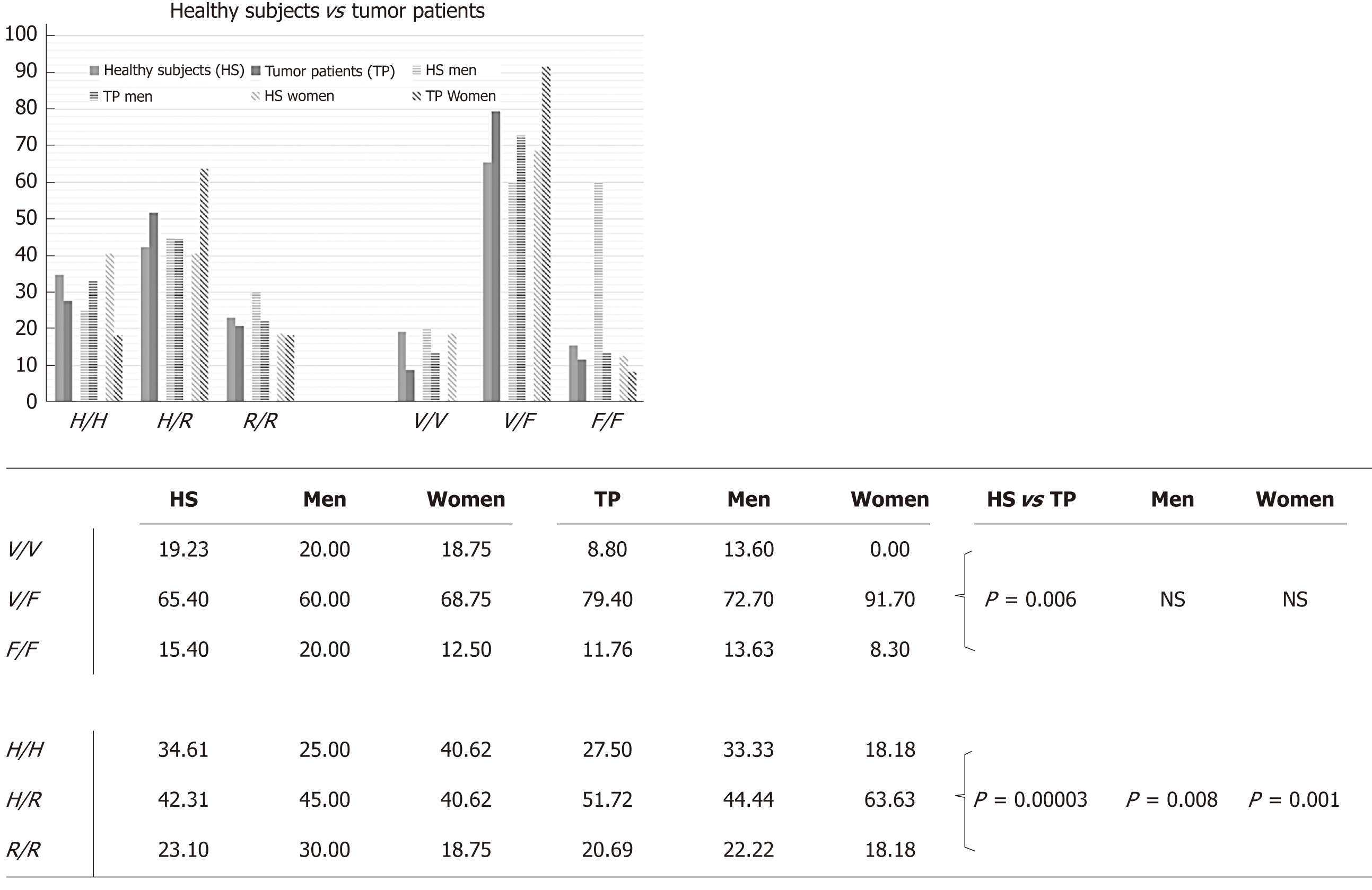
FcγRIIIa and FcγRIIa polymorphisms: The frequencies of the FcγRIIIa and FcγRIIa polymorphisms are shown in Table 2. Multivariate PCA statistical studies show that, in healthy individuals, the polymorphisms of FcγRIIIa and FcγRIIa combine in pairs, which follow different orders of combinations, depending on gender (Figure 4). These results demonstrate in the global group of healthy subjects that the combinations occur in the following ways (Figure 4A): FcγRIIa-131H/H combines with FcγIIIa-158V/V (H/H-V/V); FcγRIIa-131R/R with FcγIIIa-158F/F (R/R-FF) and FcγRIIa-131H/R with FcγRIIIa-158V/F (H/R-V/F). In the female group, the combinations follow the same trend (Figure 4E), while in the male one there are different combinations: H/H-V/V combines in the same way; while H/H combines with F/F (HH-FF) and R/R with V/F (RR-VF) (Figure 4C).
| HS | Men | Women | Men | Women | Men | Women | |||
| H/H | H/R | R/R | |||||||
| V/V | 38.89 | 40.00 | 38.46 | 9.01 | 11.11 | 7.70 | 8.33 | 16.67 | 0.00 |
| V/F | 55.55 | 40.00 | 61.54 | 68.18 | 55.55 | 76.92 | 75.00 | 83.33 | 66.67 |
| F/F | 5.50 | 20.00 | 0.00 | 22.72 | 33.33 | 15.38 | 16.67 | 0.00 | 33.33 |
| TP | |||||||||
| V/V | 25.00 | 33.33 | 0.00 | 6.67 | 12.50 | 0.00 | 0.00 | 0.00 | 0.00 |
| V/F | 75.00 | 66.67 | 100.00 | 80.00 | 75.00 | 85.71 | 100.00 | 100.00 | 100.00 |
| F/F | 0.00 | 0.00 | 0.00 | 13.33 | 12.50 | 14.28 | 0.00 | 0.00 | 0.00 |
| HS vs TP | |||||||||
| V/V | P = 0.035 | NS | P < 0.0001 | NS | NS | P = 0.004 | P = 0.0033 | P < 0.0001 | NS |
| V/F | P = 0.004 | P = 0.0002 | P < 0.0001 | NS | P = 0.004 | NS | P < 0.0001 | P < 0.0001 | P < 0.0001 |
| F/F | P = 0.017 | P < 0.0001 | NS | NS | P = 0.0005 | NS | P < 0.0001 | NS | P < 0.0001 |
In addition, an interesting observation is that, in the healthy state, when there is an increase in IL10 in the immune response network, the H/H-V/V is the most significant combination to preserve the redox-immune homeostasis in all the groups taken into consideration. On the other hand, when IL6 is increased, the most significant combination is the H/R-F/F in the male group (Figure 4C) and H/R-V/F in the female one (Figure 4E). When there are increased levels of IFNγ, IL4 and IL2, the most relevant combination is R/R-V/F in the male group (Figure 4C) and R/R-F/F in the female one (Figure 4E).
By analyzing patients’ data, it is possible to see that in non-homeostasis of the tumor state, the pathophysiological network of the immune response is not regulated through these FcγRIIIa and FcγRIIa polymorphism combinations (Figure 4B). Consequently, it is possible to define them as biomarkers of non-risk of disease.
Furthermore, since the multivariate statistical evaluation showed a gender-specific significant difference of FcγRIIa genotypes between healthy subjects and patients (Figure 5), we evaluated if this difference could be useful to personalize disease biomarkers by establishing indices of gender-risk (Table 2). When evaluating this, we noticed a different index of gender-risk, depending on the FcγR combination observed (Table 3). Considering together FcγRIIa and FcγRIIIa, we found that the H/H-F/F and R/R-V/V combinations in the male group and the H/H-V/V, H/R-V/V and the RR-F/F combinations in female one could be biomarkers of non-risk of disease progression. The H/R-F/F pair is linked to a moderate risk but only in the male group. HH-VF and RR-V/F could be high-risk biomarkers in both genders but HR-V/F only for the male group.
| Sensitivity | Specificity | Likelihood ratio | Predictive value | |||||
| Positive | Negative | Positive | Negative | |||||
| Men | No risk | |||||||
| HH/FF | P < 0.0001 | 0.00% | 80.00% | 0.00 | 1.25 | 0.00% | 40.00% | |
| RR/VV | P < 0.0001 | 0.00% | 83.33% | 0.00 | 1.20 | 0.00% | 55.56% | |
| Moderate risk | ||||||||
| HR/FF | P = 0.0005 | 12.50% | 66.67% | 0.37 | 1.31 | 25.00% | 46.15% | |
| High risk | ||||||||
| HH/VF | P = 0.0002 | 66.67% | 60.00% | 1.67 | 0.56 | 66.67% | 60.00% | |
| HR/VF | P = 0.004 | 75.00% | 44.44% | 1.35 | 0.56 | 54.55% | 66.67% | |
| RR/VF | P < 0.0001 | 100.00% | 16.67% | 1.20 | 0.00 | 44.44% | 100.00% | |
| Women | No risk | |||||||
| HH/VV | P < 0.0001 | 0.00% | 61.54% | 0.00 | 1.62 | 0.00% | 80.00% | |
| HR/VV | P = 0.004 | 0.00% | 92.31% | 0.00 | 1.08 | 0.00% | 63.16% | |
| RR/FF | P < 0.0001 | 0.00% | 66.67% | 0.00 | 1.50 | 0.00% | 66.67% | |
| High risk | ||||||||
| HH/VF | P < 0.0001 | 100.00% | 38.46% | 1.62 | 0.00 | 20.00% | 100.00% | |
| RR/VF | P < 0.0001 | 100.00% | 33.33% | 1.50 | 0.00 | 33.33% | 100.00% | |
In order to support these assumptions, it is possible to notice in the patients graphs (Figure 4D) that these FcγRIIa and FcγRIIIa polymorphisms were positively related to the serum profile parameters, which are biomarkers of the loss of redox-immune homeostasis. In fact, in the male group (Figure 4D), H/H is related to a combined increase in TGFβ, IL4 and IL10 (expansion of Th9 cells, Figure 1), and R/R and V/F are related to an increase in RTrx1 (redox unbalance). Furthermore, in the female group (Figure 4F), H/R is related to a combined increase in TGFβ, IL6 and IL10 (expansion of Th17 cells, Figure 1). On the other hand, R/R and F/F are related to an increase in Trx1 (redox unbalance).
In Supplement Figures 1 and 2, the KIR gene-content genotypes in healthy, adenoma subjects and patients are shown. Table 4 shows the levels of the phenotypic antigen expression. We evaluated if the number of aKIRs and iKIRs could be prognostic for disease stage progression. This was done by evaluating their correlations in the network. Our results show that (Figure 6A) aKIR = 0 and aKIR = 2 are the most significant biomarkers in relation to the disease stage progression. The number of iKIR receptors seems, instead, to be irrelevant in this correlation. Furthermore, aKIR = 2 is more closely related to disease progression than aKIR = 0 and could be considered as the principal biomarker of sCD30 increase. Instead, aKIR = 0 is the biomarker of IL6, sIL2R, TGFβ and Trx1 increase.
| HS | Men | Women | TP | Men | Women | |
| CD3 | 59.8 ± 1.3 | 60.9 ± 1.8 | 58.3 ± 1.9 | 49.0 ± 2.3 | 46.7 ± 2.8 | 52.4 ± 4.0 |
| CD8 | 25.3 ± 0.9 | 25.0 ± 1.3 | 26.1 ± 1.2 | 20.0 ± 1.3 | 19.2 ± 1.5 | 21.4 ± 2.3 |
| CD16 | 12.5 ± 1.1 | 16.5 ± 1.8 | 7.7 ± 0.8 | 15.5 ± 1.3 | 15.4 ± 1.5 | 14.7 ± 2.3 |
| CD56 | 11.8 ± 1.2 | 14.6 ± 1.9 | 8.5 ± 0.9 | 16.4 ± 1.6 | 17.5 ± 2.0 | 14.9 ± 2.5 |
| CD57 | 23.7 ± 1.2 | 25.9 ± 1.8 | 21.5 ± 1.3 | 23.7 ± 1.8 | 22.3 ± 1.8 | 26.2 ± 4.0 |
| CD3-CD56+ | 19.7 ± 0.1 | 19.7 ± 0.1 | 19.7 ± 0.1 | 12.7 ± 3.2 | 18.0 ± 5.5 | 7.3 ± 2.1 |
| CD8-CD57+ | 11.4 ± 1.6 | 12.4 ± 2.0 | 8.4 ± 2.2 | 7.2 ± 3.1 | 15.5 ± 0.1 | 4.5 ± 1.9 |
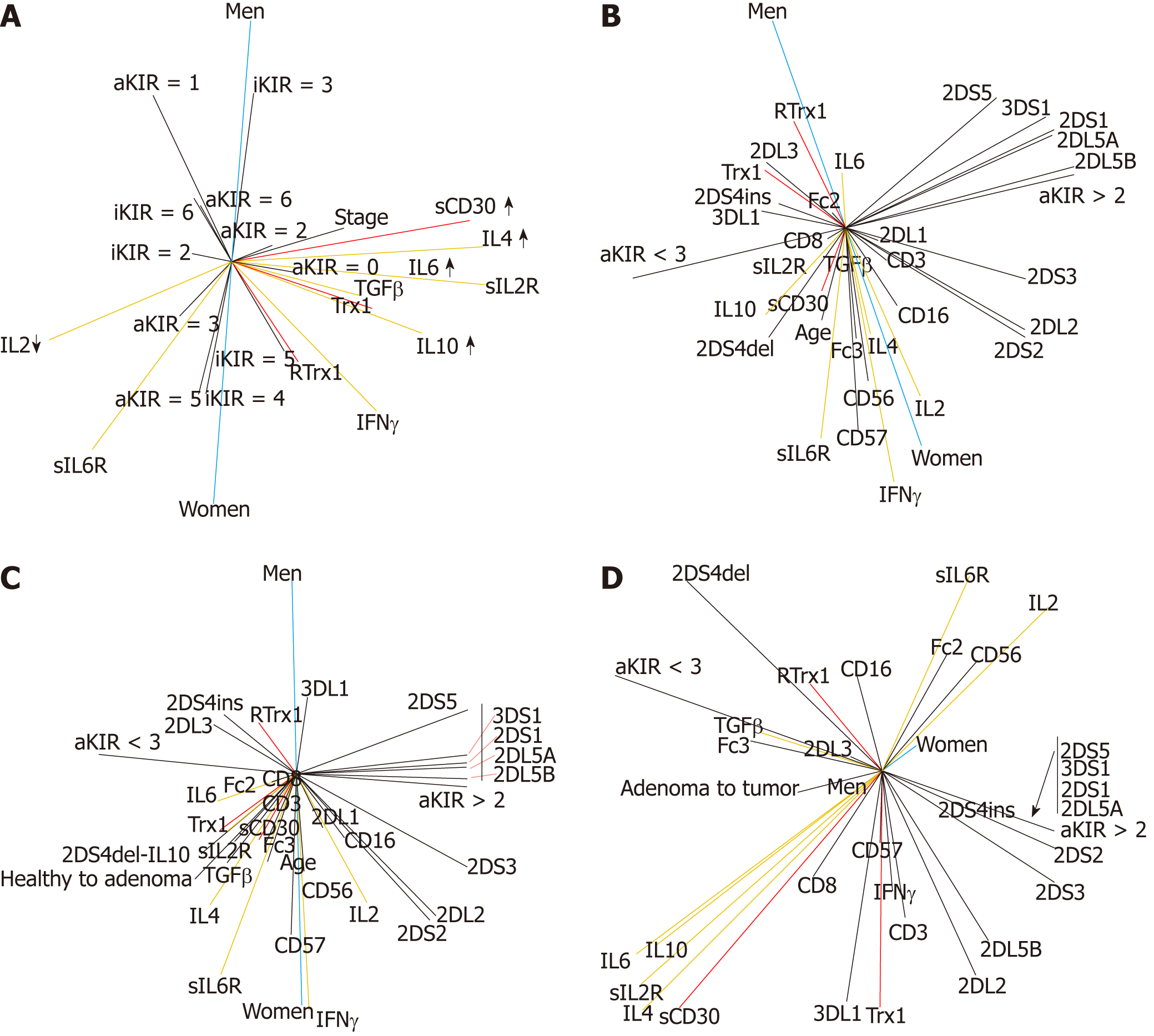
According to these results we established that the “aKIR > 2” and “aKIR < 3” parameters could, respectively, be the limits of non-risk and risk of disease, and we used them as elements of stratification to evaluate the significance of our parameters as prognostic biomarkers of disease risk. This study was performed by evaluating the correlation between the KIR genetic polymorphisms and age increase (Figure 6B) in the group of healthy subjects. Moreover, the progression from the normal condition to an adenoma (Figure 6C) in the group constituted by both healthy subjects and patients with adenoma was evaluated. In both adenoma and tumor patients, the progression from the adenoma to the tumor (Figure 6D) was studied. In all these conditions, the relationship with gender was also evaluated.
Our results show in healthy individuals (Figure 6B) that the age increase is related to an increase in disease risk. The female group is more affected by the physiological process of aging than the male one. The age and female gender vectors are very close, while for the male group, age and male gender vectors are not.
In addition (Figure 6B), our results clarify that the increased disease risk in females during aging is related to the loss of homeostasis of the immune system. In fact, it is possible to observe a positive relationship between the increase in IL4, TGFβ and IL10 levels (Figure 6B). However, the relationship between the increase in TGFβ and IL6 levels is still highly negative (Figure 6B). This confirms the value of this pair of cytokines as a non-risk biomarker in the female group.
In contrast, increased disease risk in the male group is related to loss of the redox system homeostasis (Figure 6B). It is possible to observe the increase in redox molecules. Furthermore, in the male group, it is interesting to note that the relationship between the increases in TGFβ and IL4 levels is still negative (Figure 6B). This confirms the value of this pair of cytokines as a non-risk biomarker in the male group.
Considering the stepwise nature of the CRC tumor and the identification of colon adenoma as a precursor of CRC[3], our results show that, in the passage from a healthy condition to an adenoma (Figure 6C), the female gender, once again, has a higher risk related to aging. The “healthy to adenoma” age and female gender vectors are all close to each other, but the male vector is not close to them. Moreover, the higher risk, mainly in women, is caused by the additive effect of the loss of the redox system homeostasis (Figure 6C). This is due to the increase in Trx1 level (without the support of RTrx1) and related to the combined increase in TGFβ/IL6 and IL10. Furthermore, we observed that the biomarkers of this condition are FcγRIIIa, FcγRIIa and 2DS4del polymorphisms. On the other hand, in the male group, the passage from healthy to adenoma (Figure 6C) is mainly due to the loss of redox system homeostasis, for the increase was only in RTrx1 (without the support of Trx1). The biomarkers of this condition are 2DS4ins and 2DL3.
Finally, the results show that the male group has a higher risk than the female one to pass from the adenoma to the tumor (Figure 6D). The male vector is really close to the “adenoma to tumor” vector and to the aKIR < 3 risk vector. The biomarkers of this passage are FcγRIIIa and 2DL3 and the increase in both TGFβ and RTrx1. The female vector behaves differently. It is located opposite to the “adenoma to tumor” vector and close to the aKIR > 2 non-risk vector (Figure 6D). The biomarkers of this condition are FcγRIIa and 2DS4del and the increase in RTrx1 without the increase in Trx1 (Figure 6D).
Our results reveal that Trx1/CD30 is also a biomarker of NK cell functionality. In the healthy condition (Figure 7A), the combined activity of the CD3-CD56+ and CD8-CD57+ cell populations protects the redox immune physiological homeostasis during aging. This CD3-CD56+ and CD8-CD57+ cell activity, however, works through the balance of the Trx1/RTrx1 and sCD30 levels. In Figure 7A, it is possible to note that the vectors of Trx1/RTrx1 (in red) are positively combined, and the increase in sCD30 (in red) is related to the non-risk biomarker aKIR > 2 (in black) and to the IFNγ and IL2 cytokines (in yellow). On the other hand, in the healthy state, the increase in the CD3-CD56+ NK cell balances the effects of the risk and non-risk of disease. In fact, as shown in Figure 7A, the vector CD3-CD56+ (in green) is perfectly placed between the vectors of risk and non-risk biomarkers of disease aKIR < 3 and aKIR > 2 (both in black). Conversely, during aging the CD8-CD57+ NK population is related to an increased risk of disease. The CD8-CD57+ green vector is aligned with the aKIR < 3 black vector. The CD8-CD57+ effect is strongly connected to the decrease in the IL2 level: the respective CD8-CD57+ and IL2 vectors are precisely opposite one another. Therefore, the homeostasis of CD3-CD56+ and CD8-CD57+ cell activity guarantees a healthy state during aging because they regulate the homeostasis of the immune response. Our results show also that the FcγRIIIa and FcγRIIa polymorphisms regulate NK cell activity and the disease risk increase. In fact, as shown in Figure 7A, it is possible to observe that the FcγRIIa-131H/H is strongly correlated to aKIR > 2 and to the increases in sCD30, IL2 and IFNγ. This is a condition of higher NK functionality and of non-risk of disease. In fact, the respective vectors are very close to each other principally in the female group. Conversely the VF, VV, FF, HR, RR genotypes of FcγRIIIa and FcγRIIa polymorphisms, in order of increasing importance, decrease NK activity and increase disease risk. In fact, their vectors (in purple), which are in ascending order of importance, move the CD3-CD56+ vector closer to the aKIR < 3 risk vector (in black).
For these reasons, we can affirm that the positive combined increase in the Trx1/RTrx1 and sCD30, IFNγ and IL2 serum levels is also a biomarker of NK functionality in the clinical evaluation of the selection of personalized patient treatment.
In order to confirm this, it is possible to observe that, in the tumor state (Figure 7B), Trx1/CD30 no longer has the features of the homeostasis state. In fact, there is no parallel increase in the Trx1/RTrx1 serum levels because the disease stage progression is only related to the increase in the Trx1 level. In addition, the increase in sCD30 serum level is related to the IL6, IL4 and TGFβ increase and to the IL2 decrease. In this situation, the male group has also a higher risk than the female one. The male group vector is really close to the aKIR < 3 risk vector, while the female one is strongly connected to the aKIR > 2 non-risk vector.
According to the age of our healthy subjects, we evaluated the correlations between the levels in the Trx1/RTrx1/sCD30 and Treg/Th1/Th17 cytokines with the following age ranges: Less than 46 years old (< 46), more than 65 years old (> 65) and more than 83 years old (> 83).
Our results show that, by comparing the < 46 and > 65 age groups (Figure 7C), the most significant correlation in the youngest group is with the homeostasis of the redox response. The “< 46” vector is close to the RTrx1 and Trx1 vectors. On the other hand, when we analyzed the “> 65” group we found a different situation with a more relevant homeostasis of the immune response. In Figure 7C, the “> 65” vector is close to the immune vectors.
When the “> 83” age group was included in the network evaluation, we observed that the vector of this group was not placed near the “> 65” group, as expected, but it was close to the “< 46” vector. This shows a strong positive correlation to the redox system homeostasis. These results reveal that the possibility of living a long and healthy life is primarily determined by the homeostasis of system redox regulation, in particular of sCD30, Trx1 and RTrx1. This is also true when there is an increased level in IL6. The “> 83” and “< 46” vectors are closely related to each other and to the RTrx1, Trx1, sCD30 and IL6 vectors (Figure 7D).
Research on different pathological conditions show a very heterogeneous and confused representation of how the interaction between redox and immune systems orchestrate inflammatory diseases[29]. However, it appears that a correct redox balance is required to maintain an immune condition that prevents the progress of immune-mediated inflammatory diseases[29]. Modifications of the physiological pathways regulating redox and immunological systems have been identified in a variety of types of tumors such as CRC, non-small cell lung carcinoma and breast cancer. The possible use of Trx1 and CD30 as tumor targets and biomarkers has been broadly described in the literature. In our previous work[3], we showed that serum level values of Trx1 or of sCD30 were elevated in several human primary cancers, including colorectal[1], and were generally related to tumor aggressiveness and inhibition of the immunological system. In addition, Schwertassek et al[8] demonstrated that the serum levels of sCD30 mirror Trx1 levels in physiological networks: as Trx1 levels increase in infections, allergies, autoimmune events and cancer, so do sCD30 levels; likewise Trx1 levels modulate CD30 receptor immunological functions during the immune response as do sCD30 levels. With the aim of clarifying whether it was more effective to use Trx1 and CD30 in combination, as a dual target, we have carried out this study.
Our results support that it is more effective to target Trx1 and CD30 together and that gender and genetic parameters are useful to improve disease risk classification. We demonstrated that Trx1/CD30 controls redox immunological homeostasis of the immune response both in the male and female groups but through gender specific redox-immune pathways. In these pathways, the increase in the serum level combinations of Trx1/RTrx1 (positively correlated), TGFβ/IL6 and TGFβ/IL4 (negatively correlated) and the increase in the serum of sCD30 IFNγ and IL2 are fundamental for the preservation of the microenvironment homeostasis of IL10, TGFβ, IL4, IL6 and IL2 cytokines. This homeostasis also controls inflammation in the healthy state during aging. Instead, the unbalance between the serum levels of Trx1/RTrx1 (which has become negative), TGFβ/IL6 and TGFβ/IL4 (which has become positive) and the decrease in the serum levels of sCD30, IFNγ and IL2, generates non-homeostasis in the microenvironment of both the cytokines and cells network; gender specific redox-immune pathway alterations; a non- homeostasis redox immunological of gender immune response; a non-control of inflammation and gender specific chronic degenerative diseases or disease progression[6,11-17]. Consequently, the balance of these parameters is a biomarker of control of inflammation and is prognostic of non-risk of disease or progression in both healthy subjects and patients.
To our knowledge, this is the first time that Trx1/CD30 is studied together with FcγR and KIR gene polymorphisms. This permitted us to clarify the importance of the Trx1/CD30 redox immune homeostasis in order to allow the positive interaction between NK and T cell function and health protection during aging.
As described extensively in the results section, the FcγRIIa and FcγRIIIa polymorphisms have been identified as stratification parameters of low/intermediate and high risk of disease depending on the gender and on the serum level of specific cytokines.
Regarding the KIR gene polymorphisms, we have showed their importance as stratification parameters for the individual risk of disease and its progression in order to select early-personalized biomarkers. Our study demonstrates that the most predictive conditions for the disease development or progression are when aKIRs are completely absent or there are only two of them. Instead, the iKIRs numbers did not show any relevance in this correlation.
It is interesting to note in the patient group that we demonstrated that the KIR polymorphisms are prognostic biomarkers of specific pathology polarization in both genders. In fact, as shown in Supplement Figure 3, the Trx1/CD30 controls the redox immunological homeostasis of the TGFβ, IL6, IL10, IL2, IFNγ and IL4 cytokine pathway interactions, which are specific to each sex, and the loss of this control generates the pathological polarization of T cell subsets. KIR polymorphisms are valid classification parameters for the individual risk of losing the physiological redox immunological homeostasis between the Th1, Th2, Treg, Th9, Th17 cells that are all regulated by the Trx1/CD30. For this reason, they are suitable as early prognostic biomarkers of the individual risk of disease (healthy subjects) or of its progression (patients).
In the male group, 3DL1 is the highest risk biomarker because it is correlated with a decrease in IL2 and an increase in IL4, which are prognostic for Th9 cell generation. Instead, 2DL5B is the highest non-risk male biomarker because it is positively correlated with both IL2 and IFNγ increases, which are prognostic for immunological response homeostasis.
Moreover, in the male group, the 2DS1 and 2DL5A and an increasing number of aKIRs are also non-risk biomarkers because they are associated with the TGFβ decrease. Likewise, in the female group, 2DS4del is the highest risk biomarker during aging since it is correlated with the IL2 decrease. Furthermore, 2DL5B is also the highest non-risk biomarker in the female group because it is correlated with an IL2 increase.
In addition, the 2DS2/2DL2 pair is also a female non-risk biomarker. It is correlated with TGFβ decrease. We showed that the 2DL2+/2DS2+ pair hinders tumor growth, while the 2DS2+/2DL2- or 2DS2-/2DL2+ pair leads to its development. In fact, the strong positive correlation between 2DL2 and 2DS2 is present in the healthy state. Moreover, it is still significant in the transition from the healthy to adenoma state, but it is no longer significant in the shift from the adenoma to tumor states. Furthermore, we clarify that this protection happens because the presence of the 2DL2+/2DS2+ pair is linked to a positive interaction between innate and adaptive response thanks to the Trx1 and RTrx1 combined involvement that favors the immunological redox homeostasis.
The protective function of the 2DL2+/2DS2+ pair against diseases is a recurring question in this research area but to which a response has not been given yet[21,30]. Furthermore, our results also clarify the importance of the balance of the Trx1/CD30 in the positive interaction between NK and T cell function and health protection during aging.
An important contribution for the improvement of immunotherapy in tumors is the discovery that Trx1/CD30 is a useful tool as a gender-specific target and biomarker of the NK cell activity. We found that the changes in the Trx1/RTrx1, sCD30 and Treg, Th1, Th9, Th17 cytokines are efficient biomarkers of the functionality/non-functionality of these cells.
Moreover, in the normal healthy state, the FcγRIIIa V/V, FcγRIIIa F/F, FcγRIIa H/R and FcγRIIa R/R genotypes are biomarkers, in ascending order, of the non-functionality of NK cells.
Therefore, we state that the redox system homeostasis has a primary contribution in the control of inflammation in the healthy state during aging. In fact, for both genders the passage to adenoma and eventually to cancer is caused by the redox system unbalance. We suggest that the secret of healthy longevity in an advanced age is in the homeostasis of the redox regulation on the immune system, even when there is an increase in IL6.
In conclusion, the selection of prognostic biomarkers for the definition of personalized treatment in aging and CRC onset and/or its progression is not a new topic, but results are still not relevant enough to improve the clinical strategies. Identifying biomarkers able to highlight the risk of developing degenerative processes linked to aging, which could lead to the onset of the disease, is a new challenge.
Oxidative stress on Trx1/CD30 is a trigger of cancer disease and the selected oxidation and immune products are a biomarker’s system in aging and cancer. Our results give new insight into how environment interacts with cell metabolism and redox immune state to influence Th cell differentiation and function. Furthermore, the mechanisms of the degenerative process that compromise redox immune function have been explained. Therefore, we propose that Trx1/CD30 and the selected biomarkers can be used for new personalized resolving treatments to target age-related immune decline. This will allow for the selection of novel therapies.
Since this is a retrospective study, additional research with larger prospective clinical trials is necessary to evaluate the impact of Trx1/CD30 for the treatment. Our finding on gender changes are of interest as is the apparently different sCD30, Trx1 and RTrx1 fingerprint for cancer patients compared to younger and older subjects. However, our results should be confirmed by other groups and in other countries. The follow up to our research will be the investigation into Trx1/CD30 in tumor tissue samples.
The selection of prognostic biomarkers for the definition of personalized treatment in aging and colorectal cancer (CRC) onset and/or its progression is relevant to improve clinical strategies. Finding biomarkers able to identify the risk of developing degenerative processes linked to aging, which could lead to the onset of the disease, is a new challenge. Alterations in the physiological pathways involved in the regulation of redox and immunological systems have been identified in various types of tumors, such as CRC. The potential use of Trx1 and CD30 as targets and biomarkers for tumors has been widely described in literature, but results are still not relevant enough to improve the clinical strategies.
We have carried out this study to clarify if it was effective to use Trx and CD30 as dual target in combination with T helper cytokines and polymorphisms of KIRs, FcγRIIa-131H/R and FcγRIIIa-158V/F. The aim was to identify alterations in markers of redox-immune homeostasis that may be useful as a predictive biomarker system in health prediction and to identify personalized treatments to target age-related immune decline and cancer.
The serum measures of Trx1/CD30, RTrx1, multiple cytokine levels and polymorphisms of KIR and FcγRIIa were used to estimate the effect of disease, age and gender interactions to describe the variants in the biology of the redox immune system. Using multivariate statistical procedures of correlation analysis and a matrix of correlation of all the redox immune and clinical parameters we have identified changes in the biology of the redox immune system relationships in both healthy aging and tumor disease progression. Through these procedures we determined the redox immune fingerprint of health, aging and cancer states.
We found positive increases between Trx1/RTrx1 levels and sCD30 level and increased age. With respect to the gender relationships, there are distinct differences: females show a primary relationship between TGFβ with Trx1 while males for TGFβ and RTrx1. Trx1/CD30 controls the redox immune homeostasis, and an imbalance in the relationship between Trx1/RTrx1 and sCD30 levels is linked to the onset and progression of tumor (but through different cytokine pathways in the male and female subjects). The study confirmed that the serum levels of Trx1/RTrx1, TGFβ/IL6 and TGFβ/IL4 combinations and the sCD30 IFNγ and IL2 combination are a predictive gender specific biomarker system for clinical screening to detect the risk of the potential development or progression of a tumor.
Results give new insight into how environment interacts with cell metabolism and redox immune state to influence Th cell differentiation and functions. Furthermore, the mechanisms of degenerative processes that compromise redox immune function have been explained. Results support the goals of translational medicine that tries to promote personalized strategies for the prevention and treatment of cancer.
Trx1/CD30 and selected biomarkers can be used for new personalized resolving treatments to target age-related immune decline, CRC onset and/or its progression. This will permit to select novel therapies. Given the retrospective nature of this study, additional research with larger prospective clinical trials is necessary to assess the impact of Trx1/CD30 for the treatment.
We thank Nunziatina Cherubini for the high competence and professionalism in the administrative, technical and material support.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aurello P, De Silva AP, Guo YM, He J S- Editor: Ji FF L- Editor: Filipodia E- Editor: Bian YN
| 1. | Berghella AM, Pellegrini P, Del Beato T, Ciccone F, Contasta I. The potential role of thioredoxin 1 and CD30 systems as multiple pathway targets and biomarkers in tumor therapy. Cancer Immunol Immunother. 2011;60:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Blagosklonny MV. Answering the ultimate question "what is the proximal cause of aging?". Aging (Albany NY). 2012;4:861-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Egea J, Fabregat I, Frapart YM, Ghezzi P, Görlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG, Olaso-Gonzalez G, Petry A, Schulz R, Vina J, Winyard P, Abbas K, Ademowo OS, Afonso CB, Andreadou I, Antelmann H, Antunes F, Aslan M, Bachschmid MM, Barbosa RM, Belousov V, Berndt C, Bernlohr D, Bertrán E, Bindoli A, Bottari SP, Brito PM, Carrara G, Casas AI, Chatzi A, Chondrogianni N, Conrad M, Cooke MS, Costa JG, Cuadrado A, My-Chan Dang P, De Smet B, Debelec-Butuner B, Dias IHK, Dunn JD, Edson AJ, El Assar M, El-Benna J, Ferdinandy P, Fernandes AS, Fladmark KE, Förstermann U, Giniatullin R, Giricz Z, Görbe A, Griffiths H, Hampl V, Hanf A, Herget J, Hernansanz-Agustín P, Hillion M, Huang J, Ilikay S, Jansen-Dürr P, Jaquet V, Joles JA, Kalyanaraman B, Kaminskyy D, Karbaschi M, Kleanthous M, Klotz LO, Korac B, Korkmaz KS, Koziel R, Kračun D, Krause KH, Křen V, Krieg T, Laranjinha J, Lazou A, Li H, Martínez-Ruiz A, Matsui R, McBean GJ, Meredith SP, Messens J, Miguel V, Mikhed Y, Milisav I, Milković L, Miranda-Vizuete A, Mojović M, Monsalve M, Mouthuy PA, Mulvey J, Münzel T, Muzykantov V, Nguyen ITN, Oelze M, Oliveira NG, Palmeira CM, Papaevgeniou N, Pavićević A, Pedre B, Peyrot F, Phylactides M, Pircalabioru GG, Pitt AR, Poulsen HE, Prieto I, Rigobello MP, Robledinos-Antón N, Rodríguez-Mañas L, Rolo AP, Rousset F, Ruskovska T, Saraiva N, Sasson S, Schröder K, Semen K, Seredenina T, Shakirzyanova A, Smith GL, Soldati T, Sousa BC, Spickett CM, Stancic A, Stasia MJ, Steinbrenner H, Stepanić V, Steven S, Tokatlidis K, Tuncay E, Turan B, Ursini F, Vacek J, Vajnerova O, Valentová K, Van Breusegem F, Varisli L, Veal EA, Yalçın AS, Yelisyeyeva O, Žarković N, Zatloukalová M, Zielonka J, Touyz RM, Papapetropoulos A, Grune T, Lamas S, Schmidt HHHW, Di Lisa F, Daiber A. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017;13:94-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 4. | Torrão RC, Bennett SJ, Brown JE, Griffiths HR. Does metabolic reprogramming underpin age-associated changes in T cell phenotype and function? Free Radic Biol Med. 2014;71:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Baetta R, Pontremoli M, Martinez Fernandez A, Spickett CM, Banfi C. Proteomics in cardiovascular diseases: Unveiling sex and gender differences in the era of precision medicine. J Proteomics. 2018;173:62-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Contasta I, Totaro R, Pellegrini P, Del Beato T, Carolei A, Berghella AM. A gender-related action of IFNbeta-therapy was found in multiple sclerosis. J Transl Med. 2012;10:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Uno K, Okuno K, Kato T, Tada-Oikawa S, Kan N, Saotome H, Yagi K, Hamuro J. Pre-operative intracellular glutathione levels of peripheral monocytes as a biomarker to predict survival of colorectal cancer patients. Cancer Immunol Immunother. 2010;59:1457-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Schwertassek U, Balmer Y, Gutscher M, Weingarten L, Preuss M, Engelhard J, Winkler M, Dick TP. Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 2007;26:3086-3097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Simhadri VL, Hansen HP, Simhadri VR, Reiners KS, Bessler M, Engert A, von Strandmann EP. A novel role for reciprocal CD30-CD30L signaling in the cross-talk between natural killer and dendritic cells. Biol Chem. 2012;393:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Pellegrini P, Berghella AM, Contasta I, Adorno D. CD30 antigen: not a physiological marker for TH2 cells but an important costimulator molecule in the regulation of the balance between TH1/TH2 response. Transpl Immunol. 2003;12:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 378] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 12. | Seki N, Miyazaki M, Suzuki W, Hayashi K, Arima K, Myburgh E, Izuhara K, Brombacher F, Kubo M. IL-4-induced GATA-3 expression is a time-restricted instruction switch for Th2 cell differentiation. J Immunol. 2004;172:6158-6166. [PubMed] |
| 13. | Li C, Ebert PJ, Li QJ. T cell receptor (TCR) and transforming growth factor β (TGF-β) signaling converge on DNA (cytosine-5)-methyltransferase to control forkhead box protein 3 (foxp3) locus methylation and inducible regulatory T cell differentiation. J Biol Chem. 2013;288:19127-19139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1590] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 15. | Chen Z, Laurence A, O'Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19:400-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 423] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 17. | Pellegrini P, Contasta I, Del Beato T, Ciccone F, Berghella AM. Gender-specific cytokine pathways, targets, and biomarkers for the switch from health to adenoma and colorectal cancer. Clin Dev Immunol. 2011;2011:819724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Cua DJ, Kastelein RA. TGF-beta, a 'double agent' in the immune pathology war. Nat Immunol. 2006;7:557-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346 Pt 1:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 188] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Hargreaves PG, Al-Shamkhani A. Soluble CD30 binds to CD153 with high affinity and blocks transmembrane signaling by CD30. Eur J Immunol. 2002;32:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Carrington M, Martin MP. The impact of variation at the KIR gene cluster on human disease. Curr Top Microbiol Immunol. 2006;298:225-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1264] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 23. | van de Winkel JG, Capel PJ. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 478] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 24. | Qiu WQ, de Bruin D, Brownstein BH, Pearse R, Ravetch JV. Organization of the human and mouse low-affinity Fc gamma R genes: duplication and recombination. Science. 1990;248:732-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 210] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Parren PW, Warmerdam PA, Boeije LC, Arts J, Westerdaal NA, Vlug A, Capel PJ, Aarden LA, van de Winkel JG. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J Clin Invest. 1992;90:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 273] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Randerson PF. Why do we need multivariate analysis? . In: Fry JC. Biological Data Analysis. A Pratical Approach. Oxford University Press 1993; 173-207. |
| 28. | Berghella AM, Pellegrini P, Piancatelli D, Maccarone D, Del Beato T, Giubilei D, Pomidori A, Adorno D, Casciani CU. Progression mechanisms in colon cancer: soluble interleukin-2 (IL-2) receptor, IL-2 plus anti-CD3 proliferative response and tumour stage correlations. Cancer Immunol Immunother. 1994;38:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Hoffmann MH, Griffiths HR. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: evidence from preclinical models. Free Radic Biol Med. 2018;125:62-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 30. | Canossi A, Aureli A, Del Beato T, Rossi P, Franceschilli L, De Sanctis F, Sileri P, di Lorenzo N, Buonomo O, Lauro D, Venditti A, Sconocchia G. Role of KIR and CD16A genotypes in colorectal carcinoma genetic risk and clinical stage. J Transl Med. 2016;14:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |