Published online Nov 15, 2019. doi: 10.4251/wjgo.v11.i11.1081
Peer-review started: February 26, 2019
First decision: April 16, 2019
Revised: May 13, 2019
Accepted: August 18, 2019
Article in press: August 19, 2019
Published online: November 15, 2019
Processing time: 264 Days and 23.1 Hours
The first line treatment regimen for esophageal cancer is still surgical resection and the choice of surgical scheme depends on surgeon. Now the efficacy comparison of hybrid minimally invasive esophagectomy (HMIE) and open esophagectomy (OE) is still controversial.
To compare the perioperative and postoperative outcomes of HMIE and OE in patients with esophageal cancer.
PubMed, EMBASE, and Cochrane Library databases were searched for related articles. The odds ratio (OR) or standard mean difference (SMD) with a 95% confidence interval (CI) was used to evaluate the effectiveness of HMIE and OE.
Seventeen studies including a total of 2397 patients were selected. HMIE was significantly associated with less blood loss (SMD = -0.43, 95%CI: -0.66, -0.20; P = 0.0002) and lower incidence of pulmonary complications (OR = 0.72, 95%CI: 0.57, 0.90; P = 0.004). No significant differences were seen in the lymph node yield (SMD = 0.11, 95%CI: -0.08, 0.30; P = 0.26), operation time (SMD = 0.24, 95%CI: -0.14, 0.61; P = 0.22), total complications rate (OR = 0.68, 95%CI: 0.46, 0.99; P = 0.05), cardiac complication rate (OR = 0.91, 95%CI: 0.62, 1.34; P = 0.64), anastomotic leak rate (OR = 0.95, 95%CI: 0.67, 1.35; P = 0.78), duration of intensive care unit stay (SMD = -0.01, 95%CI: -0.21, 0.19; P = 0.93), duration of hospital stay (SMD = -0.13, 95%CI: -0.28, 0.01; P = 0.08), and total mortality rates (OR = 0.70, 95%CI: 0.47, 1.06; P = 0.09) between the two treatment groups.
Compared with the OE, HMIE shows less blood loss and pulmonary complications. However, further studies are necessary to evaluate the long-term oncologic outcomes of HMIE.
Core tip: In this meta-analysis, hybrid minimally invasive esophagectomy (HMIE) was found to be associated with less blood loss and lower incidence of pulmonary complications compared to conventional open esophagectomy (OE). In the subgroup analysis, patients with HMIE using laparoscopic gastric mobilization-thoracotomy presented less blood loss, shorter hospital stay, lower incidence of total and pulmonary complications than those with OE. No significant difference was observed between the two groups in mortality. In conclusion, our study is the first meta-analysis confirming the priority of HMIE to OE.
- Citation: Yang J, Chen L, Ge K, Yang JL. Efficacy of hybrid minimally invasive esophagectomy vs open esophagectomy for esophageal cancer: A meta-analysis. World J Gastrointest Oncol 2019; 11(11): 1081-1091
- URL: https://www.wjgnet.com/1948-5204/full/v11/i11/1081.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i11.1081
Esophageal cancer is the eighth most common cancer worldwide, with nearly 17000 newly diagnosed cases and 15910 deaths recorded annually in the United States alone[1]. Despite early diagnosis and advanced therapeutic modalities, including surgical resection, radiotherapy, and chemotherapy, the 5-year overall survival rate is a dismal 15% to 20%[2]. Esophageal resection remains the major curative and palliative option for dysphagia. For middle- and lower-third esophageal cancer, the abdominal and right thoracic approach is selected due to good loco-regional control. However, post-esophagectomy morbidity and mortality rates are 30%-50% and 2%-10%, respectively[3], mainly due to endocrinal and metabolic changes. The most frequent complications of esophagectomy are the major pulmonary complications (MPPCs), such as pneumonia and acute respiratory distress syndrome. Almost 50% of the postoperative deaths are attributed to MPPCs, which are indicative of poor prognosis.
Cuschieri et al[4] introduced endoscopic esophagectomy in 1992, which was followed by the development of minimally invasive esophagectomy (MIE), which uses a thoraco-abdominal approach and a combination of laparoscopy, thoracoscopy, and transhiatal laparoscopy. MIE can reduce surgical stress response, decrease blood loss, shorten hospital stay, and lower the incidence of complications[5-7]. However, only a few randomized controlled trials (RCTs) and low-quality meta-analysis have evaluated its clinical outcomes, in terms of tumor and lymph node clearance, and the safety profile. Hybrid MIE (HMIE) is performed using an Ivor-Lewis procedure, via a thoracoscopic-laparotomy and laparoscopic gastric mobilization-thoracotomy, for tumors of the mid-lower esophagus. A three stage McKeown’s procedure, with an additional left cervical incision, has been developed for the upper third of the esophagus. Open esophagectomy is performed by starting with an open right thoracotomy to mobilize the esophagus, followed by an open laparotomy to mobilize and pull the stomach to the neck for anastomosis. Therefore, HMIE may improve perioperative outcomes. The aim of this study was to compare the efficacy of open esophagectomy (OE) and HMIE in esophageal cancer patients.
PubMed, EMBASE, and Cochrane Library databases were searched for studies published till February 1, 2019 using the following key words: Open esophagectomy, Hybrid minimally invasive esophagectomy, minimally invasive esophagectomy, and esophageal cancer. In addition, the reference lists of the eligible studies were manually searched to include additional studies.
The inclusion criteria for the studies were as follows: (1) RCTs and non-RCTs; (2) Including patients with esophageal cancer; (3) Comparing the outcomes of OE and HMIE; and (4) Evaluating intraoperative outcomes and postoperative outcomes of both modalities. The exclusion criteria were: (1) In languages other than English; (2) Lacking comparison of OE and HMIE; and (3) Case reports and duplicate publications.
Two authors (Jiao Yang and Ling Chen) evaluated the titles, abstracts, and the reference lists of the publications, and independently extracted the data of intraoperative outcomes (lymph node yield, blood loss, and operative time) and postoperative outcomes (the rates of total complications, pulmonary complications, cardiac complications, and anastomotic leak, the duration of intensive care unit (ICU) stay and hospital stay, and total 30-d and 90-d mortality). Any disagreements were resolved by discussion with a third investigator (Ke Ge). For case-control studies, the Newcastle-Ottawa Quality Assessment Scale was used to assess the quality of the eligible studies, and those with a score ≥ 6 were included. Quality of RCTs was evaluated using the risk bias of Cochrane Collaboration tool.
All analyses were performed with the RveMan5.3 tool (Nordic Cochrane Centre, Cochrane Collaboration). Study heterogeneity was assessed using χ2 and Ι2 tests. A fixed-effects model was used when Ι2 was < 50% or P > 0.1, indicating no significant heterogeneity amongst the studies, and a random-effects model was used when Ι2 was > 50% or P < 0.1. Odds ratio (OR), standard mean difference (SMD), and 95% confidence interval (CI) were used as effect measurements, and P < 0.05 was considered statistically significant. Publication bias was evaluated by funnel plots and sensitivity analysis was applied to assess the stability of results.
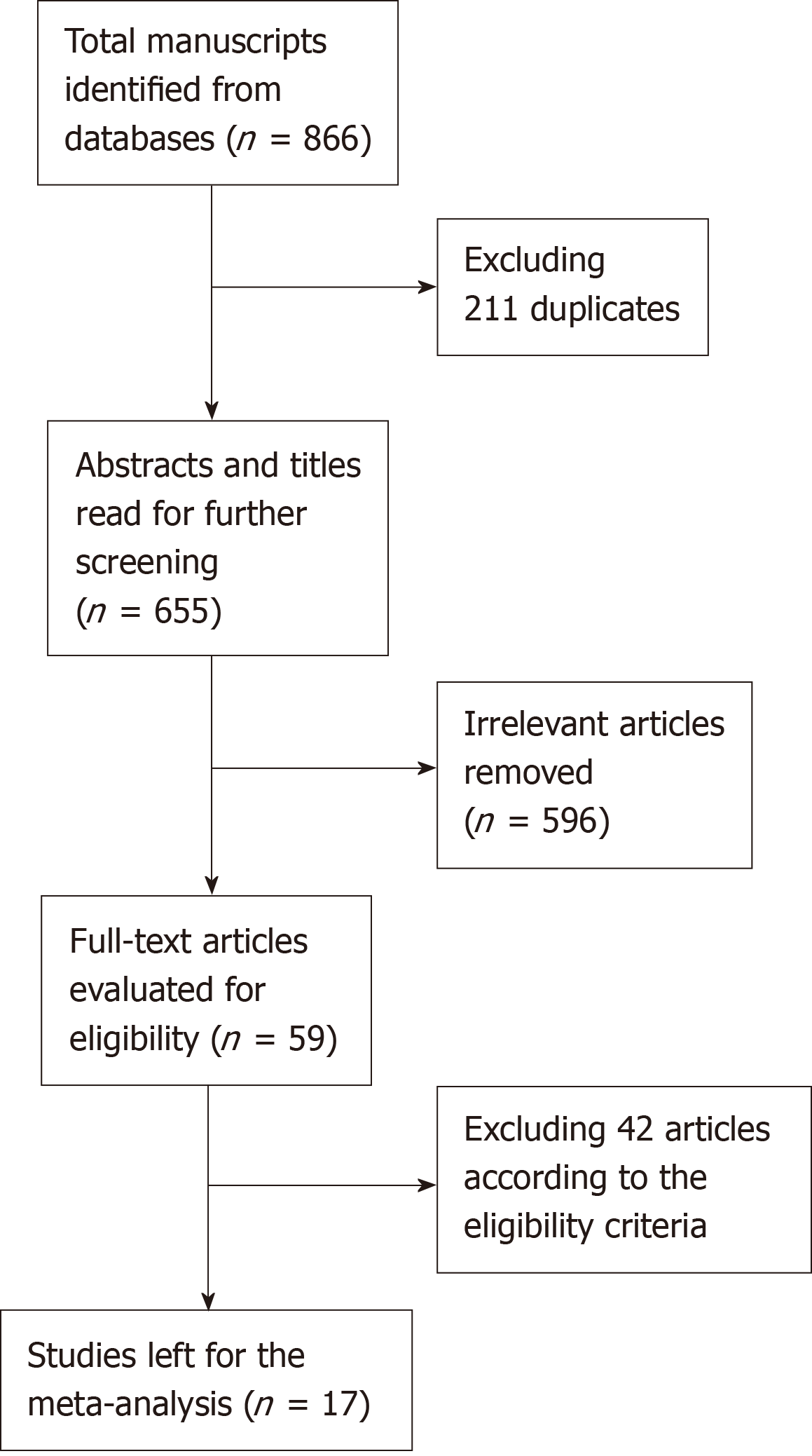
A total of 17 studies, including 2 RCTs[8,9] and 15 case-control studies[10-24], were eligible for the meta-analysis. The studies included 2397 esophageal carcinoma patients, of which 1170 received HMIE and 1227 underwent OE. The detailed search strategy is shown in Figure 1. The baseline characteristics and quality of the included studies are summarized in Table 1, Table 2, Table 3, and Table 4.
| Ref. | Time | Design | Area | Male/total | Age | Weight (kg/BMI) | |||
| HMIE | OE | HMIE | OE | HMIE | OE | ||||
| Yun et al[11] | 2017 | Retrospective | South Korea | 51/53 | 61/62 | 66 48-83 | 68 45-79 | NA | NA |
| Scarpa et al[14] | 2015 | Retrospective | Italy | 25/34 | 27/34 | 62 52-70 | 64 56-70 | NA | NA |
| Briez et al[13] | 2012 | Retrospective | France | 110/140 | 117/140 | NA | NA | NA | NA |
| Mariette et al[8] | 2019 | RCT | France | 88/103 | 87/104 | 59 23-75 | 62 41-78 | 26 16-37 | 25 18-35 |
| Glatz et al[15] | 2017 | Retrospective | Germany | 49/60 | 52/60 | 61 42-92 | 61 44-84 | 27 19-40 | 26 17-38 |
| Rinieri et al[17] | 2016 | Retrospective | France | 59/70 | 54/70 | 61.1 ± 9 | 61 ± 9 | NA | NA |
| Paireder et al[9] | 2018 | RCT | Austria | 10/14 | 10/12 | 64.5 40-75 | 62.5 49-77 | 24.08 18.07-41.45 | 26.96 17.53-35.26 |
| Rolff et al[22] | 2017 | Retrospective | Denmark | 50/56 | 125/160 | 66 39-86 | 65 28-88 | 25.8 18.8-31.2 | 26.6 15.6-43.7 |
| Parameswaran et al[10] | 2013 | Prospective | UnitedKingdom | 23/31 | 15/19 | 67 48-79 | 64 51-77 | NA | NA |
| Smithers et al[12] | 2007 | Prospective | Australia | 247/309 | 104/114 | 64 27-85 | 62.5 29-81 | 80 41-132 | 78.5 40-119 |
| Lee et al[16] | 2011 | Prospective | Taiwan | 43/44 | 61/64 | 59.7 44-78 | 56.58 30-90 | NA | NA |
| Findlay et al[19] | 2016 | Retrospective | United States | 84/95 | 69/87 | 67.76 | 65.54 | NA | NA |
| Safranek et al[20] | 2010 | Prospective | United Kingdom | 28/34 | 38/46 | 63 44-76 | 60 44-77 | NA | NA |
| Shiraishi et al[21] | 2006 | Retrospective | Japan | 32/38 | 31/37 | 62.1 ± 9 | 66.5 ± 9.3 | NA | NA |
| Kubo et al[23] | 2014 | Retrospective | Japan | 34/42 | 60/74 | 65.4 ± 9 | 62.2 ± 7.2 | NA | NA |
| Yanasoot et al[24] | 2017 | Retrospective | Thailand | 13/16 | 46/54 | 58.19± 7.78 | 61.02± 8.59 | NA | NA |
| Khan et al[18] | 2017 | Retrospective | Pakistan | 17/31 | 52/90 | 48.7 ± 13.1 | 56.5 ± 10.7 | 22.3 15-30.8 | 21.6 15-35 |
| Ref. | Tumor location | Histological subtype | Pathological stage | ASA risk score | ||||
| Upper/Middle/ Lower | ACA/SCC | 0-I-II/ III-IV | 1/2/3 | |||||
| HMIE | OE | HMIE | OE | HMIE | OE | HMIE | OE | |
| Yun et al[11] | 0/18/35 | 0/18/44 | NA | NA | 48/5 | 45/17 | NA | NA |
| Scarpa et al[14] | 0/25/9 | 0/29/5 | 24/10 | 24/10 | 29/5 | 29/5 | 5/22/7 | 4/17/13 |
| Briez et al[13] | 0/54/86 | 0/56/84 | 57/83 | 57/83 | 92/48 | 89/51 | 20/102/18 | 22/94/24 |
| Mariette et al[8] | 0/32/71 | 1/31/72 | 57/46 | 66/38 | 48/50 | 52/48 | 25/61/17 | 34/58/12 |
| Glatz et al[15] | 0/8/52 | 0/8/52 | 46/14 | 47/13 | 44/15 | 41/19 | NA | NA |
| Rinieri et al[17] | 60/10/0 | 63/7/0 | 50/20 | 55/15 | 52/18 | 49/21 | 9/48/13 | 14/40/16 |
| Paireder et al[9] | NA | NA | 10/4 | 11/1 | 7/7 | 8/4 | NA | NA |
| Rolff et al[22] | NA | NA | NA | NA | NA | NA | 17/28/12 | 41/80/39 |
| Parameswaran et al[10] | NA | NA | 27/3 | 16/3 | 18/31 | 8/11 | NA | NA |
| Smithers et al[12] | 8/68/208 | 0/3/47 | 199/74 | 100/7 | 183/108 | 36/75 | 12/200/98 | 6/68/38 |
| Lee et al[16] | 2/34/8 | 9/46/9 | 1/43 | 5/59 | 39/6 | 49/15 | NA | NA |
| Findlay et al[19] | NA | NA | NA | NA | NA | NA | NA | NA |
| Safranek et al[20] | 0/1/24 | 0/1/20 | 29/3 | 43/3 | 18/16 | 17/29 | NA | NA |
| Shiraishi et al[21] | NA | NA | NA | NA | NA | NA | NA | NA |
| Kubo et al[23] | 8/21/13 | 3/36/34 | NA | NA | 28/14 | 41/33 | NA | NA |
| Yanasoot et al[24] | 2/8/6 | 11/28/15 | 1/15 | 5/49 | 6/10 | 19/35 | NA | A |
| Khan et al[18] | NA | NA | 28/3 | 65/25 | 4/91 | 5/83 | NA | NA |
| Ref. | Selection | Comparability | Exposure | Total Score | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Parameswaran et al[10] | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| Yun et al[11] | Y | Y | Y | Y | Y | Y | 6 | ||
| Smithers et al[12] | Y | Y | Y | Y | Y | Y | Y | 7 | |
| Briez et al[13] | Y | Y | Y | Y | Y | Y | Y | 7 | |
| Scarpa et al[14] | Y | Y | Y | Y | Y | Y | 6 | ||
| Glatz et al[15] | Y | Y | Y | Y | Y | Y | 6 | ||
| Lee et al[16] | Y | Y | Y | Y | Y | Y | Y | 7 | |
| Rinieri et al[17] | Y | Y | Y | Y | Y | Y | Y | 7 | |
| Khan et al[18] | Y | Y | Y | Y | Y | Y | 6 | ||
| Findlay et al[19] | Y | Y | Y | Y | Y | Y | Y | 7 | |
| Safranek et al[20] | Y | Y | Y | Y | Y | Y | 6 | ||
| Shiraishi et al[21] | Y | Y | Y | Y | Y | Y | 6 | ||
| Rolff et al[22] | Y | Y | Y | Y | Y | Y | 6 | ||
| Yanasoot et al[24] | Y | Y | Y | Y | Y | Y | 6 | ||
| Kubo et al[23] | Y | Y | Y | Y | Y | Y | 6 | ||
| Ref. | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| Paireder et al[9] | Low risk | High risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Mariette et al[8] | Low risk | Low risk | Low risk | High risk | Unclear risk | Unclear risk | Unclear risk |
Lymph node yield: Nine studies reported the lymph node yield, with no significant difference between the HMIE with different approaches and OE groups (SMD = 0.11; 95%CI: -0.08, 0.30; P = 0.26; Table 5). Since significant heterogeneity (I2 = 65% and P = 0.004) was observed amongst the studies, a random-effects model was utilized. Then, subgroup analysis was used to compare HMIE with laparoscopy and thoracotomy and OE. Patients with laparoscopy and thoracotomy (HMIE) presented no more lymph node yield compared to those with OE (SMD = 0.19; 95%CI: -0.00, 0.37; P = 0.05; Table 5).
| Perioperative outcomes | SMD and 95%CI | P value | |
| Lymph node yield | Total HMIE vs OE | 0.11 (-0.08, 0.30) | 0.26 |
| HMIE with A vs OE | 0.19 (-0.00, 0.37) | 0.05 | |
| Blood loss | Total HMIE vs OE | -0.43 (-0.66, -0.20) | 0.0002 |
| HMIE with A vs OE | -0.51 (-0.74, -0.27) | <0.0001 | |
| Operative time | Total HMIE vs OE | 0.24 (-0.14, 0.61) | 0.22 |
| HMIE with A vs OE | 0.1 (-0.33, 0.52) | 0.65 | |
Blood loss: Six trials evaluated blood loss, which was also analyzed using the random-effects model due to significant heterogeneity (I2 = 58% and P = 0.04). HMIE with different strategies resulted in significantly lower blood loss compared to OE (SMD = -0.43; 95%CI: -0.66, -0.20; P = 0.0002; Table 5). In the subgroup analysis, HMIE using laparoscopy and thoracotomy showed priority to OE in decreasing the blood loss (SMD = -0.51; 95%CI: -0.74, -0.27; P < 0.0001; Table 5)
Operative time: Twelve studies involving 1630 patients recorded the operative time, and displayed significant heterogeneity in the outcome (I2 = 92% and P < 0.00001). However, HMIE with different approaches or HMIE with laparoscopy and thoracotomy did not significantly decrease the duration of operation (SMD = 0.24; 95%CI: -0.14, 0.61; P = 0.22 and SMD = 0.10; 95%CI: -0.33, 0.52; P = 0.65, respectively; Table 5).
Complications: Fourteen trials provided data of the total complications, and showed no significant differences between the HMIE with different approaches group and OE group (OR = 0.68; 95%CI: 0.46, 0.99; P = 0.05; Table 6). However, patients with HMIE using laparoscopy and thoracotomy presented less total complications than those with OE (OR = 0.62; 95%CI: 0.41, 0.94; P = 0.02; Table 6). Total HMIE and HMIE with laparoscopy and thoracotomy were associated with less pulmonary complications than OE (OR = 0.72; 95%CI: 0.57, 0.90; P = 0.004 and OR = 0.69; 95%CI: 0.53, 0.90; P = 0.005, respectively; Table 6), whereas the incidences of cardiac complications (OR = 0.91; 95%CI: 0.62, 1.34; P = 0.64 and OR = 0.97; 95%CI: 0.65, 1.43; P = 0.86, respectively; Table 6) and anastomotic leak (OR = 0.95; 95%CI: 0.67, 1.35; P = 0.78 and OR = 0.99; 95%CI: 0.67, 1.46; P = 0.96, respectively; Table 6) were similar.
| Postoperative outcomes | OR or SMD, 95%CI | P value | |
| ICU stay | Total HMIE vs OE | -0.01 (-0.21, 0.19) | 0.93 |
| HMIE with A vs OE | -0.05 (-0.37, 0.27) | 0.76 | |
| Hospital stay | Total HMIE vs OE | -0.13 (-0.28, 0.01) | 0.08 |
| HMIE with A vs OE | -0.37 (-0.64, -0.09) | 0.009 | |
| Total complications | Total HMIE vs OE | 0.68 (0.46, 0.99) | 0.05 |
| HMIE with A vs OE | 0.62 (0.41, 0.94) | 0.02 | |
| Pulmonary complications | Total HMIE vs OE | 0.72 (0.57, 0.90) | 0.004 |
| HMIE with A vs OE | 0.69 (0.53, 0.90) | 0.005 | |
| Cardiac complications | Total HMIE vs OE | 0.91 (0.62, 1.34) | 0.64 |
| HMIE with A vs OE | 0.97 (0.65, 1.43) | 0.86 | |
| Anastomotic leak | Total HMIE vs OE | 0.95 (0.67, 1.35) | 0.78 |
| HMIE with A vs OE | 0.99 (0.67, 1.46) | 0.96 | |
| Total mortality | Total HMIE vs OE | 0.7 (0.47, 1.06) | 0.09 |
| HMIE with A vs OE | 0.65 (0.4, 1.07) | 0.09 | |
| 30-d mortality | Total HMIE vs OE | 1.00 (0.45, 2.23) | 0.99 |
| HMIE with A vs OE | 1.10 (0.47, 2.59) | 0.82 | |
| 90-d mortality | Total HMIE vs OE | 0.80 (0.43, 1.48) | 0.47 |
| HMIE with A vs OE | 0.80 (0.43, 1.48) | 0.47 | |
Hospital and ICU stays: Thirteen studies reported duration of hospital stay with significant heterogeneity (I2 = 57% and P = 0.006), and total HMIE was not associated with significantly reduced duration of hospital stay (SMD = -0.13; 95%CI: -0.28, 0.01; P = 0.08; Table 6). However, shorter hospital stay showed in patients with HMIE using laparoscopy and thoracotomy than those with OE (SMD = -0.37; 95%CI: -0.64, -0.09; P = 0.009; Table 6).
In addition, the duration of ICU stay was similar in total HMIE or HMIE with laparoscopy and thoracotomy group and OE group (SMD = -0.01; 95%CI: -0.21, 0.19; P = 0.93 and SMD = -0.05; 95%CI: -0.37, 0.27; P = 0.76, respectively; Table 6).
Mortality: No significant heterogeneity was detected amongst the studies reporting the total, 30-d, and 90-d mortality rates, which were similar in total HMIE or HMIE with laparoscopy and thoracotomy group and OE group (total mortality: OR = 0.70, 95%CI: 0.47, 1.06, P = 0.09 and OR = 0.65, 95%CI: 0.4, 1.07, P = 0.09, respectively; 30-d mortality: OR = 1.00, 95%CI: 0.45, 2.23, P = 0.99 and OR = 1.10, 95%CI: 0.47, 2.59, P = 0.82, respectively; 90-d mortality: OR = 0.80, 95%CI: 0.43, 1.48, P = 0.47 and OR = 0.80, 95%CI: 0.43, 1.48, P = 0.47, respectively; Table 6).
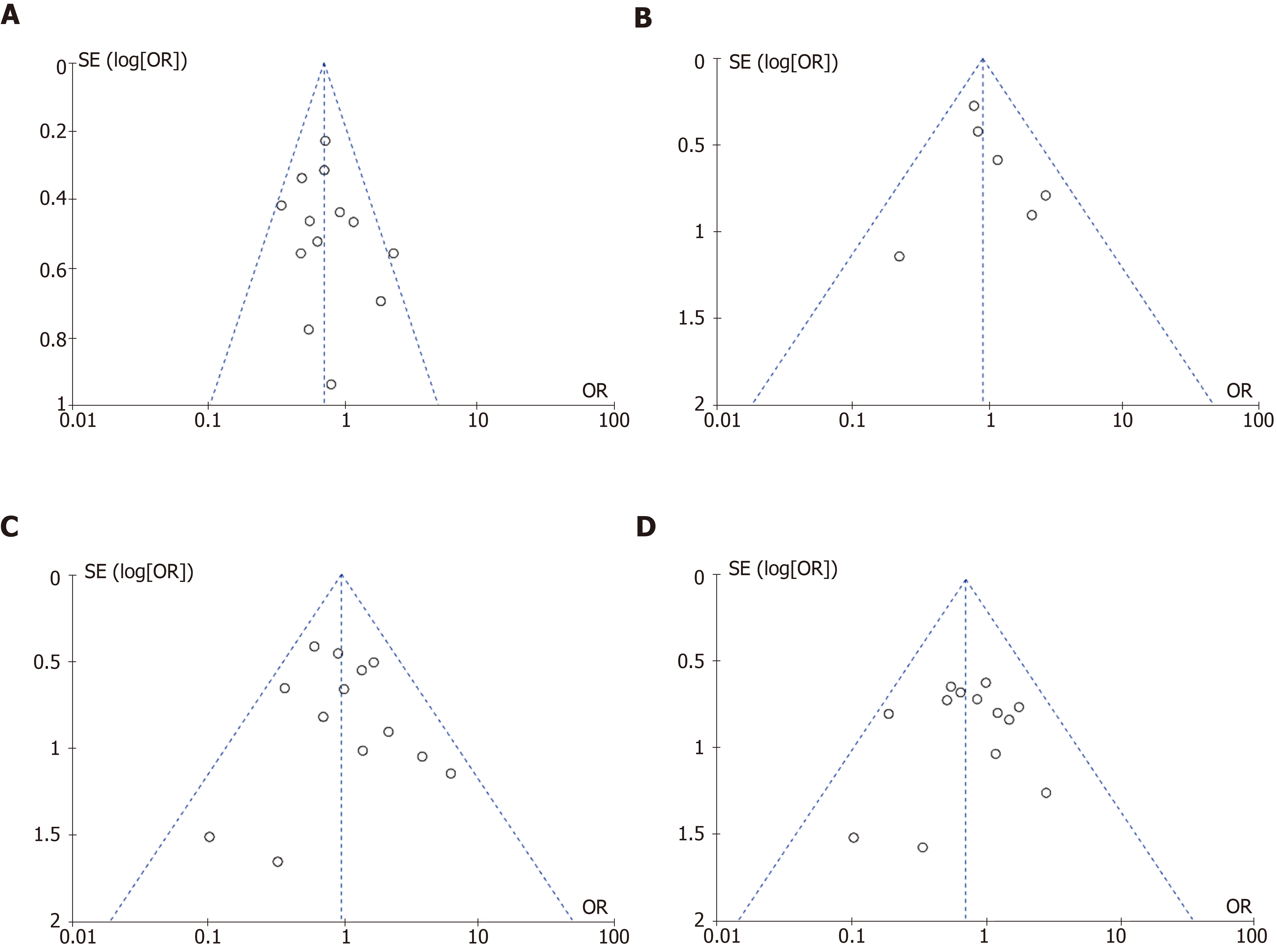
Publication bias: Publication bias was evaluated for the outcomes of pulmonary complications, cardiac complications, anastomotic leak, and total mortality and none was detected (Figure 2).
We removed any single trial, chose different effect models, and conducted subgroup analysis, and the outcomes presented no significant changes, suggesting that the results were stable.
Esophageal cancer is the sixth leading cause of cancer-related deaths worldwide. Surgical resection is the first line of treatment, and includes OE, total MIE, and HMIE. Depending on the surgeon and the hospital, the choice and sequence of surgical approaches differ significantly (transthoracic vs transhiatal, intrathoracic vs cervical anastomosis, and the degree of lymphadenectomy). OE is associated with a significantly higher risk of surgical trauma, as well as higher morbidity and mortality compared to other surgeries[25]. Sunpaweravong et al[25] conducted a meta-analysis to compare the efficacy of OE and MIE, and found that MIE resulted in fewer perioperative complications and less mortality. In addition, patients with MIE had better quality of life scores compared to those with OE in the global health, pain, and physical activity domains[26]. Therefore, total MIE would be the ideal choice. But the technical difficulties, the long learning curve, and low reproducibility of the anastomosis limit its use. HMIE has a shorter learning curve while sharing the advantages of MIE. The transition from OE to HMIE may be acceptable. The above information of MIE does not distinguish between the total MIE and HMIE approaches, so whether HMIE is prior to OE is still controversial. In this meta-analysis, we first compared the intraoperative and postoperative outcomes of HMIE and OE in patients with esophageal cancer.
Many studies show that radical lymph node resection and greater extent of lymphadenectomy are closely associated with higher survival rates[27-30]. In this study, there was no significant difference in terms of the number of harvested lymph nodes between total HMIE and OE groups, which is consistent with a previous RCT[8]. But there was a trend for patients with HMIE using laparoscopy and thoracotomy with a high rate of lymphadenectomy. Some studies once reported a higher or lower number of lymph nodes harvested in MIE group[31,32]. Those discrepancies may be explained by the inconsistency of Current Procedure Terminology codes reported by the operating surgeons.
Smithers et al[12] reported that patients who underwent HMIE had less blood loss than those undergoing OE, while Yanasoot et al[24] showed no significant difference. In our meta-analysis also, the total HMIE group and the HMIE with laparoscopy and thoracotomy group had less blood loss, which could be attributed to the relatively minimal trauma in HMIE.
Studies also report a longer operative duration of MIE compared to OE[33-35], which can result in atelectasis and pneumonia. In our meta-analysis, the operative time was similar for both surgeries.
Postoperative complications, especially pulmonary complications, significantly influence the survival of esophageal cancer patients. The incidences of total complications in patients with total HMIE and OE were 50.2% and 60.1%, respectively, although the lower occurrence after HMIE was not statistically significant. In the subgroup analysis, HMIE with laparoscopy and thoracotomy could largely lower the incidence of total complications than OE (46.55% vs 57.74%). The TIME trial showed that MIE resulted in a 70% lower incidence of pneumonia at 2 weeks post-surgery compared to OE[36], which is consistent with our slightly higher incidence of pulmonary complications in OE compared to total HMIE or HMIE with laparoscopy and thoracotomy (25.37% vs 32.08% or 24.59% vs 31.23%). In contrast, the incidence of cardiac complications and anastomotic leak was not affected by the type of surgery.
Less pulmonary complications in the total HMIE group did not translate into a significant reduction in the duration of ICU and hospital stay. But HMIE with laparoscopy and thoracotomy presented a more reduction in the duration of hospital stay on the basis of its lower total complications and pulmonary complications.
Some studies indicate that the prolonged survival associated with HMIE is due to the lower incidence of postoperative complications[37-39]. In our meta-analysis, the overall, 30-d, and 90-d mortality rates in the total HMIE group were 4.16%, 2.52%, and 4.00%, respectively vs 6.02%, 2.40%, and 4.70% in the OE group, indicating a lack of short-term survival benefit with total HMIE. Patients with HMIE using laparoscopy and thoracotomy presented no priority in short-term survival compared to those with OE. Wang et al reported that 6-year overall survival and disease-free survival were 44.7% and 46.1%, respectively, for MIE, indicating that MIE is safe[40]. A score-matched study showed that the 2-year overall survival rates based on same pathologic stage were similar between MIE and OE[41]. But further studies are still needed to clarify the long-term survival outcomes.
Our study has several limitations that need to be addressed. First, only two out of the 17 studies were RCTs and the remaining were case-control studies which might have influenced the reliability of the results, although they were consistent with that of one eligible RCT. Second, the studies had variable follow-up duration, neoadjuvant chemoradiotherapy, operating surgeons, pathological stages, histological types and location of the tumor, and baseline characteristics of the recruited population. Third, the meta-analysis did not compare the long-term oncological outcomes between HMIE and OE. Last but not the least, we made subgroup analysis between HMIE with laparoscopy and thoracotomy and OE group. But the information associated with HMIE using thoracoscopic-laparotomy approach is little and ambiguous. Therefore, the real impact of laparoscopy compared to thoracoscopy is unclear, and data that can confirm which part of esophagectomy would play an important role in MIE is lacking.
Taken together, HMIE, especially HMIE with laparoscopy and thoracotomy, has the advantages of reduced blood loss and lower incidence of pulmonary complications compared to OE for patients with esophageal cancer. However, there is no significant difference in overall survival in the two groups. These findings should be explained with caution because our study doesn’t provide the data associated with cancer-specific survival and recurrence.
The first line treatment regimen for esophageal cancer is still surgical resection and the choice of surgical scheme depends on the surgeon.
Now the efficacy comparison of hybrid minimally invasive esophagectomy (HMIE) and open esophagectomy (OE) is still controversial.
To compare the perioperative and postoperative outcomes of HMIE and OE in patients with esophageal cancer.
PubMed, EMBASE, and Cochrane Library databases were searched for related articles.
Seventeen studies including a total of 2397 patients were selected. HMIE was significantly associated with less blood loss (SMD = -0.43, 95%CI: -0.66, -0.20; P = 0.0002) and lower incidence of pulmonary complications (OR = 0.72, 95%CI: 0.57, 0.90; P = 0.004).
Compared with OE, HMIE shows less blood loss and pulmonary complications.
Further studies are necessary to evaluate the long-term oncologic outcomes of HMIE.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ahmed M, Chiu KW, Chen XZ, Shimizu Y S-Editor: Ji FF L-Editor: Wang TQ A E-Editor: Liu MY
| 1. | Howlader N. Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2013. National Cancer Institute. Bethesda, MD. Available from: https://seer.cancer.gov/archive/csr/1975_2013/. |
| 2. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1960] [Article Influence: 163.3] [Reference Citation Analysis (5)] |
| 3. | Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 4. | Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb. 1992;37:7-11. [PubMed] |
| 5. | van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1208] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 6. | Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1901] [Cited by in RCA: 1816] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 7. | Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Lee HJ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg. 2016;263:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 493] [Article Influence: 54.8] [Reference Citation Analysis (1)] |
| 8. | Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, D'Journo XB, Brigand C, Perniceni T, Carrère N, Mabrut JY, Msika S, Peschaud F, Prudhomme M, Bonnetain F, Piessen G; Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Group. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med. 2019;380:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 474] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 9. | Paireder M, Asari R, Kristo I, Rieder E, Zacherl J, Kabon B, Fleischmann E, Schoppmann SF. Morbidity in open versus minimally invasive hybrid esophagectomy (MIOMIE): Long-term results of a randomized controlled clinical study. Eur Surg. 2018;50:249-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Parameswaran R, Titcomb DR, Blencowe NS, Berrisford RG, Wajed SA, Streets CG, Hollowood AD, Krysztopik R, Barham CP, Blazeby JM. Assessment and comparison of recovery after open and minimally invasive esophagectomy for cancer: an exploratory study in two centers. Ann Surg Oncol. 2013;20:1970-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Yun JS, Na KJ, Song SY, Kim S, Jeong IS, Oh SG. Comparison of perioperative outcomes following hybrid minimally invasive versus open Ivor Lewis esophagectomy for esophageal cancer. J Thorac Dis. 2017;9:3097-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Smithers BM, Gotley DC, Martin I, Thomas JM. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg. 2007;245:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 13. | Briez N, Piessen G, Torres F, Lebuffe G, Triboulet JP, Mariette C. Effects of hybrid minimally invasive oesophagectomy on major postoperative pulmonary complications. Br J Surg. 2012;99:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Scarpa M, Cavallin F, Saadeh LM, Pinto E, Alfieri R, Cagol M, Da Roit A, Pizzolato E, Noaro G, Pozza G, Castoro C. Hybrid minimally invasive esophagectomy for cancer: impact on postoperative inflammatory and nutritional status. Dis Esophagus. 2016;29:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Glatz T, Marjanovic G, Kulemann B, Sick O, Hopt UT, Hoeppner J. Hybrid minimally invasive esophagectomy vs. open esophagectomy: a matched case analysis in 120 patients. Langenbecks Arch Surg. 2017;402:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Lee JM, Cheng JW, Lin MT, Huang PM, Chen JS, Lee YC. Is there any benefit to incorporating a laparoscopic procedure into minimally invasive esophagectomy? The impact on perioperative results in patients with esophageal cancer. World J Surg. 2011;35:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Rinieri P, Ouattara M, Brioude G, Loundou A, de Lesquen H, Trousse D, Doddoli C, Thomas PA, D'Journo XB. Long-term outcome of open versus hybrid minimally invasive Ivor Lewis oesophagectomy: a propensity score matched study. Eur J Cardiothorac Surg. 2017;51:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Khan M, Ashraf MI, Syed AA, Khattak S, Urooj N, Muzaffar A. Morbidity analysis in minimally invasive esophagectomy for oesophageal cancer versus conventional over the last 10 years, a single institution experience. J Minim Access Surg. 2017;13:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Findlay L, Yao C, Bennett DH, Byrom R, Davies N. Non-inferiority of minimally invasive oesophagectomy: an 8-year retrospective case series. Surg Endosc. 2017;31:3681-3689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Safranek PM, Cubitt J, Booth MI, Dehn TC. Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg. 2010;97:1845-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Shiraishi T, Kawahara K, Shirakusa T, Yamamoto S, Maekawa T. Risk analysis in resection of thoracic esophageal cancer in the era of endoscopic surgery. Ann Thorac Surg. 2006;81:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Rolff HC, Ambrus RB, Belmouhand M, Achiam MP, Wegmann M, Siemsen M, Kofoed SC, Svendsen LB. Robot-Assisted Hybrid Esophagectomy Is Associated with a Shorter Length of Stay Compared to Conventional Transthoracic Esophagectomy: A Retrospective Study. Minim Invasive Surg. 2017;2017:6907896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Kubo N, Ohira M, Yamashita Y, Sakurai K, Toyokawa T, Tanaka H, Muguruma K, Shibutani M, Yamazoe S, Kimura K, Nagahara H, Amano R, Ohtani H, Yashiro M, Maeda K, Hirakawa K. The impact of combined thoracoscopic and laparoscopic surgery on pulmonary complications after radical esophagectomy in patients with resectable esophageal cancer. Anticancer Res. 2014;34:2399-2404. [PubMed] |
| 24. | Yanasoot A, Yolsuriyanwong K, Ruangsin S, Laohawiriyakamol S, Sunpaweravong S. Costs and benefits of different methods of esophagectomy for esophageal cancer. Asian Cardiovasc Thorac Ann. 2017;25:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Sunpaweravong S, Ruangsin S, Laohawiriyakamol S, Mahattanobon S, Geater A. Prediction of major postoperative complications and survival for locally advanced esophageal carcinoma patients. Asian J Surg. 2012;35:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Maas KW, Cuesta MA, van Berge Henegouwen MI, Roig J, Bonavina L, Rosman C, Gisbertz SS, Biere SS, van der Peet DL, Klinkenbijl JH, Hollmann MW, de Lange ES, Bonjer HJ. Quality of Life and Late Complications After Minimally Invasive Compared to Open Esophagectomy: Results of a Randomized Trial. World J Surg. 2015;39:1986-1993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Rizk NP, Ishwaran H, Rice TW, Chen LQ, Schipper PH, Kesler KA, Law S, Lerut TE, Reed CE, Salo JA, Scott WJ, Hofstetter WL, Watson TJ, Allen MS, Rusch VW, Blackstone EH. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010;251:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 28. | Kang CH, Kim YT, Jeon SH, Sung SW, Kim JH. Lymphadenectomy extent is closely related to long-term survival in esophageal cancer. Eur J Cardiothorac Surg. 2007;31:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Martin DJ, Church NG, Kennedy CW, Falk GL. Does systematic 2-field lymphadenectomy for esophageal malignancy offer a survival advantage? Results from 178 consecutive patients. Dis Esophagus. 2008;21:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM, Hölscher A, Lerut T, Law S, Rice TW, Ruol A, van Lanschot JJ, Wong J, DeMeester TR. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 388] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 31. | Espinoza-Mercado F, Imai TA, Borgella JD, Sarkissian A, Serna-Gallegos D, Alban RF, Soukiasian HJ. Does the Approach Matter? Comparing Survival in Robotic, Minimally Invasive, and Open Esophagectomies. Ann Thorac Surg. 2019;107:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Naffouje SA, Salloum RH, Khalaf Z, Salti GI. Outcomes of Open Versus Minimally Invasive Ivor-Lewis Esophagectomy for Cancer: A Propensity-Score Matched Analysis of NSQIP Database. Ann Surg Oncol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Xiong WL, Li R, Lei HK, Jiang ZY. Comparison of outcomes between minimally invasive oesophagectomy and open oesophagectomy for oesophageal cancer. ANZ J Surg. 2017;87:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Chen X, Yang J, Peng J, Jiang H. Case-matched analysis of combined thoracoscopic-laparoscopic versus open esophagectomy for esophageal squamous cell carcinoma. Int J Clin Exp Med. 2015;8:13516-13523. [PubMed] |
| 35. | Mu J, Yuan Z, Zhang B, Li N, Lyu F, Mao Y, Xue Q, Gao S, Zhao J, Wang D, Li Z, Gao Y, Zhang L, Huang J, Shao K, Feng F, Zhao L, Li J, Cheng G, Sun K, He J. Comparative study of minimally invasive versus open esophagectomy for esophageal cancer in a single cancer center. Chin Med J (Engl). 2014;127:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, Gisbertz SS, Klinkenbijl JH, Hollmann MW, de Lange ES, Bonjer HJ, van der Peet DL, Cuesta MA. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1206] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 37. | Markar S, Gronnier C, Duhamel A, Mabrut JY, Bail JP, Carrere N, Lefevre JH, Brigand C, Vaillant JC, Adham M, Msika S, Demartines N, Nakadi IE, Meunier B, Collet D, Mariette C; FREGAT (French Eso-Gastric Tumors) working group, FRENCH (Fédération de Recherche EN CHirurgie), and AFC (Association Française de Chirurgie). The Impact of Severe Anastomotic Leak on Long-term Survival and Cancer Recurrence After Surgical Resection for Esophageal Malignancy. Ann Surg. 2015;262:972-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 38. | Rutegård M, Lagergren P, Rouvelas I, Mason R, Lagergren J. Surgical complications and long-term survival after esophagectomy for cancer in a nationwide Swedish cohort study. Eur J Surg Oncol. 2012;38:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Aahlin EK, Olsen F, Uleberg B, Jacobsen BK, Lassen K. Major postoperative complications are associated with impaired long-term survival after gastro-esophageal and pancreatic cancer surgery: a complete national cohort study. BMC Surg. 2016;16:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Qi W, Zixiang W, Tianwei Z, Shuai F, Sai Z, Gang S, Ming W. Long-term outcomes of 530 esophageal squamous cell carcinoma patients with minimally invasive Ivor Lewis esophagectomy. J Surg Oncol. 2018;117:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Wang H, Shen Y, Feng M, Zhang Y, Jiang W, Xu S, Tan L, Wang Q. Outcomes, quality of life, and survival after esophagectomy for squamous cell carcinoma: A propensity score-matched comparison of operative approaches. J Thorac Cardiovasc Surg. 2015;149:1006-14; discussion 1014- 5.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |