Published online Dec 15, 2018. doi: 10.4251/wjgo.v10.i12.505
Peer-review started: September 19, 2018
First decision: October 15, 2018
Revised: October 24, 2018
Accepted: November 25, 2018
Article in press: November 26, 2018
Published online: December 15, 2018
Processing time: 86 Days and 10.7 Hours
To evaluate the efficacy and safety of modified FOLFIRINOX as a second-line treatment for gemcitabine (GEM)-refractory unresectable pancreatic cancer (PC).
This study was a prospective, multicenter, one-arm, open-label, phase II trial. Patients with unresectable PC, who showed disease progression during GEM-based chemotherapy were enrolled. All patients were administered FOLFIRINOX with reduced irinotecan and oxaliplatin (RIO; irinotecan 120 mg/m2 and oxaliplatin 60 mg/m2), which was set according to the phase I study of FOLFIRINOX. The objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), adverse events were evaluated. Additionally, changes in quality of life (QoL) were assessed using a questionnaire on QoL.
Between August 2015 and May 2016, a total of 48 patients were enrolled. The median follow-up time was 259 d with a median of 8.5 cycles. The ORR and DCR were 18.8% and 62.5%, respectively, including one patient who showed complete remission. The median PFS was 5.8 mo [95% confidence interval (CI): 3.7-7.9] and median OS was 9.0 mo (95%CI: 6.4-11.6). Neutropenia (64.6%) was the most common grade 3-4 adverse event, followed by febrile neutropenia (16.7%). Although 14.6% of patients experienced grade 3 fatigue, most non-hematologic AEs were under grade 2. In the QoL analysis, the global health status score before treatment was not different from the score at the last visit after treatment (45.43 ± 22.88 vs 48.66 ± 24.14, P = 0.548).
FOLFIRINOX with RIO showed acceptable toxicity and promising efficacy for GEM-refractory unresectable PC. However, this treatment requires careful observation of treatment-related hematologic toxicities.
Core tip: For gemcitabine (GEM)-refractory unresectable pancreatic cancer (PC), there are limited options of second-line chemotherapy regimen. To find new treatment option for GEM-refractory unresectable PC, we conducted a multicenter phase II trial, which evaluated the efficacy and safety of uniquely modified FOLFIRINOX with reduced irinotecan and oxaliplatin. In our results, FOLFIRINOX with reduced irinotecan and oxaliplatin showed acceptable toxicity and promising efficacy. With careful observation of treatment-related hematologic toxicities, this chemotherapy regimen is a promising option for patients with GEM-refractory PC after first-line treatment failure.
- Citation: Chung MJ, Kang H, Kim HG, Hyun JJ, Lee JK, Lee KH, Noh MH, Kang DH, Lee SH, Bang S, Pancreatobiliary Cancer Study Group of Korean Society of Gastrointestinal Cancer. Multicenter phase II trial of modified FOLFIRINOX in gemcitabine-refractory pancreatic cancer. World J Gastrointest Oncol 2018; 10(12): 505-515
- URL: https://www.wjgnet.com/1948-5204/full/v10/i12/505.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i12.505
Pancreatic cancer (PC) is among the major causes of cancer-related deaths in the United States[1]. In South Korea, PC is the eighth highest-diagnosed cancer and the fifth most common cause of cancer-related death[2]. Metastatic pancreatic cancer (MPC) accounts for 60% of all cases; the median survival of patients with MPC is 3-6 mo. Systemic chemotherapy is pivotal for treating such patients; however, effective regimens remain limited. Recently, two first-line combination regimens-FOLFIRINOX [a combination of oxaliplatin, folinic acid (FA), irinotecan, and 5-fluorouracil (5-FU)] and nanoparticle albumin-bound (nab) paclitaxel in combination with GEM-prolonged survival compared to gemcitabine (GEM) monotherapy and became standard treatments[3,4]. However, the median progression-free survival (PFS) of these new treatment regimens was only 6.4 and 5.5 mo, respectively.
Proper second-line treatment can improve survival of patients with locally advanced pancreatic cancer (LAPC) or MPC who fail first-line treatment. Although some previous phase III trials showed survival improvement with their study regimens, the standard treatment remains unclear[5-7].
Patients who received first-line FOLFIRINOX or nab-paclitaxel plus GEM may benefit from a novel second-line treatment, although toxicity should also be considered. FOLFIRINOX, a standard first-line treatment, has been proposed as a second-line treatment for patients with good performance status who failed GEM-based chemotherapy. However, as the condition of many patients deteriorates after first-line chemotherapy, second-line therapy requires administration at attenuated doses and/or schedules, even in patients who maintain a preserved comorbidity profile.
Standard FOLFIRINOX has limited broad use as a second-line therapy because of toxicity; it includes irinotecan (180 mg/m2), oxaliplatin (85 mg/m2), 5-FU (400 mg/m2 administered as a bolus followed by 2400 mg/m2 administered as a 46-h continuous infusion), and leucovorin (400 mg/m2) every 2 wk[3]. Several FOLFIRINOX trials have investigated reducing dosages while maintaining efficacy[8-10]. However, studies focused on the efficacy and safety of a modified dose of FOLFIRINOX for patients with GEM-refractory PC are still rare. Therefore, we conducted a prospective, multicenter, one-arm, open-label, phase II trial using a modified FOLFIRINOX with reduced oxaliplatin and irinotecan (RIO) to minimize adverse events (AEs). Our aim was to evaluate the efficacy and safety of FOLFIRINOX with (RIO) in patients with unresectable PC who had earlier been treated with a GEM-based regimen until disease progression.
This study was a prospective, multicenter, one-arm, open-label, phase II trial and conducted in eight Korean university hospitals. The inclusion criteria for this study were patients between 19 and 75 years old; Eastern Cooperative Oncology Group performance status ≤ 2; cytologically or histologically proven unresectable pancreatic adenocarcinoma that progressed after first-line GEM-based chemotherapy; adequate bone marrow function (white blood cell count ≥ 3500/μL, absolute neutrophil count ≥ 1500/μL, and platelet count ≥ 100000/μL); adequate hepatic function (total bilirubin ≤ 1.5 × the upper limit of the normal range [ULN], serum aspartate and alanine transaminase ≤ 3 × ULN, and alkaline phosphatases ≤ 3 × ULN or ≤ 5 × ULN in case of liver metastasis); adequate renal function (serum creatinine ≤ 1.5 mg/dL); and adequate cardiopulmonary function. Patients were excluded if they had a concurrent malignancy other than PC; a serious, uncontrollable medical condition; or a psychiatric disorder. The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Written, informed consent was obtained from each participant after potential treatment complications had been fully explained. The institutional review boards at all participating institutions approved this study. This trial is registered with ClinicalTrials.gov, number NCT02440958.
The primary endpoints were objective response rate [ORR; complete remission (CR) + partial response (PR)] and disease control rate (DCR; CR + PR + stable disease (SD)). The secondary endpoints were PFS, overall survival (OS), changes in quality of life (QoL), and safety. OS was calculated from the date of enrollment until death from any cause. In the absence of an event, data were censored on the last day of survival confirmation. PFS was calculated from the initiation of treatment until either imaging-confirmed disease progression or death from any cause; in their absence, data for such patients were censored on the day of their last imaging procedure.
In the phase I study of FOLFIRINOX, febrile neutropenia, prolonged (≥ 7 d) severe neutropenia, and severe non-hematologic AEs were not reported at a dose level of 120 mg/m2 irinotecan and 60 mg/m2 oxaliplatin[11]. Based on these data, we set the study drug regimen – FOLFIRINOX with RIO – to 120 mg/m2 irinotecan (66.6% of standard dose) and 60 mg/m2 oxaliplatin (70.5% of standard dose) with standard dose of bolus and infusional 5-FU.
Oxaliplatin was first administered as a 2-h intravenous infusion (IVF); 1 h later, irinotecan was administered as a 90-min IVF. Leucovorin (400 mg/m²) was administered as a 90-min IVF immediately after oxaliplatin and irinotecan. The 5-FU dose was a 400 mg/m2 bolus followed by 2400 mg/m2 administered over 46 h of IVF. Each cycle of FOLFIRINOX with RIO was administered every 2 wk and repeated until either evidence of progressive disease (PD), significant clinical deterioration, or withdrawal of patient consent. All patients routinely received palonosetron 30 min before the initiation of chemotherapy as a prophylactic anti-emetic agent. Atropine was administered to patients with irinotecan-caused cholinergic reactions. High-dose loperamide was administered for delayed diarrhea, followed by prophylactic oral fluoroquinolones if diarrhea continued for over 48 h. Granulocyte colony-stimulating factor (G-CSF) was administered for severe neutropenia. AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03) before each cycle. In the event of predefined hematologic or non-hematologic AEs, protocol-specified treatment modifications or delays were performed to minimize additional treatment-related AEs.
For each patient, the study lasted up to 15 cycles with drugs donated by the pharmaceutical manufacturers; patients who completed these cycles without PD were admitted to a post-study phase and continued chemotherapy according to the study protocol at their own expense. Treatment was discontinued if PD or intolerable toxicity was observed, if the patient withdrew from the study, or at the physician’s discretion.
Pre-treatment evaluations included taking a complete medical history, physical examination, and laboratory tests. Evaluations were performed within 2 wk before, and every 2 wk during treatment. Tumor responses were assessed according to the Response Evaluation Criteria in Solid Tumors (version 1.1) based on high-resolution computed tomography scans every 8 wk. QoL was assessed every 8 wk using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30)[12] and its supplement for patients with pancreatic cancer (QLQ-PAN26)[13]. Additionally, changes in body weight and pain scale were checked every 2 wk. The Korean version of the questionnaire, officially translated and distributed by EORTC, was used. All patients filled-out and submitted the questionnaire by themselves on the day of visit. QoL changes between baseline and the last visit were analyzed, considering that the participation period varied among patients. Scores of all QoL scales range from 0 to 100; a higher score indicates a better functional status or a worse symptom.
When this clinical trial was being designed, the previously reported ORR of second-line chemotherapy for unresectable PC with GEM failure ranged from 0% to 11.4%[5,14-17]. With this background, this trial was performed according to a Simon optimal two-stage design (P0 = 0.100, P1 = 0.250, alpha = 0.050, and beta = 0.200; P0 and P1 are the response proportions of a poor and good drug, respectively)[18,19]. In the first stage, accepting a type I error of 10% and a power of 80%, 46 patients were planned for enrollment. If three or fewer of the 22 enrolled patients demonstrated an objective response, we would terminate the experiment at that stage based on the regimen’s low efficacy. Otherwise, the regimen would be recommended for further testing and accrual would continue to 46 patients (assuming a 15% dropout rate).
All patients who received the study regimen at least once were included in the intention-to-treat (ITT) and toxicity analysis populations. All efficacy assessments were based on the ITT analyses. PFS and OS were estimated using Kaplan-Meier methods with 95% confidence interval (CIs). When comparing data (QoL questionnaire, weight, and pain scale) between baseline and last visit, the paired t-test was used for normally distributed data while the Wilcoxon signed rank test was used for non-normally distributed data. All statistical analyses were performed using IBM SPSS (version 23.0, IBM Corp., Armonk, NY, United States). A P-value < 0.05 was considered statistically significant.
Between August 2015 and May 2016, 48 patients were enrolled. The median age at the time of enrollment was 63.5 years [interquartile range (IQR), 57.5–69.0 years]. All patients had cytologically or histologically confirmed adenocarcinoma according to the inclusion criteria. Also, all patients had LAPC or MPC including 38 patients (79.2%) with accompanying distant metastasis (Table 1). Close to 80% of the patients received GEM plus erlotinib as their first-line GEM-based treatment. Because GEM plus nab-paclitaxel became available in January 2016 in Korea, only one patient was administered this regimen prior to the study.
| Characteristics of the patients | n = 48 | Percent | |
| Age (yr) | median (IQR) | 63.5 (57.5-69.0) | |
| 40-49 | 4 | 8.3 | |
| 50-59 | 10 | 20.8 | |
| 60-69 | 25 | 52.1 | |
| 70-79 | 9 | 18.8 | |
| Sex | Male | 23 | 47.9 |
| Female | 25 | 52.1 | |
| ECOG-PS | 0 | 22 | 45.8 |
| 1 | 24 | 50 | |
| 2 | 2 | 4.2 | |
| Duration since diagnosis (mo) | median (IQR) | 7.0 (3.0-12.0) | |
| Location of pancreatic cancer | Head | 18 | 37.5 |
| Body and tail | 17 | 35.4 | |
| Recurrence after resection | 13 | 27.1 | |
| Number of metastatic site | 0 | 10 | 20.8 |
| 1 | 18 | 37.5 | |
| 2 | 14 | 29.2 | |
| ≥ 3 | 6 | 12.5 | |
| Metastatic sites (> 5%) | Liver | 28 | 58.3 |
| Peritoneum | 16 | 33.3 | |
| Distant lymph node | 8 | 16.7 | |
| Lung | 6 | 12.5 | |
| Level of CA 19-9 | Normal | 10 | 20.8 |
| > ULN | 38 | 79.2 | |
| Prior GEM CTx | GEM monotherapy | 6 | 12.5 |
| GEM + Erlotinib | 38 | 79.2 | |
| GEM + Capecitabine | 2 | 4.2 | |
| GEM + Cisplatin | 1 | 2.1 | |
| GEM + Nab-paclitaxel | 1 | 2.1 | |
| Period of prior CTx (mo) | median (IQR) | 4.1 (1.9-7.8) | |
| Prior treatment other than CTx | Operation | 13 | 27.1 |
| CCRT | 6 | 12.5 | |
A flowchart of the 48 patients’ treatments is shown in Supplementary Figure 1; at the time of analysis, 38 of these patients had died while two patients remained on FOLFIRINOX with RIO. Treatment was discontinued prior to completing 15 cycles in 33 patients, including 14 who showed PD, 10 who had treatment delays for unresolved infections (n = 2) or grade 3/4 toxicities (n = 8), five who declined further treatment, two who died after treatment (one of septic shock and the other of unknown reasons at another location), one who had acute cerebral infarction, and one who showed radiologic CR. All patients combined received a total of 493 cycles of chemotherapy. The median follow-up time was 259 d (IQR, 103.3–427.8 d), and the median number of chemotherapy cycles per patient was 8.5 (IQR, 3.0–16.5), with a median treatment duration of 145 d (IQR, 30.5–286.3 d). The relative dose intensity (proportion of the administered accumulated dose relative to the planned accumulated dose) of bolus 5-FU, infusional 5-FU, combined bolus plus infusional 5-FU, irinotecan, and oxaliplatin was 93.60% ± 15.86%, 93.60% ± 15.86%, 93.60% ± 15.86%, 95.65% ± 8.16%, and 95.65% ± 8.16%, respectively.
Tumor responses and survival analysis are shown in Table 2. The ORR and DCR of all patients were 18.8% and 62.5%, respectively. The DCR was 80% in ten LAPC patients, but no CR or PR was reported. Among 38 MPC patients (79.2% of all patients), the ORR and DCR were 23.7% and 57.9%, respectively. A sixty-year-old female patient, who progressed to multiple liver metastasis after GEM monotherapy, achieved radiologic CR after 12 cycle of FOLFIRINOX with RIO. After twelfth cycle, the patient had not experienced disease recurrence on serial radiologic studies without chemotherapy for a year, until peritoneal seeding and liver metastasis were confirmed.
| All (n = 48) | LAPC (n = 10) | MPC (n = 38) | |
| Response, n (%) | |||
| CR | 1 (2.1) | 0 (0.0) | 1 (2.6) |
| PR | 8 (16.7) | 0 (0.0) | 8 (21.1) |
| SD | 21 (43.8) | 8 (80.0) | 13 (34.2) |
| PD | 7 (14.6) | 1 (10.0) | 6 (15.8) |
| Could not be evaluated | 11 (22.9) | 1 (10.0) | 10 (26.3) |
| ORR | 9 (18.8) | 0 (0.0) | 9 (23.7) |
| DCR | 30 (62.5) | 8 (80.0) | 22 (57.9) |
| Survival, mo (95%CI) | |||
| Median PFS | 5.8 (3.7-7.9) | 8.8 (6.0-11.6) | 5.4 (2.9-7.9) |
| Median OS (from 2nd-line CTx) | 9.0 (6.4-11.6) | 12.5 (4.9-20.1) | 8.4 (5.4-11.4) |
| Median OS (from 1st-line CTx) | 17.1 (10.6-23.6) | 19.1 (13.8-24.4) | 16.8 (8.8-24.8) |
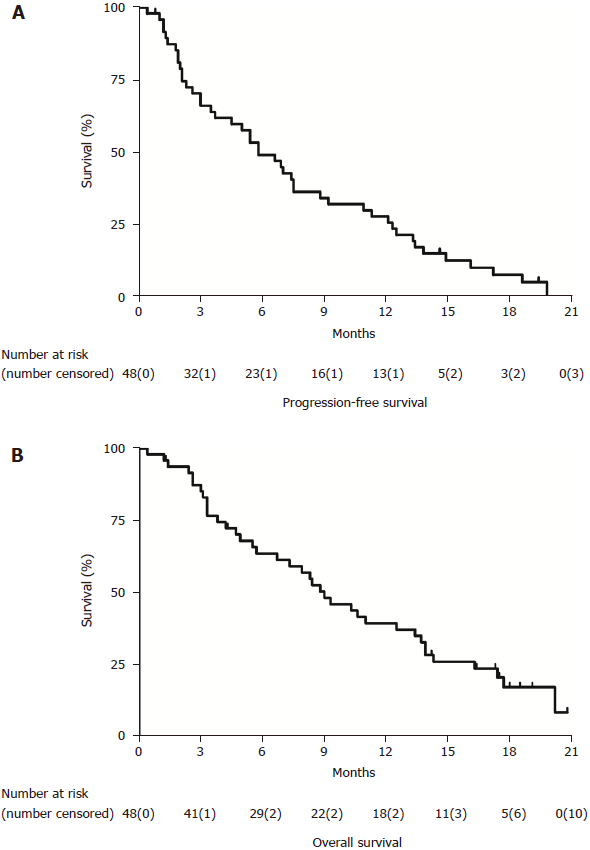
The median PFS was 5.8 mo (95%CI: 3.7–7.9 mo) and the median OS was 9.0 mo (95%CI: 6.4–11.6 mo) for all patients (Figure 1). The PFS rates at 6, 12, and 18 mo were 47.9%, 27.1%, and 6.3%, respectively, while the OS rates at 6, 12, and 18 mo were 60.4%, 37.5% and 10.4%, respectively. Eighteen patients (37.5%) survived more than 1 year. The estimated OS from the beginning of first-line treatment was 17.1 mo (95%CI: 10.6–23.6 mo). The median PFS and OS were respectively 5.4 and 8.4 mo for MPC patients, and 8.8 and 12.5 mo for LAPC patients. Analysis of changes in laboratory tests between before and after therapy showed that the median CA19-9 significantly decreased from 366.3 (1–16351) to 311.7 (2–16287) U/mL (P = 0.041).
AEs that occurred in more than 5% of the 48 patients are listed in Table 3. Common AEs observed in more than 20% of patients were neutropenia (68.8%), fatigue (22.9%), nausea and vomiting (66.7%), diarrhea (35.4%), oral mucositis (31.3%), anorexia (20.8%), and fever (20.8%). Of a total of 511 AEs, 358 (70.1%) were considered related to therapy, and 163 (31.9%) were severe AEs (grade 3 or 4). The most common severe AE was neutropenia (64.6%), followed by febrile neutropenia and fatigue (16.7% for both). None of the patients experienced severe nausea/vomiting or constipation. One patient died of septic shock related to grade 4 neutropenia after treatment.
| N = 48 | n (%) | Intensity according to the NCI-CTCAE v4.03 | |||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Non-hematologic | |||||
| Fatigue | 11 (22.9) | 3 (6.3) | 0 | 8 (16.7) | - |
| Nausea and vomiting | 32 (66.7) | 17 (35.4) | 15 (31.3) | 0 | 0 |
| Diarrhea | 17 (35.4) | 7 (14.6) | 9 (18.8) | 1 (2.1) | 0 |
| Constipation | 8 (16.7) | 4 (8.3) | 4 (8.3) | 0 | 0 |
| Oral mucositis | 15 (31.3) | 4 (8.3) | 10 (20.8) | 1 (2.1) | 0 |
| Anorexia | 10 (20.8) | 9 (18.8) | 0 | 1 (2.1) | 0 |
| Peripheral neuropathy | 7 (14.6) | 6 (12.5) | 0 | 1 (2.1) | 0 |
| Biliary tract infection | 3 (6.3) | 0 | 0 | 3 (6.1) | 0 |
| Fever | 10 (20.8) | 1 (2.1) | 9 (18.8) | 0 | 0 |
| Hematologic | |||||
| Neutropenia | 33 (68.8) | 0 | 2 (4.2) | 11 (22.9) | 20 (41.7) |
| Thrombocytopenia | 6 (12.5) | 0 | 1 (2.1) | 1 (2.1) | 4 (8.3) |
| Febrile neutropenia | 8 (16.7) | - | - | 5 (10.4) | 3 (6.3) |
The average body weight was 58.9 ± 9.81 kg at baseline and 59.0 ± 9.83 kg at the last visit. The average pain scale (Visual Analogue Scale) at baseline and the last visit were 2.12 ± 2.31 and 1.90 ± 2.15, respectively. There were no significant changes in body weight and pain scale (P = 0.93 and P = 0.71, respectively). QoL questionnaires were available for 31 patients. The global health status scores of the EORTC QLQ-C30 did not worsen after treatment (P = 0.548). In general, most functional scores were not significantly decreased except role and cognitive functioning (P = 0.044 and P = 0.015, respectively) (Supplementary Table 1). Among symptom scores, fatigue and dyspnea were significantly worse than those in the pre-treatment period (P = 0.021 and P = 0.038, respectively). Among separate QLQ-PAN26 questions, worsening of dry mouth was observed (P = 0.011). In patients who achieved disease control (n = 29), the global health status score did not worsen after treatment, but some individual items including cognitive function, fatigue, digestive symptoms, and dry mouth were significantly worsened. Only one item (future worries) was significantly improved (Supplementary Table 2). In patients who completed 15 cycles, constipation and pancreatic pain were significantly improved by the end of treatment; only digestive symptoms were aggravated (Supplementary Table 3).
FOLFIRINOX with RIO showed an acceptable toxicity profile and promising efficacy as a second-line treatment for GEM-refractory unresectable PC. Although severe neutropenia occurred in almost 65% of participants, other severe AEs, particularly non-hematologic AEs, were infrequently reported. Moreover, the global health status scores of the EORTC QLQ-C30 were not changed significantly after treatment.
Second-line chemotherapy may be considered for many patients[20]. At present, there is no recognized standard for patients with unresectable PC who experience PD after first-line chemotherapy, and PFS is consistently < 4 mo in patients receiving second-line chemotherapy. A meta-analysis showed that median OS was 6.0 mo with chemotherapy versus 2.8 mo with best supportive care[21]. Patients whose cancers progress after first-line therapy have difficulty undergoing second-line chemotherapy since they are often older, unwell, and at risk of rapid deterioration[22].
Only a few prospective trials have shown encouraging results with oxaliplatin plus 5-FU using various doses and schedules[5,6,14,17,23]. Two phase III trials produced conflicting results. The CONKO-003 trial comparing 5-FU plus FA (FF) and oxaliplatin plus FF (OFF) showed survival benefits of second-line OFF in patients with unresectable GEM-refractory PC[6]. In contrast, the PANCREOX trial evaluating the modified FOLFOX6 (mFOLFOX6) found no difference in PFS, while the OS of mFOLFOX6 was inferior to that of FF[24].
Research on irinotecan plus 5-FU as second-line chemotherapy for PC was also performed[15,25]. The NAPOLI-1 phase III trial comparing nanoliposomal irinotecan (nal-IRI) alone or combined with FF showed that the combination of nal-IRI and FF was more effective than FF alone, but caused more frequent severe AEs[7].
A recent comparative systematic review of four randomized trials evaluating oxaliplatin- or irinotecan-containing regimens as post-GEM therapies for patients with unresectable PC showed significant dissimilarity between them; therefore, it is unclear which regimen is best-suited for patients with unresectable PC previously treated with GEM[26]. Table 4 summarizes clinical trials of second-line treatment for GEM-pre-treated unresectable PC. Our results using second-line FOLFIRINOX with RIO showed results that were superior to those in most previous trials.
| Author (yr) | Type of study | Regimen | Patients, n | KPS ≥ 90 or ECOG ≤ 1, % | MPC, % | ORR, % | DCR, % | PFS/TTP, mo | OS, mo |
| Yoo et al[15] 2009 | II | Modified FOLFOX | 30 | 97 | 100 | 7 | 17 | 6.0 wk | 14.9 wk |
| Modified FOLFIRI.3 | 31 | 100 | 100 | 0 | 23 | 8.3 wk | 16.6 wk | ||
| Novarino et al[14] 2009 | II | Oxaliplatin/5-FU/LV | 23 | 73.9 | 69.6 | 0 | 23.5 | 11.6 wk1 | 17.1 wk |
| Pelzer et al[5] 2011 | III | BSC | 23 | 52.2 | 69.6 | 0 | NA | NA | 2.3 |
| Oxaliplatin/5-FU/LV (OFF) | 23 | 47.8 | 73.9 | 0 | NA | NA | 4.8 (P = 0.008) | ||
| Chung et al[23] 2013 | II | FOLFOX4 | 44 | NA | 100 | 11.4 | 40.9 | 9.9 wk1 | 31.1 wk |
| Oettle et al[6] 2014 | III | 5-FU/LV (FF) | 84 | 47.6 | 88.1 | NA | NA | 2 | 3.3 |
| Oxaliplatin/5-FU/LV (OFF) | 84 | 53.9 | 88.2 | NA | NA | 2.9 (P = 0.019) | 5.9 (P = 0.01) | ||
| Zaanan et al[17] 2014 | Prospective cohort | FOLFOX2 | 27 | 44.4 | 100 | 0 | 36.4 | 1.7 | 4.3 |
| Wang-Gillam et al[7] 2016 | III | 5-FU/LV | 119 | 48 | 100 | 1 | NA | 1.5 | 4.2 |
| Nal-IRI/5-FU/LV | 117 | 59 | 100 | 16 | NA | 3.1 (P < 0.001) | 6.1 (P = 0.01) | ||
| Nal-IRI | 151 | 57 | 100 | 6 | NA | 2.7 (P = 0.1) | 4.9 (P = 0.94) | ||
| Gill et al[24] 2016 | III | Modified FOLFOX6 | 54 | 88.9 | 92.6 | 13.2 | 44.7 | 3.1 | 6.1 |
| Infusional 5FU/LV | 54 | 94.3 | 94.4 | 8.5 (P = 0.36) | 55.3 | 2.9 (P = 0.99) | 9.9 (P = 0.02) | ||
| Present study | II | FOLFIRINOX with RIO | 48 (MPC: 38) | 95.8 | 79.2 | 18.8 (MPC: 23.7) | 62.5 (MPC: 57.9) | 5.8 (MPC: 5.4) | 9 (MPC: 8.4) |
Toxicities associated with standard FOLFIRINOX have prompted trials evaluating modifications of FOLFIRINOX[8-10]. These previous studies of FOLFIRINOX modifications suggested that upfront dose attenuations of standard FOLFIRINOX can improve tolerability without reducing efficacy. A recent phase II trial showed that the efficacy of first-line FOLFIRINOX with reduced doses of the 5-FU bolus and irinotecan was comparable to that of the standard regimen; furthermore, neutropenia, vomiting, and fatigue were significantly reduced[10]. However, only a few studies have evaluated FOLFIRINOX for patients with unresectable PC after failure of GEM-based chemotherapy; patients’ performance statuses are likely to deteriorate after first-line treatment; necessitating second-line dose reductions as in our study. A Japanese phase II trial used modified FOLFIRINOX reducing only irinotecan for 18 MPC patients[27]. Findings of that trial were consistent with our results except the PFS, which was longer in the present study (2.8 mo vs 5.8 mo).
FOLFIRINOX and nab-paclitaxel plus GEM are two of the most effective first-line treatments for MPC, but the appropriate sequence of administration is unclear. A previous study in France concluded that nab-paclitaxel plus GEM appears to be effective, with a manageable toxicity profile, after FOLFIRINOX failure in patients with MPC[28]. However, studies evaluating the reverse sequence of administration have not been performed to date. Only three studies have been performed evaluating second-line FOLFIRINOX following first-line GEM-based treatment (Table 5)[27,29,30]. To our knowledge, this study is the first prospective multicenter phase II trial, which evaluated the efficacy and safety of FOLFIRINOX in GEM-refractory PC using unique dose modification called “FOLFIRINOX with RIO”.
| Study characteristics | Patients characteristics | Treatment outcomes | Grade ≥ 3 AE (%) | ||||||||||||
| Author (yr) | Type | Dose modification | Patients, n | Age, median (range) | ECOG | Cancer status (%) | ORR, % | DCR, % | PFS, mo | OS, mo | NP | Febrile NP | Fatigue | Nausea | Diarrhoea |
| Assaf et al[29] 2011 | Retro | Standard | 27 | 63 (45-83) | 1-3 | MPC (100) | 18.5 | 62.9 | 3 | 8.5 | 56 | 3.7 | NA | 11 | 11 |
| Lee et al[30] 2013 | Retro | Standard | 18 | 57 (44-68) | 0-1 | MPC (88.9) | 27.8 | 55.6 | 2.8 | 8.4 | 38.9 | 11.1 | NA | 38.9 | 0 |
| LAPC (11.1) | |||||||||||||||
| Kobayashi et al[27] 2017 | II | Irinotecan 56% or 67% | 18 | 63 (46-68) | 0-1 | MPC (100) | 22.2 | 61.1 | 2.8 | 9.8 | 66.7 | 5.6 | NA | 0 | 0 |
| Present study | II | Irinotecan 67% | 48 | 64 (40-79) | 0-2 | MPC (79.2) | All: 18.8 | All: 62.5 | All: 5.8 | All: 9.0 | 64.6 | 16.7 | 16.7 | 0 | 2.1 |
| Oxaliplatin 71% | LAPC (20.8) | MPC: 23.7 | MPC: 57.9 | MPC: 5.4 | MPC: 8.4 | ||||||||||
| LAPC: 0.0 | LAPC: 80.0 | LAPC: 8.8 | LAPC: 12.5 | ||||||||||||
In our study, the relatively higher incidences of severe neutropenia may be related to the patients’ deteriorated physical status after first-line chemotherapy and the lack of prophylactic G-CSF support. For non-hematologic AEs, no grade 3 or 4 vomiting was observed, unlike in the PRODIGE 4/ACCORD 11 trial[3]. This improved non-hematologic tolerability may be related to the routine administration of prophylactic palonosetron during every cycle of treatment. Peripheral neuropathy was also significantly reduced compared with the previous study, likely because of oxaliplatin dose reduction. In comparison with nal-IRI plus 5-FU regimen of the NAPOLI-1 trial, the preferred second-line therapy for MPC in current guidelines[31,32], most severe non-hematologic AEs of the present study occurred at lower rates (diarrhea, 13% vs 2.1%; vomiting, 11% vs 0%; anorexia, 4% vs 2.1%)[7]. However, the rate of severe neutropenia was much higher in the present study (27% vs 64.6%).
Considering QoL, some functional scales such as role and cognitive functioning were significantly reduced in our patients, and some symptom scales such as fatigue and dyspnea were significantly worsened. However, these changes were predictable given our patients’ ages and performance statuses. Global QoL indicators did not significantly deteriorate; moreover, the “physical functioning” QoL score (regarded as one of the strongest prognostic values[33]) did not significantly worsen throughout treatment.
This study had several limitations. First, it was a non-randomized, single arm trial with a relatively small sample size. A prospective randomized trial including sufficient patients is warranted to provide the clinical recommendation about the treatment sequence for MPC. Second, we included patients with LAPC and MPC who were treated with various prior GEM-based regimens. This heterogeneity in patient population and first-line chemotherapy regimens needs to be improved in future research.
In conclusion, FOLFIRINOX with RIO showed encouraging results in terms of efficacy, with an acceptable safety profile. In addition to nal-IRI plus 5-FU regimen, FOLFIRINOX with RIO may be considered as a treatment option in patients with GEM-refractory unresectable PC. Because the condition of such patients can quickly deteriorate owing to rapid disease progression and treatment toxicity, this regimen may provide acceptable tolerability for patients in terms of patient QoL. However, the presence of hematologic toxicities should be carefully observed, nevertheless, and the routine use of G-CSF should be considered to minimize the risk of hematologic toxicities.
Proper second-line treatment can improve survival of patients with unresectable pancreatic cancer (PC) who fail first-line treatment with gemcitabine (GEM)-based regimen. Although some previous phase III trials showed survival improvement with their study regimens, the standard second-line treatment remains unclear.
FOLFIRINOX, a standard first-line treatment for PC, has been proposed as a second-line treatment regimen; however, concerns about relatively high toxicity limited broad use of FOLFIRINOX as a second-line therapy.
We evaluated the efficacy and safety of modified dose of FOLFIRINOX as a second-line treatment for GEM-refractory unresectable PC.
In this prospective, multicenter, one-arm, open-label, phase II trial, unresectable PC patients, who showed disease progression during GEM-based therapy were enrolled. All patients were administered FOLFIRINOX with reduced irinotecan and oxaliplatin (RIO; irinotecan 120 mg/m2 and oxaliplatin 60 mg/m2), which was set according to the previous phase I study of FOLFIRINOX, with the standard dose of 5-fluorouracil (5-FU). The objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), adverse events, and changes in quality of life (QoL) were evaluated.
A total of 48 patients were enrolled in eight Korean centers. The ORR and DCR were 18.8% and 62.5%, respectively, including one patient who showed complete remission. The median PFS was 5.8 mo [95% confidence interval (CI): 3.7-7.9] and median OS was 9.0 mo (95%CI: 6.4-11.6). Neutropenia (64.6%) was the most common grade 3-4 adverse event. Although 14.6% of patients experienced grade 3 fatigue, most non-hematologic AEs were under grade 2. In the QoL analysis, the global health status score before treatment was not different from the score at the last visit after treatment (45.43 ± 22.88 vs 48.66 ± 24.14, P = 0.548).
FOLFIRINOX with RIO showed acceptable tolerability for patients in terms of patient QoL and may be considered as a treatment option in patients with GEM-refractory unresectable PC. However, the presence of hematologic toxicities should be carefully observed and the routine use of granulocyte colony-stimulating factor should be considered to minimize the risk of hematologic toxicities.
Prospective study with larger population comparing the efficacy and safety between FOLFIRINOX with RIO and 5-FU plus leucovorin needs to be conducted.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chandrasinghe PC, Kuo SH, Yang F S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12187] [Article Influence: 1523.4] [Reference Citation Analysis (3)] |
| 2. | National Cancer Information Center (Republic of Korea). Cancer Statistics in Korea. Ministry of Health and Welfare, Korea Central Cancer Registry, National Cancer Center. 2017; Available from: https://ncc.re.kr/main.ncc?uri=english/sub04_Statistics. |
| 3. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5640] [Article Influence: 402.9] [Reference Citation Analysis (1)] |
| 4. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4889] [Article Influence: 407.4] [Reference Citation Analysis (0)] |
| 5. | Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dörken B, Riess H, Oettle H. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 6. | Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, Görner M, Mölle M, Greten TF, Lakner V. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32:2423-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 7. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 830] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 8. | Mahaseth H, Brutcher E, Kauh J, Hawk N, Kim S, Chen Z, Kooby DA, Maithel SK, Landry J, El-Rayes BF. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Ghorani E, Wong HH, Hewitt C, Calder J, Corrie P, Basu B. Safety and Efficacy of Modified FOLFIRINOX for Advanced Pancreatic Adenocarcinoma: A UK Single-Centre Experience. Oncology. 2015;89:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, Staugaard C, Indukala D, Boustani AM, Patel V. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Ychou M, Conroy T, Seitz JF, Gourgou S, Hua A, Mery Mignard D, Kramar A. An open phase I study assessing the feasibility of the triple combination: oxaliplatin plus irinotecan plus leucovorin/5-fluorouracil every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2003;14:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11469] [Article Influence: 358.4] [Reference Citation Analysis (0)] |
| 13. | Fitzsimmons D, Johnson CD, George S, Payne S, Sandberg AA, Bassi C, Beger HG, Birk D, Büchler MW, Dervenis C. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer. 1999;35:939-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 190] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Novarino A, Satolli MA, Chiappino I, Giacobino A, Bellone G, Rahimi F, Milanesi E, Bertetto O, Ciuffreda L. Oxaliplatin, 5-fluorouracil, and leucovorin as second-line treatment for advanced pancreatic cancer. Am J Clin Oncol. 2009;32:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Yoo C, Hwang JY, Kim JE, Kim TW, Lee JS, Park DH, Lee SS, Seo DW, Lee SK, Kim MH. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101:1658-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Chung MJ, Park JY, Bang S, Park SW, Song SY. Phase II clinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancer. Cancer Immunol Immunother. 2014;63:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Zaanan A, Trouilloud I, Markoutsaki T, Gauthier M, Dupont-Gossart AC, Lecomte T, Aparicio T, Artru P, Thirot-Bidault A, Joubert F. FOLFOX as second-line chemotherapy in patients with pretreated metastatic pancreatic cancer from the FIRGEM study. BMC Cancer. 2014;14:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2789] [Cited by in RCA: 2948] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 19. | Conroy T, Paillot B, François E, Bugat R, Jacob JH, Stein U, Nasca S, Metges JP, Rixe O, Michel P. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer--a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. 2005;23:1228-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Nagrial AM, Chin VT, Sjoquist KM, Pajic M, Horvath LG, Biankin AV, Yip D. Second-line treatment in inoperable pancreatic adenocarcinoma: A systematic review and synthesis of all clinical trials. Crit Rev Oncol Hematol. 2015;96:483-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Rahma OE, Duffy A, Liewehr DJ, Steinberg SM, Greten TF. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol. 2013;24:1972-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2206] [Article Influence: 147.1] [Reference Citation Analysis (2)] |
| 23. | Chung JW, Jang HW, Chung MJ, Park JY, Park SW, Chung JB, Song SY, Bang S. Folfox4 as a rescue chemotherapy for gemcitabine-refractory pancreatic cancer. Hepatogastroenterology. 2013;60:363-367. [PubMed] |
| 24. | Gill S, Ko YJ, Cripps C, Beaudoin A, Dhesy-Thind S, Zulfiqar M, Zalewski P, Do T, Cano P, Lam WYH. PANCREOX: A Randomized Phase III Study of Fluorouracil/Leucovorin With or Without Oxaliplatin for Second-Line Advanced Pancreatic Cancer in Patients Who Have Received Gemcitabine-Based Chemotherapy. J Clin Oncol. 2016;34:3914-3920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 25. | Gebbia V, Maiello E, Giuliani F, Borsellino N, Arcara C, Colucci G. Irinotecan plus bolus/infusional 5-Fluorouracil and leucovorin in patients with pretreated advanced pancreatic carcinoma: a multicenter experience of the Gruppo Oncologico Italia Meridionale. Am J Clin Oncol. 2010;33:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Vogel A, Ciardiello F, Hubner RA, Blanc JF, Carrato A, Yang Y, Patel DA, Ektare V, de Jong FA, Gill S. Post-gemcitabine therapy for patients with advanced pancreatic cancer - A comparative review of randomized trials evaluating oxaliplatin- and/or irinotecan-containing regimens. Cancer Treat Rev. 2016;50:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Kobayashi N, Shimamura T, Tokuhisa M, Goto A, Endo I, Ichikawa Y. Effect of FOLFIRINOX as second-line chemotherapy for metastatic pancreatic cancer after gemcitabine-based chemotherapy failure. Medicine (Baltimore). 2017;96:e6769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Portal A, Pernot S, Tougeron D, Arbaud C, Bidault AT, de la Fouchardière C, Hammel P, Lecomte T, Dréanic J, Coriat R. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer. 2015;113:989-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | Assaf E, Verlinde-Carvalho M, Delbaldo C, Grenier J, Sellam Z, Pouessel D, Bouaita L, Baumgaertner I, Sobhani I, Tayar C. 5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology. 2011;80:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Lee MG, Lee SH, Lee SJ, Lee YS, Hwang JH, Ryu JK, Kim YT, Kim DU, Woo SM. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with advanced pancreatic cancer who have progressed on gemcitabine-based therapy. Chemotherapy. 2013;59:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Sohal DP, Mangu PB, Khorana AA, Shah MA, Philip PA, O’Reilly EM, Uronis HE, Ramanathan RK, Crane CH, Engebretson A. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2784-2796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 32. | Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v56-v68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 930] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 33. | Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Boige V. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 342] [Article Influence: 26.3] [Reference Citation Analysis (0)] |