Published online Nov 15, 2018. doi: 10.4251/wjgo.v10.i11.431
Peer-review started: August 8, 2018
First decision: September 12, 2018
Revised: September 19, 2018
Accepted: October 12, 2018
Article in press: October 12, 2018
Published online: November 15, 2018
Processing time: 100 Days and 10.5 Hours
To analyze the survival data between patients diagnosed with right-sided primary (RSP) tumors and patients diagnosed with left-sided primary (LSP) tumors after hepatic arterial infusion chemotherapy (HAIC) at our center.
A retrospective analysis of pretreated metastatic colorectal cancer patients who received HAIC from May 2006 to August 2015 was conducted. A Cox proportional hazard regression analysis was used to assess the long-term survival outcomes. The mean and median age of patients was 61 years (range 27-85 years). There were 115 males and 53 females in our study.
One hundred sixty-eight patients were enrolled in this study. The overall response rate was 28.9% in LSP patients and 27.3% in RSP patients. The disease control rate was 76.3% in LSP patients and 69.7% in RSP patients. The median overall survival in response to HAIC was 16.3 mo in the LSP arm and 9.3 mo in the RSP arm (P = 0.164). The median progression-free survival was 5.7 mo in the LSP arm and 4.2 mo in the RSP arm (P = 0.851).
There was no significant difference in survival between LSP patients and RSP patients after HAIC. Further prospective studies are needed to confirm these findings.
Core tip: Our study shows that the prognosis of left-sided colorectal cancer liver metastasis patients is superior to that of right-sided patients, but no significant difference in survival was found between left-sided primary and right-sided primary patients in response to treatment with hepatic arterial infusion chemotherapy.
- Citation: Zhang HY, Guo JH, Gao S, Chen H, Wang XD, Zhang PJ, Liu P, Cao G, Xu HF, Zhu LZ, Yang RJ, Li J, Zhu X. Effect of primary tumor side on survival outcomes in metastatic colorectal cancer patients after hepatic arterial infusion chemotherapy. World J Gastrointest Oncol 2018; 10(11): 431-438
- URL: https://www.wjgnet.com/1948-5204/full/v10/i11/431.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i11.431
Colorectal cancer is the third leading cause of cancer death in both men and women in the Western world[1]. In China, the incidence of colorectal cancer is gradually increasing and has become the fourth most frequent cancer in women and the fifth in men[2]. Gene expression-based subtyping is now widely accepted as a predictive model of survival, including the mutually exclusive RAS and BRAF pathways, as well as the Wnt pathway[3,4]. In addition, increasing evidence indicates that patients with a left-sided primary (LSP) tumor have a survival advantage compared to those with a right-sided primary (RSP) tumor, indicating that primary location could be a predictive factor[5]. The distinguishing prognosis is ascribed to differences in biology, pathology, and epidemiology of colorectal cancer based on primary tumor location. LSP tumors arise from the hindgut at their embryological beginnings and are supplied by the inferior mesenteric artery, while RSP tumors arise from the midgut and are supplied by the superior mesenteric artery. There are also biological and molecular pathway variations between these two subtypes[6-9].
Due to the dissimilar genotype and phenotype of LSP and RSP tumors, the location of primary tumor has turned out to be predictive of outcome[10,11]. Subsequent studies have found that RSP patients have an inferior outcome in first-line chemotherapy[12], and targeted agents, such as anti-epidermal growth factor receptor (EGFR) monoclonal antibody and anti-vascular EGFR monoclonal antibody, show differential efficacy in RSP and LSP patients[5,13,14].
Metastasis occurs in approximately 50% of patients during disease[15]. Without efficient treatment, metastatic colorectal cancer (mCRC) patients who fail to respond to systemic chemotherapy only survive approximately 3.5 mo[16]. The survival benefit of third-line chemotherapies is 4.5-10.5 mo[17]. However, interventional treatments are potential choices for mCRC patients. Transarterial chemoembolization and hepatic arterial infusion chemotherapy (HAIC) can achieve a higher local response rate than systemic chemotherapy and remain effective when patients have failed to respond to previous chemotherapy[18,19]. Chemo-refractory patients treated with HAIC can survive 7.7-19 mo[20-23]. However, no studies have reported the relationship between the efficacy of HAIC and the primary tumor side. We gathered survival information on mCRC patients after HAIC in our center to clarify this issue.
This was a retrospective analysis of the survival and efficacy of HAIC in mCRC patients. The primary criteria for inclusion were as follows: Pathological diagnosis of adenocarcinoma of the colon or rectum, inoperable liver metastases or contraindications for liver resection, systemic chemotherapy failure (experienced at least first-line chemotherapy previously), treated with HAIC in our center, and received tumor assessment after HAIC. Subject demographic variables examined included age, sex, and survival or censored data. Tumor variables examined included location, gene status, histologic grade (well, moderate, or poor), and extrahepatic metastasis. Treatment variables examined included previous treatment, combined liver radiotherapy or radiofrequency ablation, and combined molecular targeted drugs.
RSP patients have a tumor site in the cecum, ascending colon, hepatic flexure, or transverse colon, while LSP patients present tumors in the splenic flexure, descending colon, sigmoid colon, or rectum. Disease evaluation was repeated every two cycles using computed tomography scans, and the Response Evaluation Criteria in Solid Tumors 1.1 criteria was applied. The primary end-point of this study was the overall survival (OS) difference between RSP and LSP patients. Secondary end-points were progression-free survival (PFS) and efficacy of several different chemotherapy regimens. Our retrospective study was in accordance with the ethical standards of the Beijing Cancer Hospital Ethics Committee.
OS was defined from the first day of HAIC until death from any cause. PFS was defined from the first day of HAIC until the first objective observation of disease progression or death from any cause. The SPSS software program (version 19; SPSS, Chicago, IL, United States) was used for analyses. The Graph Pad Prism 6 program (Graph Pad Software, Inc., La Jolla, CA, United States) was used to create charts. A Student’s t-test was used to analyze continuous variables, which are reported as mean ± SD if normally distributed or as a median and range if skewed. A χ2 test was used to analyze categorical variables, which are reported as a proportion (%) of the overall cohort. The Kaplan-Meier method was used to approximate PFS and OS, and the significance of survival differences between separate subgroups was assessed using the log-rank test. The Cox proportional hazards model was used to determine the univariate and multivariate hazards ratios for the study parameters. For all tests, a P-value < 0.05 was defined as statistically significant.
One hundred sixty-eight patients were included in this study between May 2006 and August 2015. The median age was 61 years (range 27-85 years), and the last follow up day was July 5, 2016. Median follow-up time was 17 mo. Among all patients included in this study, 138 patients died, 14 patients were lost during the follow-up period, and 16 patients were still alive. There were 135 LSP patients and 33 RSP patients. Extrahepatic metastases accounted for more than half of all patients (94/168). There were 17 KRAS mutation patients and 48 KRAS wild type patients among LSP tumors. There were eight KRAS mutation patients and seven KRAS wild type patients among RSP tumors. The baseline information of patients, disease, and treatment characteristics by primary tumor location are shown in Table 1. Eighty-nine (65.9%) LSP patients were previously administered first-line systemic chemotherapy, and 46 (34.1%) patients were given second-line or subsequent therapies. Twenty-four (72.7%) RSP patients received first-line systemic chemotherapy, and nine (27.3%) patients received second-line or subsequent lines of chemotherapy.
| Variable | Left side (n = 135) | Right side (n = 33) | P-value |
| Age, mean (range), years | 60.5 (27-85) | 63.8 (37-83) | 0.392 |
| Men, n (%) | 95 (70.4) | 20 (60.6) | 0.279 |
| Previous system treatment, n (%) | 0.455 | ||
| Only first line | 89 (65.9) | 24 (72.7) | |
| Second line or more | 46 (34.1) | 9 (27.3) | |
| Extrahepatic metastasis, n (%) | 73 (54.1) | 21 (63.6) | 0.321 |
| Primary tumor resected, n (%) | 0.173 | ||
| No surgery | 22 (16.2) | 10 (30.3) | |
| Palliative surgery | 49 (36.3) | 11 (33.3) | |
| Radical surgery | 64 (47.4) | 12 (36.4) | |
| Synchronous metastases, n (%) | 103 (76.3) | 26 (78.8) | 0.761 |
| Gene status, n (%) | 0.127 | ||
| KRAS mutation | 17 (35.6) | 8 (24.2) | |
| KRAS wild type | 48 (12.6) | 7 (21.2) | |
| Unknown | 70 (51.9) | 18 (54.5) | |
| Targeted therapy, n (%) | |||
| Bevacizumab treated | 27 (14.8) | 2 (6.1) | 0.21 |
| Cetuximab treated | 13 (9.6) | 3 (9.1) | |
| Other local treatment, n (%) | 31 (23) | 4 (12.1) | 0.169 |
| Repeated times of HAIC, n (%) | 0.554 | ||
| 2 | 29 (21.5) | 10 (30.3) | |
| 3-4 | 43 (21.9) | 10 (30.3) | |
| > 6 | 63 (46.7) | 13 (39.4) |
Patients were injected with 20-40 mg epirubicin hydrochloride after routine arteriography by artery catheter, and iodipin was injected when obvious blood supply was found in the arteriography. Chemotherapy agents administered through the catheter after chemoembolization included oxaliplatin (85 mg/m2) or irinotecan (180 mg/m2) over 4 h, followed by fluorouracil (2000 mg/m2) administered over approximately 44 h and cisplatin/fluorouracil (200 mg /m2) over 2-4 h vs peripheral vein, combined with/without bevacizumab (7.5 mg/kg) or cetuximab (250 mg/m2). Treatments were repeated every three weeks. One hundred fifty-three patients received oxalipatin-based chemotherapy, and only 15 patients received irinotecan-based chemotherapy. With respect to targeted therapy, 27 (20%) LSP patients were treated with bevacizumab; while another 13 (9.6%) were treated with cetuximab. In RSP patients, there were only two patients treated with bevacizumab and three with cetuximab.
No significant differences were found between RSP and LSP patients in terms of age, sex, tumor variables, or treatment variables (Table 1).
The overall response rate was 28.9% in LSP patients and 27.3% in RSP patients. There were 0.7% complete response (n = 1), 28.9% partial response (n = 39), 47.4% stable disease (n = 64), and 23% progressive disease (n = 31) in LSP patients. There were 27.3% partial response (n = 9), 42.4% stable disease (n = 14), and 30.3% progressive disease (n = 10) in RSP patients The disease control rate was 76.3% in LSP patients and 69.7% in RSP patients.
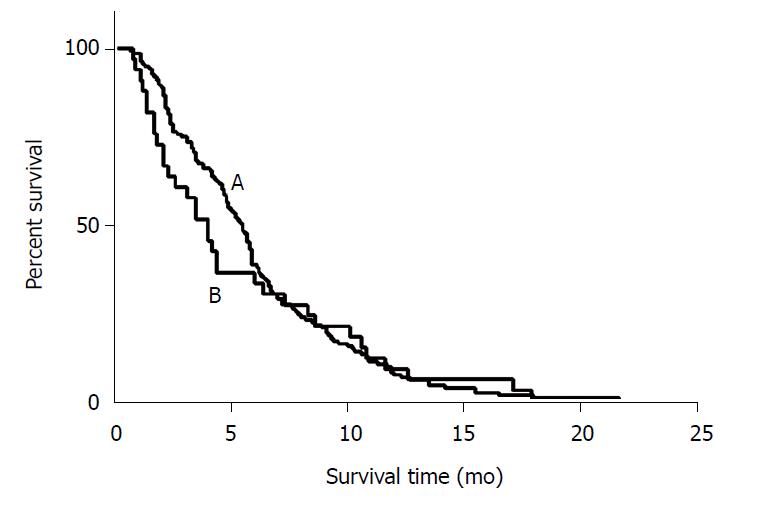
Most of the patients (n = 84) who progressed did so due to liver metastasis, while a small number of patients (n = 45) progressed due to the progression of extrahepatic metastasis, and another 23 patients exhibited both liver and extrahepatic metastasis progression. Median PFS of all included patients was 5.5 mo (95%CI: 4.9-6.0 mo). The median PFS was 5.7 mo (95%CI: 5.3-6.1 mo) in LPS patients and 4.2 mo (95%CI: 3.2-5.1 mo) in RSP patients, and no significant difference was observed between these two groups (P = 0.851) (Table 2 and Figure 1).
| Subgroup | OS events n (%) | Median OS, mo (95%CI) | Hazard ratio (95%CI) | P-value | PFS events n (%) | Median PFS, mo (95%CI) | Hazard ratio (95%CI) | P-value | ||
| Left-sided | Right-sided | Left-sided | Right-sided | |||||||
| All eligible patients (n = 168) | 138 (82.1) | 16.3 (13.5-19.0) | 9.3 (3.4-15.1) | 0.74 (0.48-1.13) | 0.164 | 151 (89.9) | 5.7 (5.3-6.1) | 4.2 (3.2-5.1) | 0.96 (0.64-1.50) | 0.851 |
| KRAS wild type (n = 55) | 44 (76.4) | 17.6 (12.3-22.9) | 15.4 (6.0-24.7) | 0.85 (0.33-2.19) | 0.74 | 51 (92.7) | 5.1 (4.2-5.9) | 4.0 (2.7-5.3) | 0.76 (0.32-1.81) | 0.529 |
| KRAS mutation (n = 25) | 18 (72) | 10.9 (0-34.6) | 9.0 (2.4-15.5) | 0.77 (0.29-2.02) | 0.6 | 22 (88) | 4.8 (2.9-6.6) | 2.1 (0-5.0) | 0.97 (0.36-2.58) | 0.956 |
| KRAS unknown (n = 88) | 78 (88.6) | 16.1 (14.1-18.1) | 9.3 (6.9-11.7) | 0.69 (0.38-1.24) | 0.218 | 78 (88.6) | 6.2 (5.1-7.3) | 6.0 (3.3-8.7) | 0.75 (0.42-1.33) | 0.324 |
| Bevacizumab (n = 29) | 21 (72.4) | 24.5 (16.6-32.3) | 9.3 (-) | 0.30 (0.06-1.43) | 0.11 | 27 (93.1) | 6.2 (4.9-7.4) | 4.0 (-) | 0.45 (0.10-2.01) | 0.285 |
| Cetuximab (n = 16) | 12 (75) | 16.5 (9.0-23.9) | 8.2 (-) | 0.21 (0.03-1.29) | 0.065 | 15 (93.8) | 3.6 (0.89-6.3) | 4.0 (-) | 0.42 (0.08-2.06) | 0.269 |
The median PFS of LSP patients was 5.5 mo in liver progression (n = 67, 54%), 4.7 mo in extrahepatic progression (n = 39, 31%), and 6.7 mo in both liver and extrahepatic progression groups (n = 18, 15%) (P = 0.155). The median PFS of RSP patients was 4.0 mo in liver progression (n = 16, 57%), 4.4 mo in extrahepatic progression (n = 7, 25%), and 4.4 mo in both liver and extrahepatic progression groups (n = 5, 18%) (P = 0.986).
LSP patients who had only first-line systemic chemotherapy exhibited a median PFS of 5.9 mo, and those who received second or more lines of treatment exhibited a median PFS of 4.6 mo (P = 0.001). RSP patients who had only first-line systemic chemotherapy exhibited a median PFS of 4.4 mo, and those who received second or more lines of treatment exhibited a median PFS of 2.3 mo (P = 0.018).
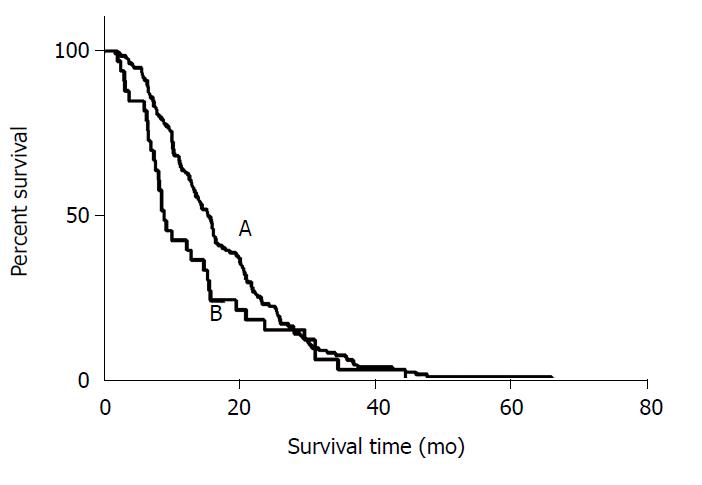
There were 112 out of 135 LSP patients and 26 out of 33 RSP patients who died during the follow-up period. The median OS from the diagnosis of CRC was 31.4 mo in LSP patients and 22.2 mo in RSP patients (P = 0.186). The OS after HAIC was 16.3 mo in LSP patients and 9.3 mo in RSP patients (P = 0.164) (Figure 2).
The median OS after HAIC in patients treated with HAIC and bevacizumab was 22 mo, and patients treated with HAIC and cetuximab or HAIC only exhibited a median OS of 15.4 mo (P = 0.162). LSP patients treated with HAIC and bevacizumab had a median OS of 24.5 mo and 15.4 mo in the cetuximab arm (P = 0.053). No significant difference was observed between the bevacizumab and cetuximab arms. Only two RSP patients were treated with bevacizumab, and their OS was 9.3 mo and 13 mo. The three RSP patients treated with cetuximab exhibited an OS of 2.6 mo, 3.8 mo, and 8.2 mo.
The median OS in KRAS wild type patients (n = 55) was 16.6 mo, 13 mo in patients with KRAS mutation (n = 25), and 15.6 mo in KRAS status unknown patients (n = 88). In KRAS wild type patients, ten were treated with cetuximab and six with bevacizumab. The median OS of these two group were 11.5 mo and 22 mo, respectively (P = 0.087) (Table 2). Among all 48 LSP KRAS wild type patients, nine were treated with bevacizumab and eleven with cetuximab. The median OS of these two different treatments was 28.1 mo and 21.1 mo, respectively (P = 0.444). There were only seven KRAS wild type patients in the RSP group.
LSP patients who progressed by liver metastases had a median OS of 18.8 mo, progression of extrahepatic metastasis was 14.6 mo, and progression of both liver and extrahepatic metastasis was 13.7 mo (P = 0.771). RSP patients who progressed by liver metastases exhibited a median OS of 8.6 mo, progression of extrahepatic metastasis was 10.1 mo, and progression of both liver and extrahepatic metastasis was 9.3 mo (P = 0.885). No significant difference was observed in survival between liver metastasis only and extrahepatic metastases patients (P = 0.493).
A prognostic factor analysis showed that different infusion agents resulted in differential survival. OXA-based infusion chemotherapy (n = 153) resulted in a median OS of 15.8 mo, while CPT-11-based chemotherapy (n = 15) reached 22.8 mo (P = 0.518). Neither LSP nor RSP patients experienced a significant difference in this treatment variable. Among all factors considered, primary tumor histology, radiofrequency ablation or liver radiotherapy, normal serum CA19-9 levels, and response to HAIC were protective factors associated with OS (Table 3).
| Variable | MST (mo) | Univariate analysis | P-value | |
| HR | 95%CI | |||
| Primary tumor site (right/left) | 9.3 vs 16.3 | 1.353 | 0.881-2.079 | 0.167 |
| Age (> 60/< 60 yr) | 16 vs 15.5 | 1.026 | 0.731-1.440 | 0.88 |
| Gender (male/female) | 16.5 vs 13 | 0.744 | 0.520-1.063 | 0.104 |
| Histology (poor/well to moderate) | 10.3 vs 15.9 | 1.706 | 1.003-2.904 | 0.049* |
| Serum CA19-9 (≥ 37U/mL/< 37 U/mL)# | 12.5 vs 21.2 | 2.108 | 1.444-3.076 | < 0.001* |
| Serum CA72-4 (≥ 6.7 U/mL/< 6.7 U/mL)# | 13 vs 20.8 | 1.605 | 1.114-2.311 | 0.011* |
| Serum CEA (≥ 5U/mL/< 5 U/mL)# | 14.6 vs 21.1 | 1.428 | 0.867-2.351 | 0.162 |
| Extrahepatic metastasis (present/absent) | 15.8 vs 15.8 | 1.172 | 0.825-1.667 | 0.376 |
| Time to liver metastasis (synchronous/ metachronous) | 14.8 vs 16.5 | 1.125 | 0.802-1.580 | 0.495 |
| Other local treatment (combined/uncombined) | 21.1 vs 14.6 | 0.651 | 0.426-0.995 | 0.047* |
| Response to HAIC | < 0.001* | |||
| PR | 21.9 | 0.234 | 0.146-0.375 | < 0.001* |
| SD | 16.1 | 0.285 | 0.185-0.439 | < 0.001* |
| PD | 7.5 | 1 | 1 | NA |
| Infusion agents (OXA/CPT-11) | 15.8 vs 22.8 | 1.225 | 0.660-2.273 | 0.52 |
| Please define what this symbol represents in the table legend below | ||||
| Please define what this symbol represents in the table legend below | ||||
Differences in survival resulting from differences in biological behavior were examined in LSP and RSP patients. In our study, we analyzed the survival data between patients with LSP tumors and those with RSP tumors after HAIC in mCRC in our center. When comparing PFS between LSP and RSP patients, no obvious advantages were found in LSP patients; however, a trend did exist. These results suggest that combined hepatic arterial infusion (HAI) does not change survival in patients with liver metastasis from either LSP or RSP colorectal cancer, which is inconsistent with the survival data for mCRC patients who undergo hepatic metastasis resection. Patients treated with hepatic metastasis surgery exhibit an OS similar to LSP and RSP patients after liver metastasis. However, this result was based on retrospective analysis, and patient selection bias was likely to have influenced the outcome. We cannot conclude that local treatment of liver metastasis reverses the worse prognosis in RSP patients.
In systemic chemotherapy, one of the most important prognostic factors is the use of molecular targeted drugs, especially with respect to differences between anti-EGFR and anti-vascular endothelial growth factor monoclonal antibodies. However, an interesting phenomenon was found in our study wherein the OS of LSP patients was significantly better in those treated with bevacizumab than in those treated with cetuximab, and the OS of RSP patients exhibited the same trend. This phenomenon is completely opposite to data concerning systemic chemotherapy in both LSP and RSP patients. Possible reasons for these discrepancies include the following: The optimal dose of bevacizumab and cetuximab in HAI treatment has not been clearly verified; only a few cases were treated with cetuximab; only KRAS genotyping was performed instead of testing all RAS genes; and HAI treatment was not a first-line treatment in our study. Another study reported that RAS gene mutations might be influenced by previous treatment. However, in LSP patients, bevacizumab treatment showed an obvious advantage compared with cetuximab, and this advantage could even be observed in RAS wild-type patients. This demonstrates that in HAIC treatment, especially in left-sided colorectal cancer liver metastasis, bevacizumab is superior to cetuximab.
In comparison with cytotoxic agents, irinotecan seems superior to oxaliplatin in OS after HAI treatment. However, in first-line treatment of all patients, the vast majority received oxaliplatin-based systemic chemotherapy, so the data could support the conclusion that irinotecan is superior to oxaliplatin in HAI treatment. However, it is worth noting that, as a second-line or subsequent treatment, HAIC obtained close to 30% objective remission rates in both LSP and RSP patients when most patients had previously received oxalipatin. The overall response rate observed in this study was obviously superior to second-line systemic chemotherapy and was similar to systemic therapy treatment using FOLFOX and bevacizumab (E3200)[24], suggesting that HAIC treatment might be superior to systemic cytotoxic chemotherapy in second-line conversion therapy for mCRC.
In conclusion, for HAIC treatment of mCRC, the survival of patients with left colon cancer remains better than that of right colon cancer patients. Subgroup analysis showed that bevacizumab might be superior to cetuximab, especially in left-sided colorectal cancer liver metastasis. However, further study is needed on the optimal dosage and mode of administration of molecular targeted drugs for HAIC treatment. Both oxaliplatin and irinotecan achieve considerable objective remission rates.
Previous studies have shown that left-sided colorectal cancer has a better survival prognosis than right-sided colorectal cancer. However, whether this prognosis difference is also present in liver metastasis colorectal cancer (CRC) patients treated with hepatic arterial infusion chemotherapy (HAIC) is still unknown.
Our study attempted to analyze for the first time, whether there would be a difference in survival and overall response rate in liver metastasis CRC patients treated with HAIC.
To analyze the overall survival and overall response rate difference of patients with liver metastasis of left-sided or right-sided colorectal cancer after HAIC.
A retrospective analysis of liver metastasis CRC patients from May 2006 to August 2015 was conducted. Cox proportional hazard regression analysis was used to assess long-term survival outcomes.
Overall response rate was 28.9% in left-sided primary (LSP) patients, and 27.3% in right-sided primary (RSP) patients. Disease control rate was 76.3% in LSP patients and 69.7% in RSP patients. Median overall survival after HAIC was 16.3 mo in the LSP arm and 9.3 mo in the RSP arm (P = 0.164). Median progression-free survival was 5.7 mo in the LSP arm and 4.2 mo in the RSP arm (P = 0.851).
The treatment response rate of HAIC in metastatic CRC patients is similar when compared by different primary tumor site. LSP patients seemed to have a superior survival compared to RSP patients when treated by HAIC but no significant difference was found.
Further large sample size and multi-center prospective studies are needed to confirm the conclusion of this study.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Park SJ, Masaki O, Ko E S- Editor: Dou Y L- Editor: Filipodia E- Editor: Tan WW
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12991] [Article Influence: 1443.4] [Reference Citation Analysis (2)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13215] [Article Influence: 1468.3] [Reference Citation Analysis (3)] |
| 3. | Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Sinicrope FA, Shi Q, Smyrk TC, Thibodeau SN, Dienstmann R, Guinney J, Bot BM, Tejpar S, Delorenzi M, Goldberg RM. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015;148:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 5. | Brulé SY, Jonker DJ, Karapetis CS, O’Callaghan CJ, Moore MJ, Wong R, Tebbutt NC, Underhill C, Yip D, Zalcberg JR. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51:1405-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 6. | Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, Townsend AR, Moore J, Roy A, Tomita Y. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer. 2015;121:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Birkenkamp-Demtroder K, Olesen SH, Sørensen FB, Laurberg S, Laiho P, Aaltonen LA, Orntoft TF. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut. 2005;54:374-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Liu LU, Holt PR, Krivosheyev V, Moss SF. Human right and left colon differ in epithelial cell apoptosis and in expression of Bak, a pro-apoptotic Bcl-2 homologue. Gut. 1999;45:45-50. [PubMed] |
| 9. | Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, Maus MK, Antoniotti C, Langer C, Scherer SJ. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107:pii: dju427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 357] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 10. | Elez E, Argilés G, Tabernero J. First-Line Treatment of Metastatic Colorectal Cancer: Interpreting FIRE-3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol. 2015;16:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? - a systematic review. Eur J Surg Oncol. 2015;41:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 314] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 12. | Modest DP, Schulz C, von Weikersthal LF, Quietzsch D, von Einem JC, Schalhorn A, Vehling-Kaiser U, Laubender RP, Giessen C, Stintzing S. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment). Anticancer Drugs. 2014;25:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 316] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 14. | Boisen MK, Johansen JS, Dehlendorff C, Larsen JS, Osterlind K, Hansen J, Nielsen SE, Pfeiffer P, Tarpgaard LS, Holländer NH. Primary tumor location and bevacizumab effectiveness in patients with metastatic colorectal cancer. Ann Oncol. 2013;24:2554-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Tabernero J, Teh C, Van Cutsem E; Jean-Nicolas Vauthey of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 16. | Tellez C, Benson AB 3rd, Lyster MT, Talamonti M, Shaw J, Braun MA, Nemcek AA Jr, Vogelzang RL. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer. 1998;82:1250-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Nielsen DL, Palshof JA, Larsen FO, Jensen BV, Pfeiffer P. A systematic review of salvage therapy to patients with metastatic colorectal cancer previously treated with fluorouracil, oxaliplatin and irinotecan +/- targeted therapy. Cancer Treat Rev. 2014;40:701-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Vogl TJ, Gruber T, Balzer JO, Eichler K, Hammerstingl R, Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology. 2009;250:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Gruber-Rouh T, Naguib NN, Eichler K, Ackermann H, Zangos S, Trojan J, Beeres M, Harth M, Schulz B, Nour-Eldin A NE. Transarterial chemoembolization of unresectable systemic chemotherapy-refractory liver metastases from colorectal cancer: long-term results over a 10-year period. Int J Cancer. 2014;134:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Levy J, Zuckerman J, Garfinkle R, Acuna SA, Touchette J, Vanounou T, Pelletier JS. Intra-arterial therapies for unresectable and chemorefractory colorectal cancer liver metastases: a systematic review and meta-analysis. HPB (Oxford). 2018;20:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Martin RC, Joshi J, Robbins K, Tomalty D, Bosnjakovik P, Derner M, Padr R, Rocek M, Scupchenko A, Tatum C. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol. 2011;18:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Hong K, McBride JD, Georgiades CS, Reyes DK, Herman JM, Kamel IR, Geschwind JF. Salvage therapy for liver-dominant colorectal metastatic adenocarcinoma: comparison between transcatheter arterial chemoembolization versus yttrium-90 radioembolization. J Vasc Interv Radiol. 2009;20:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Zhang H, Guo J, Gao S, Zhang P, Chen H, Wang X, Li X, Zhu X. Prognostic factors for transarterial chemoembolization combined with sustained oxaliplatin-based hepatic arterial infusion chemotherapy of colorectal cancer liver metastasis. Chin J Cancer Res. 2017;29:36-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1726] [Article Influence: 95.9] [Reference Citation Analysis (1)] |