Copyright
©The Author(s) 2017.
World J Gastrointest Oncol. Sep 15, 2017; 9(9): 390-396
Published online Sep 15, 2017. doi: 10.4251/wjgo.v9.i9.390
Published online Sep 15, 2017. doi: 10.4251/wjgo.v9.i9.390
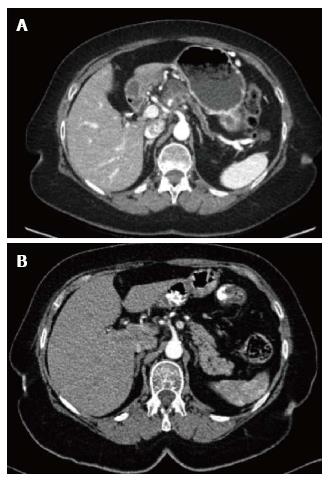
Figure 1 Computed tomography image.
A: Computed tomography (CT) image of the solid-cystic pancreatic mass with distal atrophy of the pancreas and pancreatic duct dilatation; B: CT three years before in which no pancreatic lesions were present.
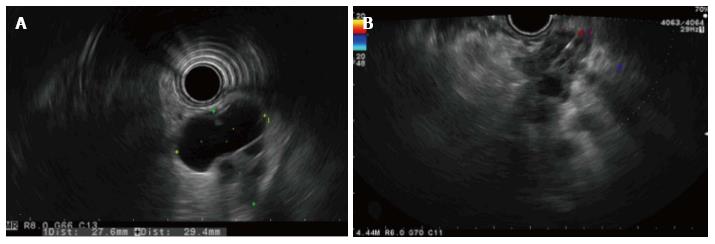
Figure 2 Endoscopic ultrasonography image.
A: Radial endoscopic ultrasonography (EUS) view of the mass; B: Lineal guided EUS fine needle aspiration of the solid component of the mass.
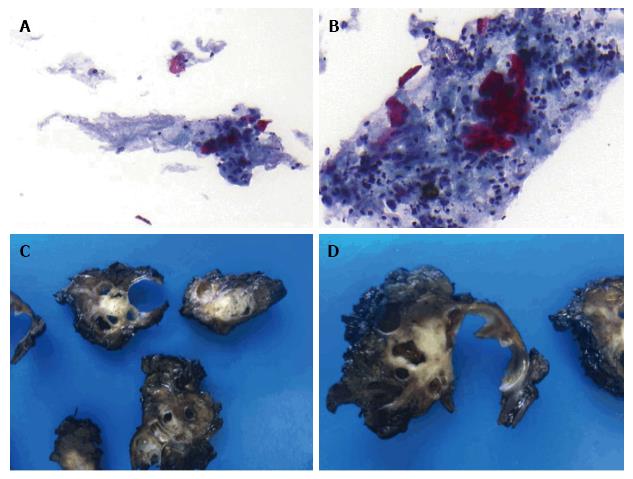
Figure 3 Endoscopic ultrasonography fine needle aspiration biopsies and surgical specimen.
A and B: Positive cytology from the pancreatic mass (adenocarcinoma with a significant keratinizing component suggestive of adenosquamous carcinoma), Papanicolaou staining 20 × and 40 ×, respectively; C and D: A solid-cystic pancreatic mass (gross pathology).
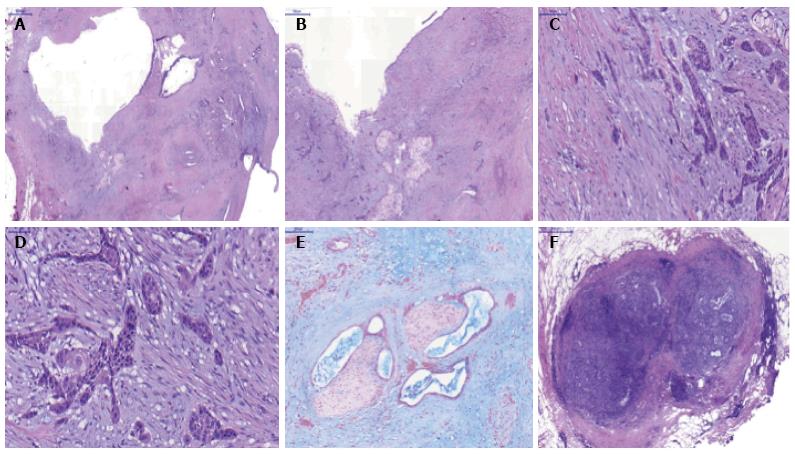
Figure 4 Microscopic pathology of the surgical specimen.
A: Intraductal papillary mucinous neoplasm with adenocarcinoma component, hematoxylin and eosin (H and E) 10 ×; B-D: Squamous metaplasia and evident infiltrative squamous carcinoma, H and E 10 ×, 20 × and 40 ×, respectively; E: Adenocarcinoma with perineural invasion, alcian blue 20 ×; F: Peripancreatic lymph node metastasis (adenocarcinoma component), H and E 20 ×.
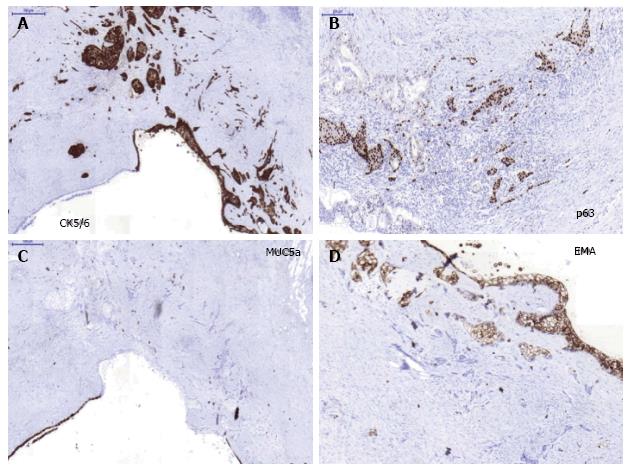
Figure 5 Immunohistochemical study in the surgical specimen.
A: CK5/6 strong positivity in squamous component (metaplasia and carcinoma), × 20; B: Strong nuclear p63 immunopositivity in the invasive squamous carcinoma. The adenocarcinoma area display poor p63 nuclear positivity, × 20; C: MUC5a negativity in the squamous carcinoma component and strong positive in ductal epithelial cells, × 20; D: MUC1/EMA positivity in the squamous metaplasic component, × 20.
- Citation: Martínez de Juan F, Reolid Escribano M, Martínez Lapiedra C, Maia de Alcantara F, Caballero Soto M, Calatrava Fons A, Machado I. Pancreatic adenosquamous carcinoma and intraductal papillary mucinous neoplasm in a CDKN2A germline mutation carrier. World J Gastrointest Oncol 2017; 9(9): 390-396
- URL: https://www.wjgnet.com/1948-5204/full/v9/i9/390.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i9.390