Copyright
©2014 Baishideng Publishing Group Co.
World J Gastrointest Oncol. Jan 15, 2014; 6(1): 11-21
Published online Jan 15, 2014. doi: 10.4251/wjgo.v6.i1.11
Published online Jan 15, 2014. doi: 10.4251/wjgo.v6.i1.11
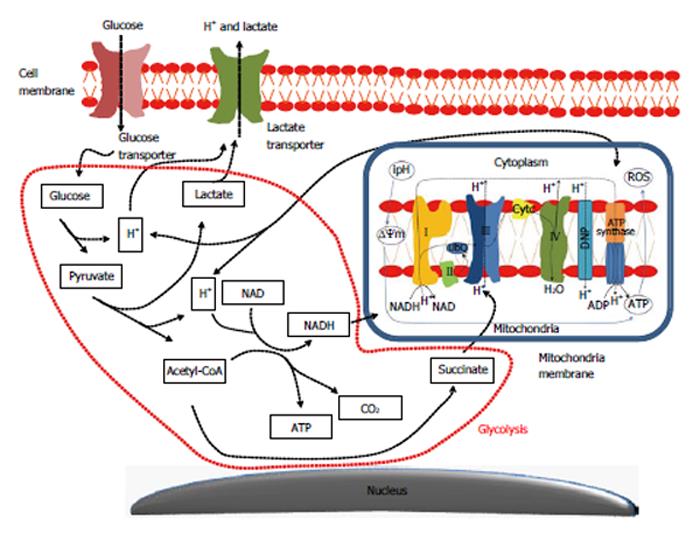
Figure 1 Main metabolic interactions lead to formation of the aerobic glycolytic metabolism in colon tumor cells.
The increased biosynthetic activity of cancer cells, as related to the activation of the aerobic glycolytic metabolism or “Warburg effect”, is based on the activation of glucose and lactate transporters supplying tumor cells not only with vast amounts of energy (glucose), but further reducing blockage-associated mechanisms due to glycolysis over usage. It seems that the lactate overproduction is compensated by the hyperactivation of lactate transporters allowing a rapid transport of this molecule across the plasma membrane together with H+ atoms, which results in an intracellular alkalinization. This event hyperpolarizes the mitochondrial membrane potential (ΔΨm) and induces a higher uptake of NADH by the first and succinate by the second mitochondrial complexes enhancing the oxidative mitochondrial phosphorylation (Krebs cycle). All together, this means that tumor cells are prone to produce higher energy amounts (ATP) than found in a normal tissue. ROS: Reactive oxygen species; CO2: Carbon dioxide; ipH: intracellular pH. NADH: Nicotinamide adenine dinucleotide phosphate-oxidase.
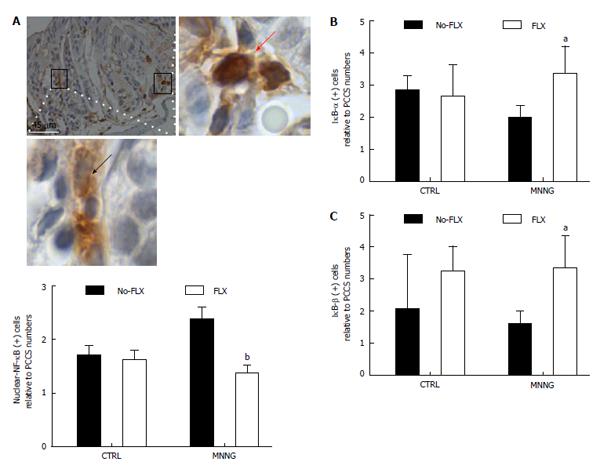
Figure 2 Fluoxetine modulates nuclear factor kappa-B nuclear activity among subepithelial colonic cells.
For this figure, groups of female C57BL/6 mice (25 g) consisted of control (CTRL) animals or received methylnitronitrosoguanidine (MNNG) treatment [four successive doses of MNNG (5 mg/mL; intrarectal deposits of 100 µL) twice a week for 2 wk], FLX treatment (30 mg/kg per day; intraperitoneal, ip) or MNNG + FLX treatment. FLX treatment was started after 2 wk from the end of MNNG treatment, and continued for the next 4 wk. All mice were euthanized by CO2 exposure at week 8. Individual autopsies were performed and colon tissue samples were fixed in paraformaldehyde buffer (4%; 24 h). All experimental protocols were approved by the Internal Animal Care, Ethical and Use Committee (n° 068/2012). Immunohistochemistry was performed with anti-nuclear factor kappa-light-chain-enhancer of activated B cells [nuclear factor kappa-B (NF-κB), p50; clone C-19], nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor (IκB), alpha (IκB-α; clone N-20), beta (IκB-β; clone H-4). Antibodies were acquired from Santa Cruz Biotechnology (Heidelberg, Germany). A: Representative histological image of a colonic-longitudinal section labeled with anti-NF-κB antibody, picture taken at × 400 magnification and scale bar of 45 μm inserted. A cytoplasmic anti-NF-κB antibody positively cell detected within cryptal area (inset below; × 1000 magnification of the boxed region, middle-left). Nuclear-NF-κB protein detected in stromal cells (inset right-side; × 1000 magnification of the boxed region, middle-right). Graph shows the relative number of nuclear-NF-κB positive cells within colonic subepithelial areas (PCCS; bP < 0.01 vs MNNG without FLX, n = 4; FLX + MNNG, n = 4); B: Relative number of IκB-α positive cells (aP < 0.05 vs MNNG without FLX, n = 4; FLX+MNNG, n = 4); and C: IκB-β positive cells within colon stromal areas (aP < 0.05 vs MNNG without FLX, n = 5; FLX + MNNG, n = 4). FLX: Fluoxetine. PCCS: Pericryptal colonic stroma.
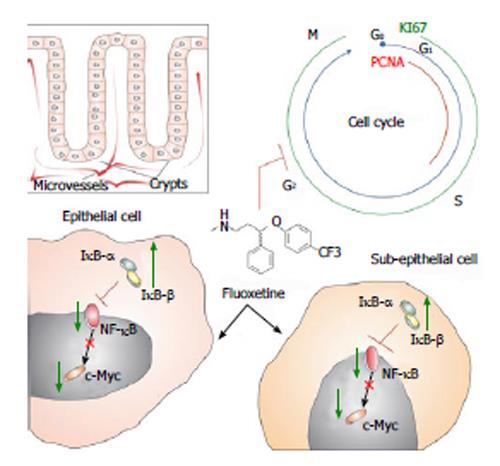
Figure 3 Schematic illustration shows fluoxetine antiproliferative activities in colon tissue.
Boxed figure shows the clear division between epithelial and subepithelial colonic areas. Considering that crypts compose the colonic epithelia, it is known that microvessels surround these gland structures. Fluoxetine (chemical structure represented at the center) blocks cell-cycle (blue line and letters) in colonic tissue. We have observed that fluoxetine treatment reduced two proliferative markers, named proliferating cell nuclear antigen (PCNA, red line) and KI67 (green line). These effects of fluoxetine treatment might be related to its enhancement on IκB-α and IκB-β proteins. This could arrest nuclear factor kappa-B (NF-κB) protein in the cytoplasm, reducing its transcriptional activity which, due to its activation over c-Myc transcription factor, would decrease this protein activation and proliferation. We believe that a similar mechanism could take a place in epithelial and subepithelial cells.
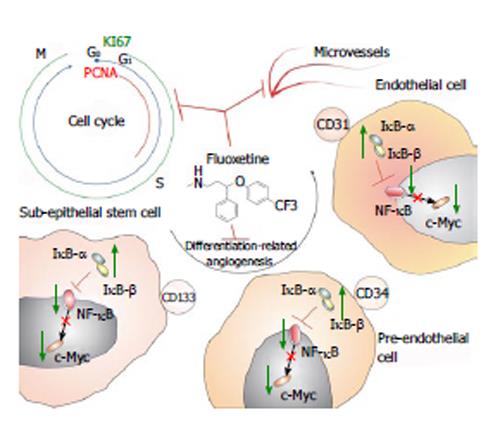
Figure 4 Schematic illustration shows fluoxetine anti-angiogenic potential in colon preneoplastic tissue.
This means that by reducing proliferation of subepithelial cells, blocking their cell-cycle, fluoxetine would reduce microvessel density. This anti-angiogenic potential was observed in a direct relationship with reduced differentiation-related angiogenesis of subepithelial stem cells. This suggests that fluoxetine would reduce the differentiation of CD133 positive cells into a CD34 phenotype, which would also not differentiate in endothelial cells, as CD31. This sequence of events would mainly be associated with the control of fluoxetine treatment on nuclear factor kappa-B signaling, as reducing proliferation and preneoplastic angiogenesis. PCNA: Proliferating cell nuclear antigen.
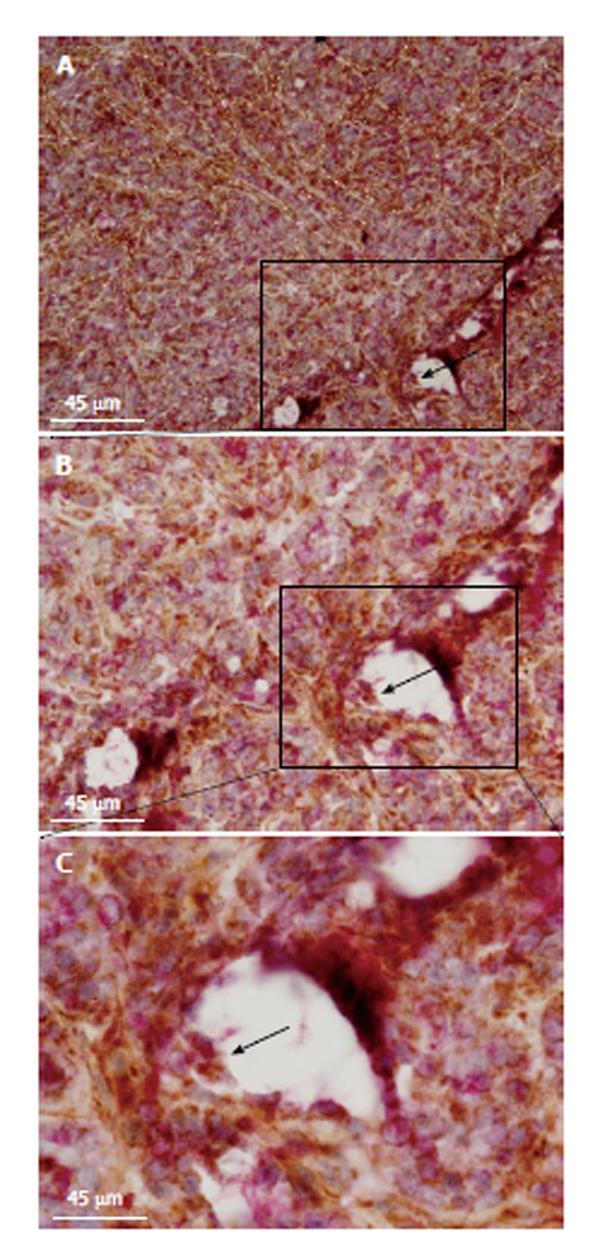
Figure 5 Tumor metabolism and malignant angiogenesis.
Histopathological images show double staining between cytochrome C oxidase (COX) and anti-CD31 antibody (clone 1A10 at 1:100; Novocastra, United States). Microvessel walls are traced with sectioned white lines (horizontal view of sectioned tumor microvessels). Black arrow indicates a microvessel lumen with double-stained cells (boxed region; transversal view of a tumor microvessel). Picture was taken at × 100 magnification and 45 μm scale bars are inserted in all images. Inset (C) shows the same boxed region at × 200 magnification. Double-stained cells are pointed out by a black arrow at the microvessel wall (inset; B). Sectioned green line circulates a niche of double-stained endothelial cells at the edge of a microvessel bifurcation. To build these images, 5 wk (20 ± 2 g) nonobese diabetic, severe combined immunodeficient mice (NOD/SCID) were subcutaneously transplanted with HT29 cells (1.5 × 106 cells per mice) in agreement with the protocol approved by the Internal Animal Care, Ethical and Use Committee (n° 121/2012). All mice were acclimated for 1 wk before starting the experiment and maintained under specific pathogen-free conditions. Tumor volume was monitored throughout the whole experimental period by measures with a caliper. Mice were sacrificed under general anesthesia (1.5% Forane in 98.5% oxygen; 2l min). Tissue samples were frozen within TissueTek (Sakura, Germany) and kept at -80 °C for immunohistochemical analyses. Double-staining was performed according to our standard methods.
- Citation: Stopper H, Garcia SB, Waaga-Gasser AM, Kannen V. Antidepressant fluoxetine and its potential against colon tumors. World J Gastrointest Oncol 2014; 6(1): 11-21
- URL: https://www.wjgnet.com/1948-5204/full/v6/i1/11.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i1.11