Copyright
©The Author(s) 2025.
World J Gastrointest Oncol. May 15, 2025; 17(5): 105027
Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.105027
Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.105027
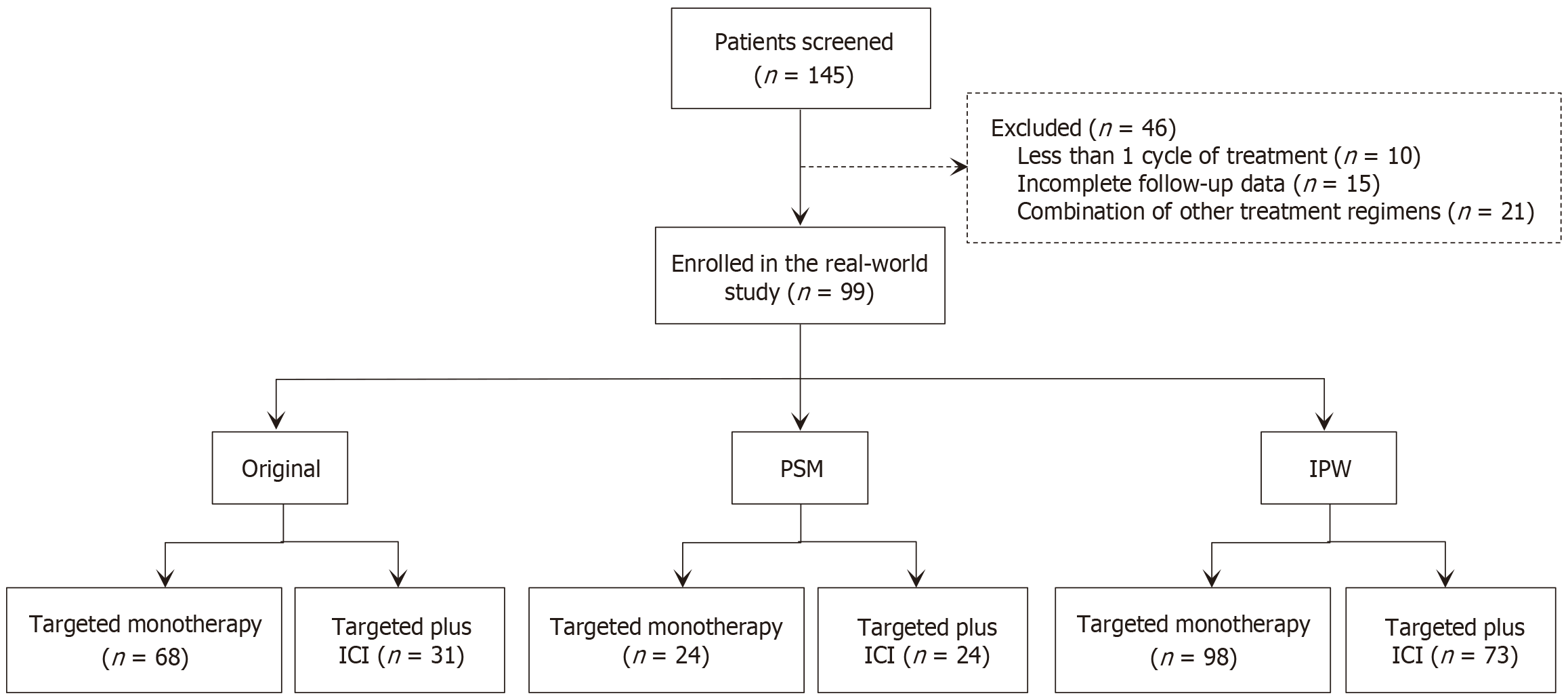
Figure 1 Flowchart illustrating the patient selection process for the study.
PSM: Propensity score matching; IPW: Inverse probability weighting; ICI: Immune checkpoint inhibitor.
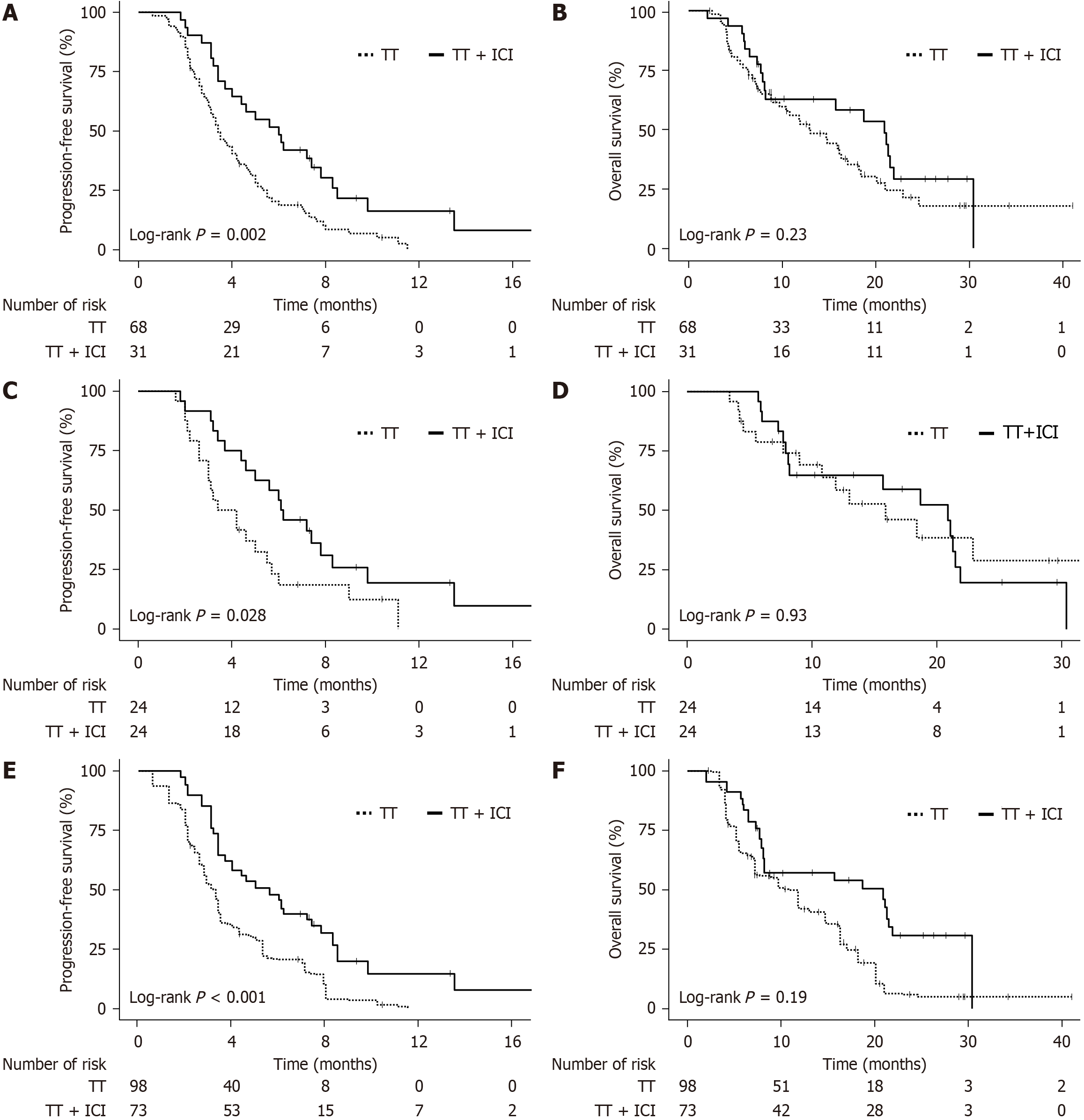
Figure 2 Kaplan-Meier survival curves comparing targeted therapy and targeted therapy + immune checkpoint inhibitor (targeted therapy combined with immune checkpoint inhibitors).
A and B: Original data: Progression-free survival (PFS; A) and overall survival (OS; B); C and D: Propensity score matching: PFS (C) and OS (D); E and F: Inverse probability weighting: PFS (E) and OS (F). Log-rank test P values are indicated on each panel. The number at risk at different time points is shown below the X-axis. TT: Targeted therapy; ICI: Immune checkpoint inhibitor.
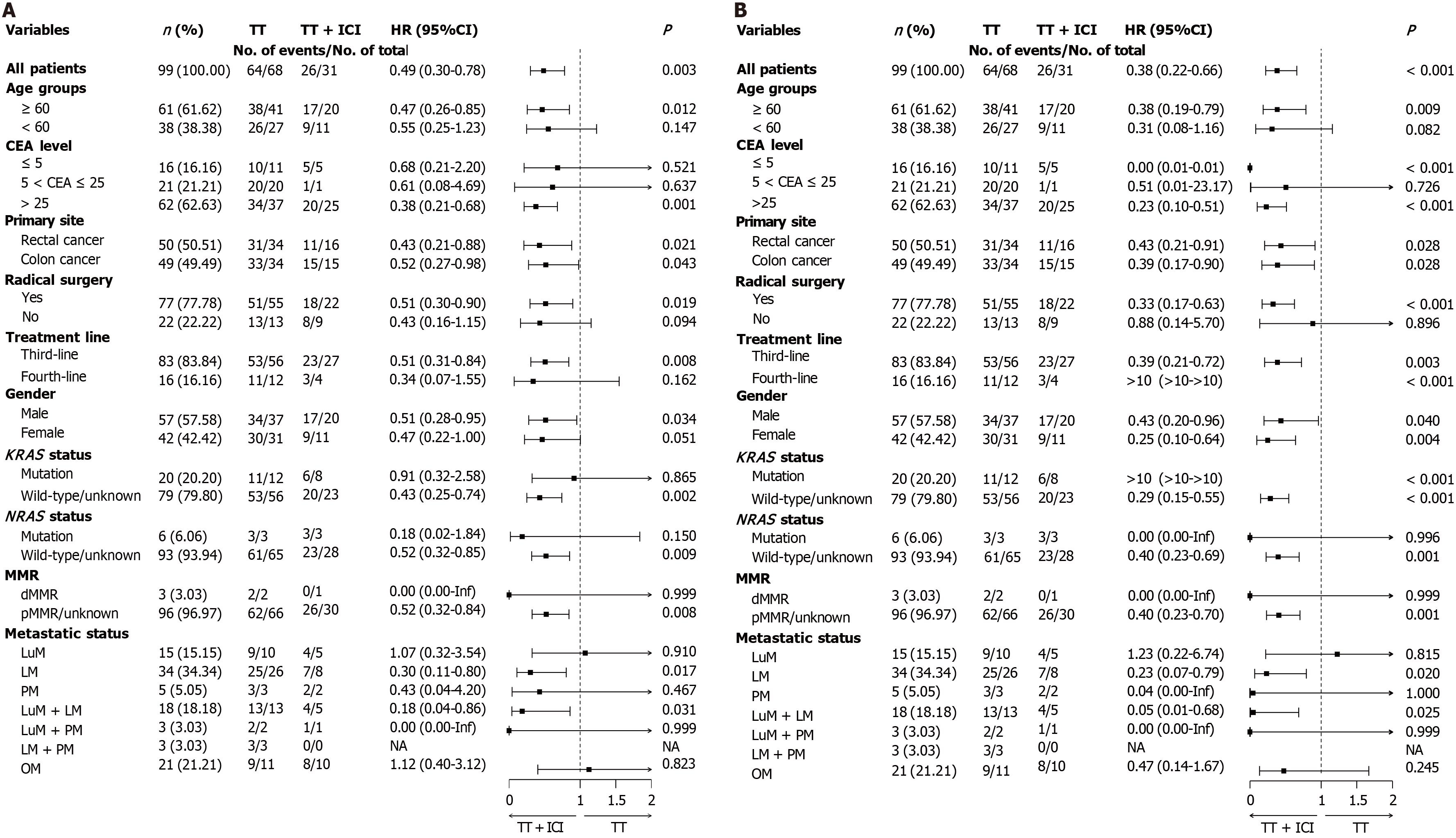
Figure 3 Subgroup analysis of progression-free survival in metastatic colorectal cancer patients.
A: Univariate subgroup analysis; B: Multifactorial subgroup analysis. TT: Targeted therapy; ICI: Immune checkpoint inhibitor; HR: Hazard ratio; 95%CI: 95% confidence interval; LM: Liver metastasis; LuM: Lung metastasis; PM: Peritoneal metastasis; OM: Other metastases.
- Citation: Wang PJ, Wang J, Yao XM, Cheng WL, Sun L, Yan J, Yu YL, Li SY, Li DP, Jia JH. Evaluation of efficacy and safety of targeted therapy and immune checkpoint inhibitors in metastatic colorectal cancer. World J Gastrointest Oncol 2025; 17(5): 105027
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/105027.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.105027