Copyright
©The Author(s) 2023.
World J Gastrointest Oncol. Oct 15, 2023; 15(10): 1823-1828
Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1823
Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1823
Figure 1 Sequence of the patient’s multiline treatments and computed tomography images, the levels of colorectal cancer biomarkers, and allele frequencies of circulating tumor DNA alterations during treatments.
A: Timeline of multiline therapies received by the patient; B: Computed tomography images of liver metastases during treatment. Lesions are indicated by the red circles. The number represents the change in the size of liver metastases compared with the previous image; C: The levels of the colorectal cancer biomarkers carcinoembryonic antigen and carbohydrate antigen 19-9 are shown by the blue and black lines, respectively. The four background colors represent each treatment line; D: The allele frequencies of circulating tumor DNA alterations are shown during cetuximab treatment. CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9; PR: Partial response; PD: Progressive disease.
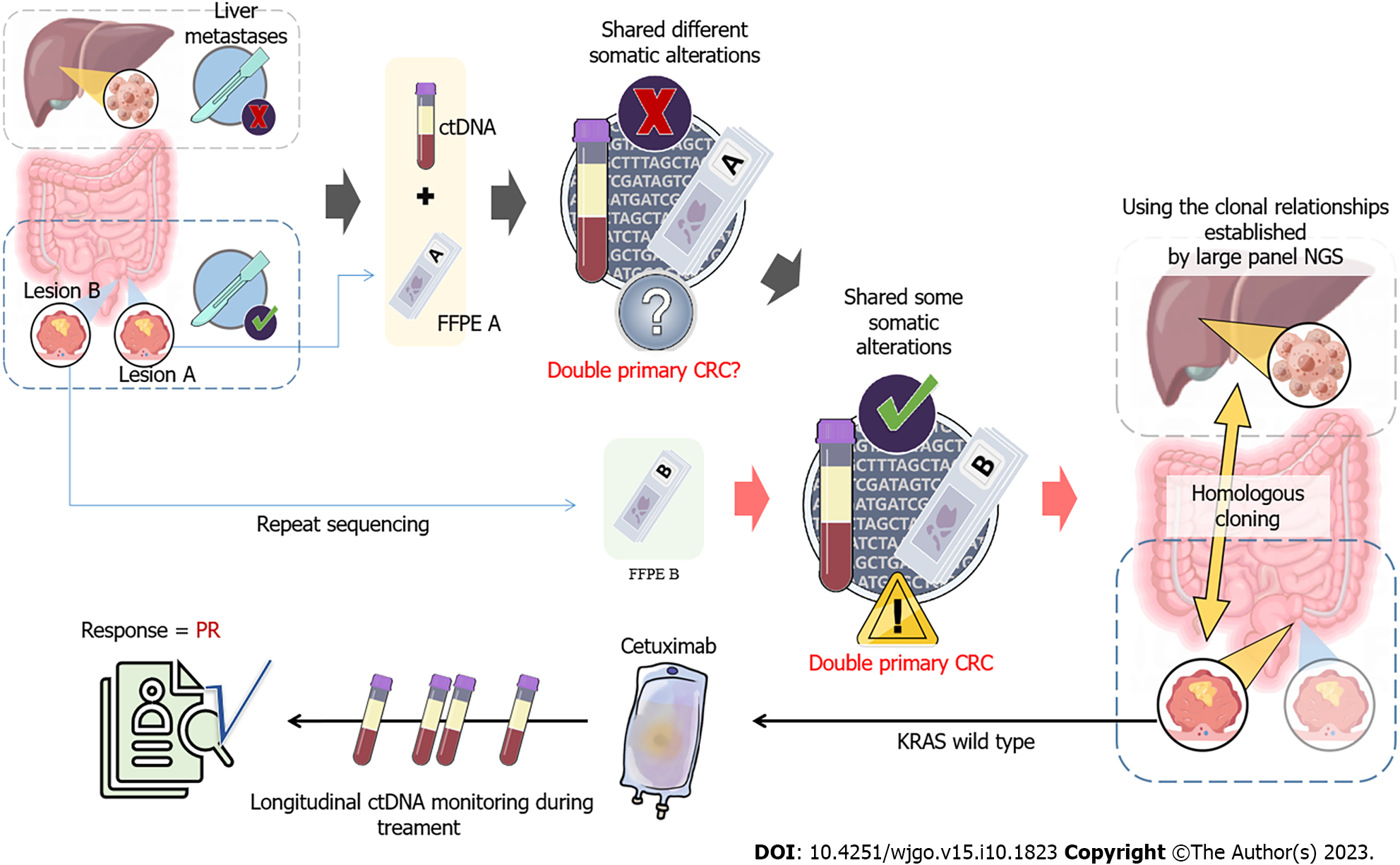
Figure 2 Schematic diagram of the use of targeted next-generation sequencing for the diagnosis of two primary colorectal tumors, and the corresponding treatment and efficacy.
NGS: Next-generation sequencing; FFPE: Formalin-fixed paraffin-embedded; CRC: Colorectal cancer.
- Citation: Qu YJ, Zhang QS, Wang B, Zhang F, Pan E, Zhao CY, Liu SY, Fang LP. Comprehensive next-generation sequencing reveals double primary colorectal carcinoma missed by diagnostic imaging: A case report. World J Gastrointest Oncol 2023; 15(10): 1823-1828
- URL: https://www.wjgnet.com/1948-5204/full/v15/i10/1823.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i10.1823