Copyright
©2009 Baishideng.
World J Gastrointest Oncol. Oct 15, 2009; 1(1): 26-33
Published online Oct 15, 2009. doi: 10.4251/wjgo.v1.i1.26
Published online Oct 15, 2009. doi: 10.4251/wjgo.v1.i1.26
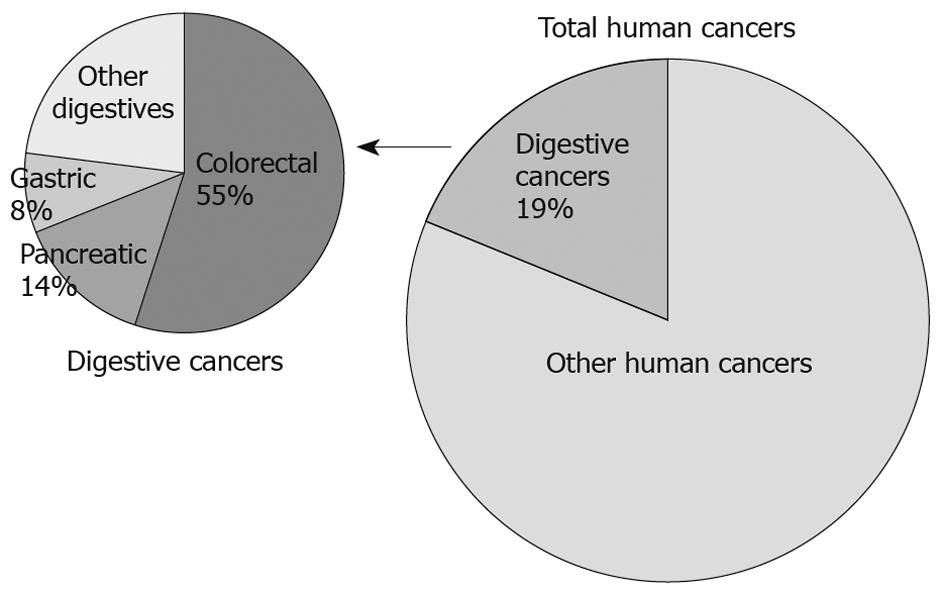
Figure 1 Prevalence and distribution of digestive cancers in USA population.
Digestive cancers make up 19% of all cancers in the United States. Colorectal cancer, pancreatic cancer and gastric cancer are the three most common and/or most deadly among them.
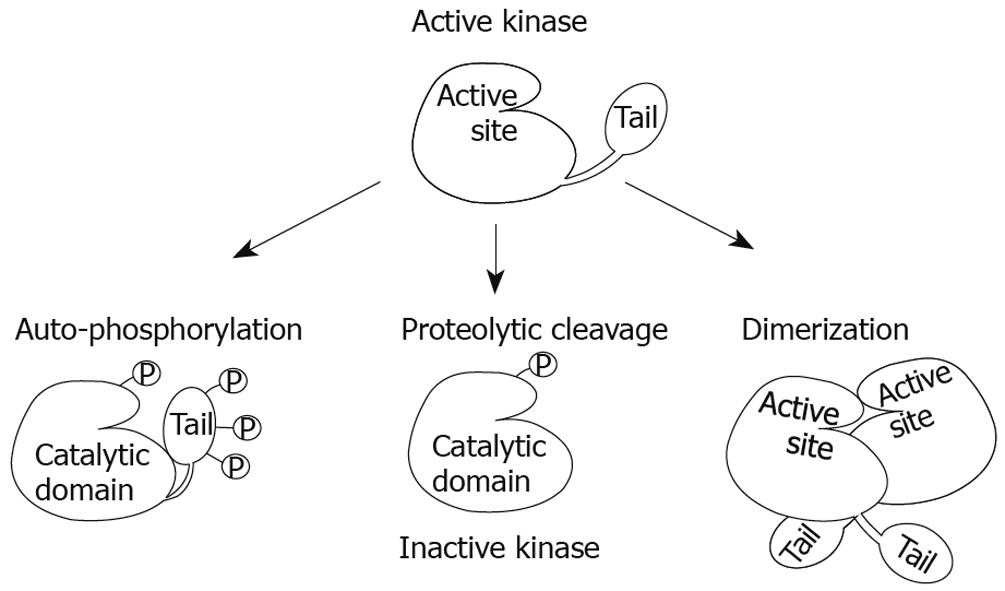
Figure 2 Models of CKIepsilon/δ regulation.
CKIepsilon/δ auto-phosphorylation at their C-terminal tail inhibits their kinase activity through a conformational change that places the tail over the active site, therefore blocking it from potential substrates. Dephosphorylation by phosphatases is the most accepted mechanism for their activation in vivo. In addition, removal of the tail by proteolytic cleavage or mutations has been used in vitro to activate these kinases; however, actual removal of the tail in vivo may have additional effects on proper function. Finally, CKIepsilon/δ dimerization at the active site could inactivate these kinases by physically blocking substrates from entering this region.
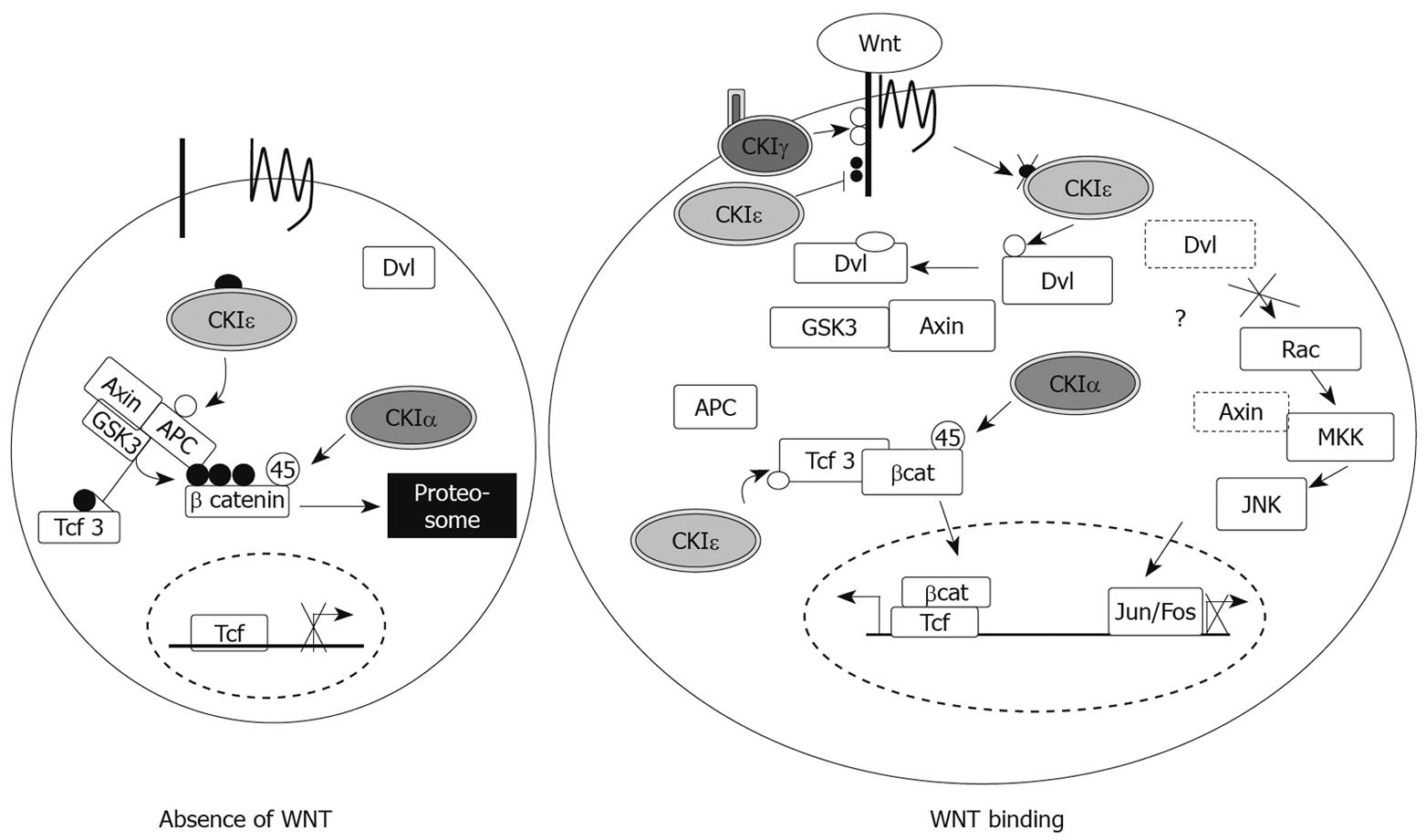
Figure 3 CKI members in canonical Wnt signaling.
In the absence of Wnt ligand, β-catenin is phosphorylated by GSK3β after priming at S45 by CKIα and becomes degraded. In this state, CKIepsilon phosphorylates APC and stabilizes the β-catenin degradation complex. Upon Wnt ligand binding to its receptor, CKIepsilon is fully activated through dephosphorylation and contributes to the disassembly of the β-catenin degradation complex by phosphorylating Dishevelled (Dvl) and facilitating its interaction with Axin and GSK3β. Therefore, even though CKIα still primes β-catenin at S45, without additional phosphorylation by GSK3β, β-catenin becomes stabilized and accumulates in the cytoplasm. CKIepsilon also promotes β-catenin stability by phosphorylating Tcf3 which competes against GSK3β for β-catenin. Stabilized β-catenin then translocates into the nucleus, where it contributes to activation of Wnt target genes. CKIepsilon also negatively regulates the Wnt pathway by phosphorylating the LRP-co-receptor, which is positively phosphorylated by CKIγ. Since epistasis analysis showed that CKIepsilon-mediated phosphorylation of LRP was downstream of its phosphorylation of Dvl, this is most likely a negative feedback loop to quickly deactivate the pathway after propagation of the signal.
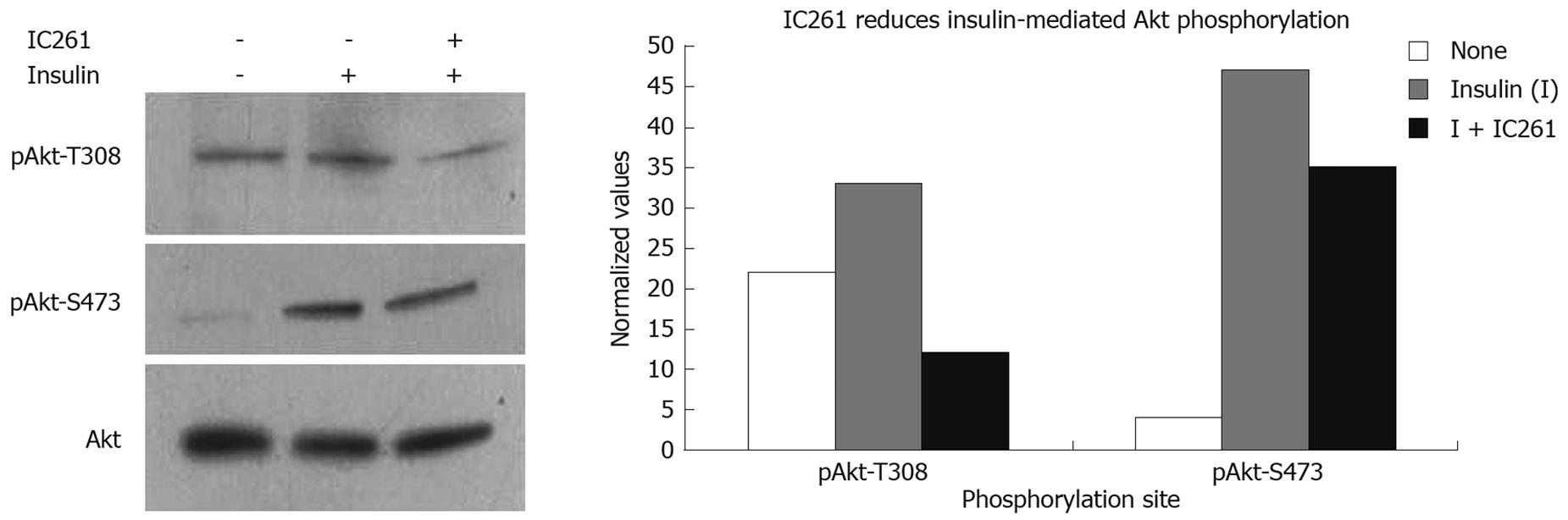
Figure 4 Pharmacological inhibition of CKIepsilon/δ reduces insulin-induced Akt phosphorylation in PDAC cells.
Cells were serum starved overnight and then incubated in 10 μg/mL insulin-containing media for 20 min in the presence or absence of the CKIepsilon/δ inhibitor IC261 (40 μmol/L). Akt activation was measured by phosphorylation at T308 and S473. Bands were quantified using Image J software and values were normalized against total Akt.
- Citation: Modak C, Chai J. Potential of casein kinase I in digestive cancer screening. World J Gastrointest Oncol 2009; 1(1): 26-33
- URL: https://www.wjgnet.com/1948-5204/full/v1/i1/26.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v1.i1.26