Published online Sep 16, 2017. doi: 10.4253/wjge.v9.i9.486
Peer-review started: February 12, 2017
First decision: March 31, 2017
Revised: July 31, 2017
Accepted: August 3, 2017
Article in press: August 4, 2017
Published online: September 16, 2017
Processing time: 226 Days and 8.5 Hours
To compare efficacy and safety of endoscopic ultrasound (EUS)-guided and surgical drainage in pancreatic fluid collection management.
Data were obtained retrospectively from January 2012 to December 2016. Patients with pancreatic fluid collection were performed EUS-guided or surgical procedure. Main outcome measures including clinical efficiency, complication, duration of procedures, hospital stay and cost were analyzed.
Thirty-six patients were enrolled into the study, including 14 in endoscopic group while 22 in the surgical group. Twelve (86%) patients were treated successfully by endoscopic approach while 21 (95%) patients benefited through surgical procedure. Endoscopic treatment had higher recurrence and complication rates than surgery, resulting in more re-interventions. Meanwhile, duration of procedure, hospital stay and cost were significantly lower in endoscopic group.
Both approaches were effective and safe. EUS-guided approach should be the first-line treatment in mild and simple cases, while surgical approach should be considered as priority in severe and complex cases.
Core tip: This retrospective study was to compare efficacy and safety of endoscopic ultrasound (EUS)-guided and surgical drainage in pancreatic fluid collection management after acute pancreatitis or pancreatic surgery. Of all the 36 patients, 14 patients were performed EUS-guided drainage while 22 patients were performed surgical procedure. Endoscopic treatment had higher recurrence and complication rates than surgery, resulting in more re-interventions. Meanwhile, duration of procedure, hospital stay and cost were significantly lower in endoscopic group. Both approaches were effective and safe. EUS-guided approach should be the first-line treatment in mild and simple cases, while surgical approach should be considered as priority in severe and complex cases.
- Citation: Hao SJ, Xu WJ, Di Y, Yao L, He H, Yang F, Jin C, Zhong L, Fu DL. Novel and supplementary management of pancreatic fluid collections: Endoscopic ultrasound-guided drainage. World J Gastrointest Endosc 2017; 9(9): 486-493
- URL: https://www.wjgnet.com/1948-5190/full/v9/i9/486.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i9.486
Pancreatic fluid collection (PFC), including acute peripancreatic fluid collection (APFC), acute necrotic collection (ANC), pseudocyst and development of walled-off necrosis (WON), arises as a complication of acute pancreatitis and pancreatic surgery[1]. Part of PFC will resolve spontaneously. The indications for PFC treatment are symptom driven, including fever, upper abdominal pain, satiety, gastric outlet obstruction, weight loss, or jaundice[2].
Open surgical drainage has long been first choice for PFC treatments[3]. However, patients often suffered tremendous injury from operation with an unsatisfied modality and mobility. During the last 20 years, endoscopic ultrasound (EUS) guided drainage, which is minimally invasive, has been increasing utilized to help manage PFCs[4]. Fluid collection can be aspirated by EUS-guided puncture. Furthermore, the procedure creates a fistula using stent or naso-cystic catheter between the PFC and the gastric lumen (cyst-gastrostomy). However, EUS-guided drainage of PFCs is technically challenging which requires experienced expertise and centers. Although both approaches have been demonstrated different efficacy in previous studies, there is still a scarcity of data to decided which one is optimal.
In this study, we tried to compare endoscopic and surgical treatment regarding clinical success, complication rate, recurrence, duration of procedures, hospital stay and cost with emphasis on selection of patients.
Thirty-six patients were enrolled retrospectively at the Department of Pancreatic Surgery and Department of Gastroenterology and Digestive Endoscopy, Huashan Hospital, Shanghai, China, from January 2012 to December 2016. All patients suffered symptomatic or asymptomatic PFCs after acute pancreatitis or pancreatic surgery. Symptomatic associated with PFC included fever, abdominal pain, biliary or gastric outlet obstruction. All patients provided written informed consent to undergo the procedures.
Patients were identified from the clinical databases, and clinical data and CT scan were individually reviewed. Patient and PFC characteristics, treatment outcomes and complications were recorded. As the EUS-guided drainage is an evolving treatment modality, the choice between endoscopic vs surgical treatment was made according to the patients’ current opinion and doctors’ experience.
Treatment success means complete resolution of PFC or a decrease in size to 2 cm or smaller on CT scan with the relief of symptoms at 72 h after procedure. Treatment failure was defined as symptoms persists or worsen with PFC increased in size or remained 2 cm in size on CT scan at 6 wk afterwards. Recurrence means PFC found on CT scan with symptoms at 72 h after an initial procedure. Re-intervention means the need for repeat procedure, surgery or endoscopy, because of persistent symptoms with PFC not less than half of the original size on follow-up imaging[5]. The cost was determined by the expenditure of procedure, anesthesia, peri-treatment medications, facility fees and hospital stay.
A contrast-enhanced abdominal CT scan was performed 24 to 48 h before undergoing either treatment. The PFC was categorized and graded according to the Atlanta classification[6], based on CT scan imaging reviewed by two experienced radiologists. All patients with pancreatic pseudocyst, or necrosis in the setting of uncontrolled pancreatitis underwent placement of naso-jejunum feeding tubes, to provide symptomatic relief and nutrition support. Third generation cephalosporin was intravenously administered in the peri-procedure period.
All EUS procedures were performed with EUS guidance by an experienced endoscopist while the patient was under conscious sedation. Once the PFC was identified, it was accessed using a 19-gauge needle, fluid was aspirated. Furthermore, a 0.035-inch guidewire was inserted into the PFC through the needle with fluoroscopic guidance. And needle was removed afterwards. needle knife was inserted through the guidewire guidance to extend a bigger fistula. Finally, a wire-guided balloon was used to dilate the gastric wall perforation to 10 mm. Two double pig-tail plastic stents or a metal stent were chosen to be deployed to facilitate the drainage of pseudocyst contents into the stomach. A naso-cystic catheter was inserted if there was necrotic debris.
All operations were performed by experienced pancreatic surgeon. An incision was made from the umbilicus to xiphoid process, to allow access to the abdomen. If the PFC were diffuse, debridement and drainage were performed and necrotic tissue were cleansed. If the PFC were localized such as pseudocyst or WON, cyst-gastrostomy was performed in the lowest point of the cyst. The abdominal drainage tubes were set if needed. Patients were discharged when pain control was adequate and a soft diet was tolerated.
During the process of drainage, all patients remained hospitalized. The cavity was lavage daily with saline solution through naso-cystic catheter after endoscopic procedure. All patients were evaluated with CT scan within 72 h after PFC drainage. If the PFC re-appears after procedure, re-intervention was considered. In patients with treatment success, cyst-gastrostomy stents, naso-cystic catheter, and the nose-jejunum feeding tube were removed. The patients with PFC decreased in size partially underwent transmural stents replacement and were re-evaluated by CT scan after 1 mo; With resolved PFC, then the patients were managed as treatment success. The patients with treatment failure suffered endoscopic therapy repeatedly or turn to surgery. All patients were follow-up during 6 mo.
Descriptive statistics for continuous variables are presented as means or medians with SD, respectively. Categorical variables are reported as absolute values and percentages. Differences between groups were analyzed for categorical variables with the χ2 test. We considered P < 0.05 as statistically significant. Statistical analysis was performed with SPSS version 20.0 for Windows.
A total of 36 patients had an intervention for PFC over the period from January 2012 to December 2016. 22 patients (61%) were treated surgically and 14 (39%) endoscopically. In 23 patients (64%), PFC were caused by acute pancreatitis in early or late stage. Of them, 5 in endoscopic group while 18 in surgical group. Thirteen patients (36%) suffered post-operative pancreatic leakage which resulted in PFC, and 9 in endoscopic group while 4 in surgical group. The type of PFC was divide into four categories. In endoscopic group, acute PFC were the leading type while pseudocyst, ANC and WON followed. In contrast, pseudocyst and WON were the majority in the surgical group (Table 1).
| Endoscopic group, n = 14 | Surgical group, n = 22 | P value | |
| Age (yr) | 56.3 | 58.7 | 0.102 |
| Gender (male, %) | 6 (43) | 9 (41) | 0.143 |
| Etiology | 0.223 | ||
| Acute pancreatitis | 5 (36) | 18 | |
| Post-op. pancreatic leakage | 9 (64) | 4 | |
| Type of PFC | 0.138 | ||
| APFC | 7 | 3 | |
| ANC | 2 | 3 | |
| Pseudocyst | 3 | 9 | |
| WON | 2 | 7 | |
| ASA grade | / | ||
| I-II | 14 | 21 | |
| III-V | 0 | 1 | |
| Occurrence time | 0.557 | ||
| Early (within 14 d) | 9 | 14 | |
| Late (after 14 d) | 5 | 8 |
Endoscopic approach was performed in the patients with PFC in the distal pancreas mainly. PFC around head of pancreas relied more on surgical treatment. Moreover, 2 patients with diffuse PFC in the abdomen have underwent surgical approach. The size of the PFC in both group had no significant difference. Overall two-third of the patients had PFC with infection, which had fever, high WBC value, or microbiologic evidence. But infection was not the influencing factor of deciding the treatment approach. Contrasted with the operation, endoscopic treatment of PFC benefits in duration of procedure, hospital stay after procedure and the medical costs (Table 2).
| Endoscopic group, n = 14 | Surgical group, n = 22 | P value | |
| Location | 0.127 | ||
| Head of pancreas | 2 | 8 | |
| Distal pancreas | 12 | 12 | |
| Peripancreatic (diffuse) | 0 | 2 | |
| Long axis (cm) | 4.32 ± 1.13 | 5.17 ± 3.18 | 0.098 |
| Infection | 0.081 | ||
| + | 10 | 14 | |
| - | 4 | 8 | |
| Duration for procedure (min) | 94.4 ± 23.5 | 127.2 ± 61.9 | 0.038 |
| Hospital stay (d) | 7.4 ± 2.8 | 12.5 ± 8.1 | 0.019 |
| Cost (RMB) | 24311.48 ± 3211.76 | 48119.93 ± 6723.25 | 0.003 |
Fourteen patients with PFC underwent endoscopic treatment in the study. Seven of them were diagnosed with APFC and simple aspiration were performed through EUS-guided puncture. Among these patients, 2 patients suffered re-intervention because of fluid collection re-appears within 72 h. Two patients with ANC were treated by combination of naso-cystic catheter and double pig-tail tube successfully. Three patients with pseudocyst were treated by different method, including naso-cystic catheter, double pig-tail tube and metal stent. Both patients with WON were treated by metal stent, one of which suffer the serious bleeding after procedure and turned to emergency surgery (Table 3).
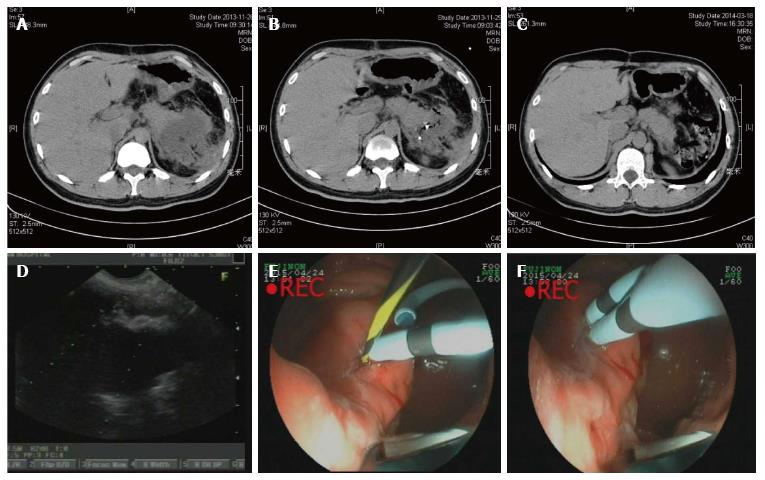
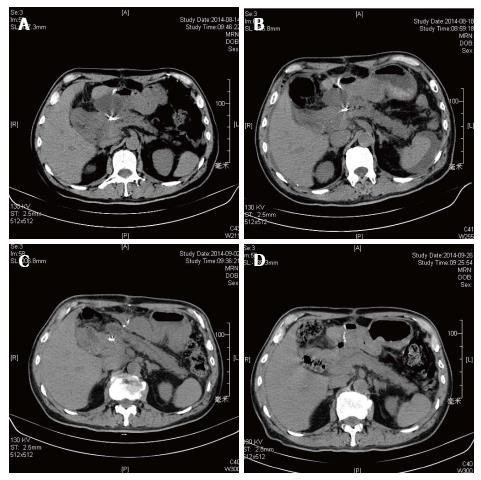
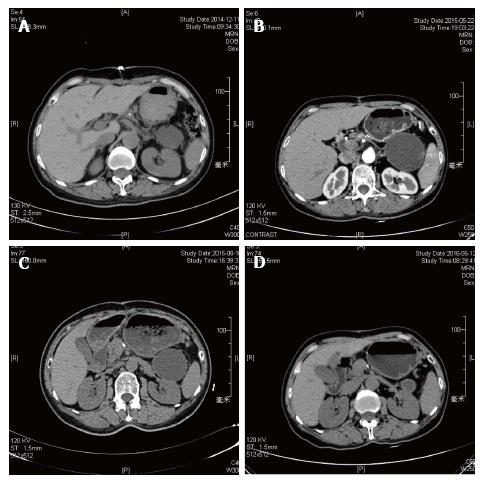
Overall, 12 patients (86%) in the endoscopic group were treated successful (Figure 1) while the surgical group were 21/22 (95%) success. One of the failed cases in the endoscopic group turned to surgery due to complication and then underwent operation successfully (No. 10). In the surgical group, the fail case died within 72 h due to serious bleeding from WON to digestive tract. Three patients in the endoscopic group suffered PFC recurrence and 2 of them underwent re-intervention (Nos. 2 and 12, Figure 2). The other one failed and chose conventional therapy and follow-up when PFC recurrence appeared (No. 3, Figure 3). Two patients in the endoscopic group had complication after procedure. One case mentioned above were serious bleeding (No. 10) and the other were secondary infection after metal stent implantation (No. 8). In the other hand, surgical group had satisfying efficiency and safety (Tables 3 and 4).
| Endoscopic group, n = 14 | Surgical group, n = 22 | P value | |
| Treatment success | 12 | 21 | 0.858 |
| Treatment failure | 2 | 1 | 0.041 |
| Recurrence | 3 | 0 | 0.035 |
| Re-interventions | 2 | 0 | 0.017 |
| Complications | 2 | 0 | 0.051 |
| Bleeding | 1 | 1 | |
| Secondary infection | 1 | 0 |
In this retrospective study, we indicate that there are no statistical differences in clinical success, complications and mortality rate between the two approaches (endoscopic drainage and surgical drainage) in the PFC treatment. Procedure time, cost and hospital stay were lower statistically in the endoscopic group. However, recurrence and re-interventions rates in the endoscopic group were significantly higher than those of surgical group.
Regarding hospital cost and stay, endoscopic approach has some advantages compared with surgery: Minimal invasion, shorter procedure time and rapid recovery. As previously reported in a retrospective analysis of 19 PFC, only about one-third of patients required hospitalization. The authors indicated that endoscopic treatment is a feasible method for some selected outpatient with PFC[7]. We found that the cost of surgical treatment was about twice cost of endoscopic approach, which agrees with the previous study[6,8]. These savings were mostly the result of early patient discharge, lower medication costs and use of conscious sedation for endoscopy. As comparison of costs in this study pertained to the China only, it may not be applicable to other countries. Due to a lower professional income for surgeons in China, we assume the gap between endoscopic group and surgery group would be larger in other countries.
In the study, the treatment success rate in short-term was 95% in surgical group vs 86% in Endoscopic group, which is agreed with previous reports[9,10]. Surgery is successful technically in almost all patients who meet the criteria for a cyst-gastrostomy, while not all PFCs may be amenable for EUS-guided drainage. Especially for patients with thick viscosity of necrotic fluid and solid debris or the PFC tracked deep into the pelvic cavity, Endoscopic treatment may be not adequate. Insufficient drainage may potentially result in persistence of symptoms and infection, thus causing recurrence, even re-intervention.
With the advantage of real-time imaging and revealing the presence intervening vasculature, EUS-guided cyst-gastrostomy is increasingly being performed in pancreatic fluid collections. Unlike walled-off necrosis, current treatment outcomes for endoscopic drainage of pseudocysts are excellent[11,12]. With increasing experience, EUS-guided drainage has extended its use in many complicate cases such as pancreatic necrosis, even with infection[13,14]. Several studies have introduced new techniques, such as large diameter, lumen-apposing, self-expanding metal stent with bilateral flanges[15] or multiple transluminal gateway treatment (MTGT)[16]. However, there is no definite evidence showing metal stents are better than plastic ones, or which kind of plastic stent is better than others[17]. Also, there have not adequate studies reported about the efficacy of MTGT in the treatment of PFC. In a study of 211 patients with symptomatic PFCs, the reported success rate for treating sterile and infective pseudocysts was 93.5%, but only 63.2% when treating a WON[11]. Another study reported by adopting endoscopic necrosectomy, a more aggressive endoscopic approach, the success rates up to 81% when treating a WON[18]. We must point out that endoscopic necrosectomy carries risks of bleeding and perforation, even costs patients’ life with internal hemorrhage. During the poorly drainage process of treatment and collapse, tiny, narrow connections were formed, causing unilocular separated into sub-cavities. Since there are undrained sub-cavities, pancreatic fluid collections are less responsible to any endoscopic treatment.
In 2011, a retrospective study was published discussing the long-term outcomes of patients underwent endoscopic treatment of PFC[19]. The results showed that the long-term success rate was 72.5% (58/80 patients), and 28% of patients turned to surgery. It was perforation in four patients, endoscopically inaccessible areas in two patients, inadequate drainage and recurrent fluid collections in sixteen patients, respectively. Surgical drainage is a multidisciplinary decision and should be considered for patients who have a high potential recurrence, or not suits endoscopic or percutaneous drainage.
In our study, 3 patients experienced recurrence due to continuous pancreatic leakage after distal pancreatectomy, 2 of which need a re-intervention with octreotide therapy afterward. The other one chose conventional treatment and the PFC absorbed significantly after one year follow up. It seems like we had higher recurrence rate than other studies, but we found the 3 cases were APFC after distal pancreatectomy, which was free and connected with main pancreatic duct while most studies focus on the pseudocyst or WON, which is limited by fibrous tissue. If we excluded the APFC case, we had no recurrence case in ANC, pseudocyst and WON cases. 2 patients suffered complications in the endoscopic group while surgical group had one. Both endoscopic complications were caused by metal stents implantation. Repeated rubbing between the stent and the cyst wall or necrotic tissue may lead to internal hemorrhage. The placement of stent may increase the risks of secondary infection in sterile pseudocyst.
Additional, another advantage of EUS-guided drainage for PFC is that an additional diagnosis is made in approximately 5% of patients with drainage of pseudocyst[10]. There is about 1.25% risk of cancer in patients with PFC and no clear evidence in imaging scans[20]. As necrotic tissue and debris in the pancreatic fluid collections are morphologically similar to mural nodules in mucinous cystic neoplasm, it is necessary to perform a contrast-enhanced ultrasound before the EUS puncture.
There are several limitations to this study. Firstly, it is a retrospective study performed by a single center. Our department is a highly specialized tertiary academic endoscopic unit, which may lead to overestimation of procedures’ results. Secondly, the small size of the enrolled patient group may also represent a bias. Another important selection bias is the fact that patients referred to surgical cyst-gastrostomy were sicker and required more definitive therapy.
In conclusion, in this retrospective study, we demonstrated that EUS-guided drainage and surgical drainage were both effective and safe technique, which were complementary to each other. For patients with uncomplicated pancreatic pseudocysts, EUS-guided cyst-gastrostomy should be the first-line treatment, because the approach has less expenditure and shorter length of hospital stay. For patients with PFC connected to pancreatic duct or with complex situations such as pancreatic necrosis, even with infection, surgical cyst-gastrostomy may be better choice due to its low recurrence rate.
Pancreatic fluid collection (PFC), arises as a complication of acute pancreatitis and pancreatic surgery. Part of pancreatic fluid collections will resolve spontaneously, while others needs aggressive management. Open surgical drainage has long been first choice for PFC treatments. However, patients often suffered tremendous injury from operation with an unsatisfied modality and mobility. Minimally invasive endoscopic ultrasound (EUS) guided drainage has been increasing utilized to help manage PFCs in decades. PFCs can be aspirated by EUS-guided puncture. Furthermore, the procedure creates a fistula using stent or naso-cystic catheter between the PFC and the gastric lumen (cyst-gastrostomy). Although both approaches have been demonstrated different efficacy in previous studies, there is still a scarcity of data to decided which one is optimal. In this study, the authors tried to compare endoscopic and surgical treatment regarding clinical success, complication rate, recurrence, duration of procedures, hospital stay and cost with emphasis on selection of patients.
Many studies have focus on the efficacy and feasibility of the new approach for PFCs, and the researches have concluded that the efficacy and safety of EUS-guided drainage is satisfying. But comparing with the surgical drainage, a traditional treatment, the endoscopic method has not shown comprehensive advantage. There is still a scarcity of data to decided which one is optimal.
Both EUS-guided and surgical drainage were both effective and safe technique, which were complementary to each other. For patients with uncomplicated pancreatic pseudocysts, EUS-guided cyst-gastrostomy should be the first-line treatment, because of cost saving and shorter length of hospital stay. For patients with PFCs connected to pancreatic duct or with necrosis or infection, surgical cyst-gastrostomy may be better choice due to its low recurrence rate.
The study suggested that EUS-guided and surgical drainage can be complementary to each other. If the patients with simple pseudocysts or acute peripancreatic fluid collection (APFC), the EUS-guided drainage should be firstly considered. If the patients suffer complicate PFCs, including necrosis or infection, surgical drainage is always be the better choice.
PFC: Pancreatic fluid collection; APFC: Acute peripancreatic fluid collection; ANC: Acute necrotic collection; WON: Walled-off necrosis.
This article is well-written.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shah OJ S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Jacobson BC, Baron TH, Adler DG, Davila RE, Egan J, Hirota WK, Leighton JA, Qureshi W, Rajan E, Zuckerman MJ. ASGE guideline: The role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastrointest Endosc. 2005;61:363-370. [PubMed] |
| 2. | Vitas GJ, Sarr MG. Selected management of pancreatic pseudocysts: operative versus expectant management. Surgery. 1992;111:123-130. [PubMed] |
| 3. | Yin WY. The role of surgery in pancreatic pseudocyst. Hepatogastroenterology. 2005;52:1266-1273. [PubMed] |
| 4. | Yusuf TE, Baron TH. Endoscopic transmural drainage of pancreatic pseudocysts: results of a national and an international survey of ASGE members. Gastrointest Endosc. 2006;63:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4306] [Article Influence: 358.8] [Reference Citation Analysis (45)] |
| 6. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-590.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 331] [Article Influence: 27.6] [Reference Citation Analysis (1)] |
| 7. | Gibbs CM, Baron TH. Outcome following endoscopic transmural drainage of pancreatic fluid collections in outpatients. J Clin Gastroenterol. 2005;39:634-637. [PubMed] |
| 8. | Varadarajulu S, Lopes TL, Wilcox CM, Drelichman ER, Kilgore ML, Christein JD. EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc. 2008;68:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Aljarabah M, Ammori BJ. Laparoscopic and endoscopic approaches for drainage of pancreatic pseudocysts: a systematic review of published series. Surg Endosc. 2007;21:1936-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Varadarajulu S, Wilcox CM, Tamhane A, Eloubeidi MA, Blakely J, Canon CL. Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc. 2007;66:1107-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Akshintala VS, Saxena P, Zaheer A, Rana U, Hutfless SM, Lennon AM, Canto MI, Kalloo AN, Khashab MA, Singh VK. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc. 2014;79:921-928; quiz 983.e2, 983.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Bhasin DK, Rana SS, Udawat HP, Thapa BR, Sinha SK, Nagi B. Management of multiple and large pancreatic pseudocysts by endoscopic transpapillary nasopancreatic drainage alone. Am J Gastroenterol. 2006;101:1780-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Mukai S, Itoi T, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Tanaka R. Novel single transluminal gateway transcystic multiple drainages after EUS-guided drainage for complicated multilocular walled-off necrosis (with videos). Gastrointest Endosc. 2014;79:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Walter D, Will U, Sanchez-Yague A, Brenke D, Hampe J, Wollny H, López-Jamar JM, Jechart G, Vilmann P, Gornals JB. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a prospective cohort study. Endoscopy. 2015;47:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Varadarajulu S, Phadnis MA, Christein JD, Wilcox CM. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011;74:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 17. | Bang JY, Hawes R, Bartolucci A, Varadarajulu S. Efficacy of metal and plastic stents for transmural drainage of pancreatic fluid collections: a systematic review. Dig Endosc. 2015;27:486-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, Kreitmair C, Meining A, Wehrmann T, Rösch T. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009;58:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 19. | Seewald S, Ang TL, Richter H, Teng KY, Zhong Y, Groth S, Omar S, Soehendra N. Long-term results after endoscopic drainage and necrosectomy of symptomatic pancreatic fluid collections. Dig Endosc. 2012;24:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Holt BA, Varadarajulu S. EUS-guided drainage: beware of the pancreatic fluid collection (with videos). Gastrointest Endosc. 2014;80:1199-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |