Published online Jun 16, 2017. doi: 10.4253/wjge.v9.i6.243
Peer-review started: February 12, 2017
First decision: March 28, 2017
Revised: April 10, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: June 16, 2017
Processing time: 139 Days and 2.3 Hours
An accurate staging is necessary to select the best treatment and evaluate prognosis in oncology. Staging usually begins with noninvasive imaging such as computed tomography, magnetic resonance imaging or positron emission tomography. In the absence of distant metastases, endoscopic ultrasound plays an important role in the diagnosis and staging of gastrointestinal tumors, being the most accurate modality for local-regional staging. Its use for tumor and nodal involvement in pre-surgical evaluation has proven to reduce unnecessary surgeries. The aim of this article is to review the current role of endoscopic ultrasound in the diagnosis and staging of esophageal, gastric and colorectal cancer.
Core tip: Endoscopic ultrasound (EUS) has an important role in staging, establishing prognosis and optimizing therapeutic decisions. Also, it has proved to be a useful alternative therapeutic modality in surgery. In terms of cost-benefit, it reduces the number of unnecessary diagnostic or therapeutic procedures, leading to lower morbidity and mortality rates and reduced cost in cancer treatment. This review summarizes the current role of EUS in the diagnosis and staging of esophageal, gastric and colorectal cancer.
- Citation: Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: An update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6): 243-254
- URL: https://www.wjgnet.com/1948-5190/full/v9/i6/243.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i6.243
Endoscopic ultrasound (EUS) was first used in 1980 as a technology prototype for pancreatic cancer evaluation[1]. It was designed as a combination of two techniques, endoscopy and ultrasound, allowing the visualization of the gastrointestinal mucosa as well as the tract wall in deep and surrounding structures. In 1989 its standardized indications in clinical practice were described[2]. Due to the constant evolution of this technology, it is now considered an important diagnostic and therapeutic method in the oncology field. EUS has an important role in staging, establishing prognosis and optimizing therapeutic decisions[3]. Also, it has proved to be a useful alternative therapeutic modality in surgery. In terms of cost-benefit, it reduces the number of unnecessary diagnostic or therapeutic procedures, leading to lower morbidity and mortality rates and reduced cost in cancer treatment[4,5]. The TNM classification (American Joint Committee on Cancer, AJCC) is the most accepted staging classification and is based on the analysis of local tumor invasion (T), lymph node involvement (N) and distant metastasis (M). Staging usually begins using noninvasive imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET), which are generally better than EUS for excluding M. In the absence of metastasis, EUS has proved to be an accurate modality for assessing T and N[2]. Moreover, the development of EUS-related technology such as fine needle aspiration (FNA), high frequency catheter probe, elastography and contrast enhancement has helped to improve EUS staging accuracy. EUS indications in oncology is therefore increasing[6]. The aim of this review is to summarize the current role of EUS in the staging of esophageal, gastric and colorectal cancer.
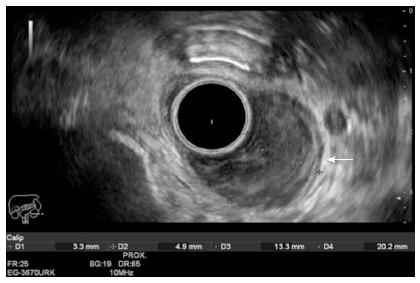
The prognosis of esophageal cancer (EC) is poor because these tumors are usually detected in an advanced stage. Surgery is not possible in most cases and has a high rate of morbidity and mortality. The level of tumor invasion and lymph node metastasis will determine treatment and prognosis. Therefore, EUS plays a vital role by providing an accurate T and N staging, which allows deciding on the best treatment[7]. The use of EUS evaluation in preoperative staging has led to a mortality reduction of 42.1% and a better recurrence-free survival rate, compared to patients with no EUS evaluation[8]. According to the TNM classification (Table 1), superficial EC includes mucosal and submucosal involvement (Tis, T1a or T1b)[9]. Patients with any nodal involvement (N+) or advance tumors (T2-T4a) (Figure 1) need preoperative neoadjuvant chemoradiotherapy, whereas T1 patients with no nodal metastasis can benefit from endoscopic (Tis, T1a N0) or surgical resection (T1bN0)[10-12]. When different staging methods were compared, CT, MRI and PET-scan showed themselves to be better than EUS in evaluating distant metastasis (M), however EUS proved superiority in the detection of tumor stage (T) and lymph nodes (N)[13-16]. One method does not have to exclude the other. The incorporation of CT, PET and EUS in preoperative staging reduces the number of unnecessary surgical procedures from 44% to 21%[17].
| Primary tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | High-grade dysplasia |
| T1 | Tumor invades lamina propria, muscularis mucosae, or submucosa |
| T1a | Tumor invades lamina propria or muscularis mucosae |
| T1b | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades adventitia |
| T4 | Tumor invades adjacent structures |
| T4a | Resectable tumor invading pleura, pericardium, or diaphragm |
| T4b | Unresectable tumor invading other adjacent structures, such as the aorta, vertebral body, and trachea |
| Regional lymph nodes (N) | |
| NX | Regional lymph node(s) cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in 1-2 regional lymph nodes |
| N2 | Metastasis in 3-6 regional lymph nodes |
| N3 | Metastasis in 7 or more regional lymph nodes |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
EC limited to the mucosa (Tis, T1a) can be treated effectively with minimally invasive endoscopic therapy, whereas submucosal (T1b) EC carries relatively high risk of lymph node metastasis and requires surgical resection. According to a meta-analysis by Puli et al[18] (49 articles), EUS sensitivity and specificity for T stage was 81.6% and 99.4%, for T1, 81.4% and 96.3%, for T2, 91.4% and 94.4%, for T3, and 92.4% and 97.4% for T4 staging, respectively. The accuracy was higher for T3-T4 lesions (> 90%) than T1-T2 (65%). However, a study by Thosani et al[19] reported, on the analysis of 1019 patients with only superficial EC, that EUS sensitivity and specificity was 85% and 87% for T1a and 86% and 86% for T1b respectively, with an overall EUS accuracy for superficial EC staging of > 93%.
The lymph node (LN) metastasis in EC is considered the main fact that influences prognosis and it depends on the number of nodes involved. This pathology has a high rate of LN involvement at an early stage. T1sm (T1b) disease has a 15% to 30% rate of LN dissemination. The 7th edition of the AJCC (Table 1) classifies the N stage according to the number of metastasized lymph nodes in N1 (1 to 2), N2 (3 to 6), and N3 (≥ 7). The use of EUS evaluation in preoperative staging has led to a mortality reduction of 42.1% and a better recurrence-free survival rate, compared to patients with no EUS evaluation[8]. According to the TNM classification (Table 1)[9], the presence of node metastasis indicates the need of neoadjuvant therapy. Therefore, identification of the N stage is mandatory. PET and CT have a low accuracy (51%) compared to EUS[20]. The evaluation of the LN features using EUS have shown that malignant nodes tend to be larger than 1 cm, round, sharply demarcated, and hypoechoic. When all these features are present there is an 85% chance of malignancy. However, only 25% of malignant LN have all four features[21]. A systematic review found that EUS has a sensitivity range of 59.5% to 100% and a specificity range of 40% to 100% for N staging[22]. Puli et al[18] described a EUS sensitivity for N stage of 85% and showed that the use of FNA substantially improves the sensitivity and specificity of EUS nodal staging from 85% to 97% and 85% to 96% respectively, with a low rate of complications, ranging from 0% to 2.3%. Chen et al[23] found an accuracy rate of 99.4% using EUS-FNA. In patients with EC, the identification of a celiac lymph node is synonymous to LN metastasis in 90% of the cases regardless of echo features and size and therefore indicates a poor prognosis[24]. EUS-FNA for celiac lymph node diagnosis has shown a sensitivity of 72% to 83%, a specificity of 85% to 98%, and an accuracy of 94%[25].
The role of EUS has some limitations. It may be less accurate for assessing the T1-T2 stage compared with T3-T4. According to some authors there is a trend to overstaging the depth of the submucosal invasion, with a low accuracy rate in early T staging (64%)[26]. The use of high frequency catheter probes may improve the diagnostic accuracy in early lesions from 83% to 92%, but the results are heterogeneous[27,28]. EUS criteria are not accurate after neoadjuvant radio-chemotherapy because EUS poorly differentiates tumor from necrosis or inflammatory reaction[29]. The presence of esophageal malignant stenosis that cannot be overcome can make TNM evaluation more difficult. A recent multi-center study suggested that routine EUS examinations may not be required in all patients with EC as the inability to advance a diagnostic gastroscope through a malignant stricture correlates 100% with locally advanced disease, so that performing a EUS does not change the treatment decision[30].
EUS has long been used to evaluate Barrett’s esophagus (BE)[6]. In the case of BE associated with high-grade dysplasia (HGD) or early (T1m) esophageal adenocarcinoma (EAC), the patient may benefit from endoscopy resection, but if EUS shows an advanced disease with tumor invading the submucosal, or beyond, or lymph node involvement, endoscopic therapy may not be warranted. Qumseya et al[31] showed in a recent meta-analysis that 14% of patients referred to EUS for BE associated with HGD or EAC will have advanced cancer (> T1sm or > N1) detected by EUS that is not amenable to endoscopic treatment and which therefore changes the therapeutic approach. With EUS it was found that 4% of these patients have advanced disease in the absence of nodules. The sensitivity and specificity for T stage was 56% and 89% and for N stage was 71% and 94 % respectively[31]. However, even the data mentioned, the American College of Gastroenterology has stated that EUS routine staging of patients with BE before EMR is unwarranted as clinical decision making will rest with the EMR findings and given the possibility of over- and under-staging in patients with superficial EAC[32-35]. In case of T1a lesions the rate of lymph node (LN) involvement is low, making these lesions optimally treated by EMR[36,37]. In patients with known T1b sm1 disease, there is conflicting data with respect to the likelihood of LN invasion[38,39]. The evidence of LN involvement, especially if substantiated by FNA, means that any attempt at endoscopic therapy would be palliative and therefore EUS may have a role in assessing and sampling regional LN, given the increased prevalence of lymph node involvement in these patients compared with less advanced disease[19].
Gastric cancer (GC) is the fourth most common cancer and the second cause of cancer-related deaths (10%)[40]. An accurate staging (Table 2) can be extremely useful in providing patients with the best therapeutic option. Patients with early gastric cancer, in the presence of favorable prognosis features (well-differentiated carcinoma, limited to the mucosa, diameter < 2 cm, absence of ulceration) and no lymph node involvement (N0) can benefit from endoscopic resection rather than surgical resection[41,42]. On the other hand, patients with advanced gastric cancer (T3-T4 tumors or N+) need to be treated with neoadjuvant therapy (chemotherapy, radiotherapy or both)[43,44].
| Primary tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ: Intraepithelial tumor without invasion of the lamina propria |
| T1 | Tumor invades lamina propria, muscularis mucosae, or submucosa |
| T1a | Tumor invades lamina propria or muscularis mucosae |
| T1b | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor penetrates subserosal connective tissue without invasion of visceral peritoneum or adjacent structures |
| T4 | Tumor invades serosa (visceral peritoneum) or adjacent structures |
| T4a | Tumor invades serosa (visceral peritoneum) |
| T4b | Tumor invades adjacent structures |
| Regional lymph nodes (N) | |
| NX | Regional lymph node(s) cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in 1-2 regional lymph nodes |
| N2 | Metastasis in 3-6 regional lymph nodes |
| N3 | Metastasis in seven or more regional lymph nodes |
| N3a | Metastasis in 7-15 regional lymph nodes |
| N3b | Metastasis in 16 or more regional lymph nodes |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
CT is a frequent imaging method for the preoperative staging of GC[45]. It has a high accuracy for distant metastasis (M), however its overall accuracy for loco-regional staging (T and N stages) is low, ranging from 65% to 85%[46,47]. The CT sensitivity and specificity for N stage is 77% and 78%, respectively[48]. No better results appear to be achievable with MRI or PET[48-50].
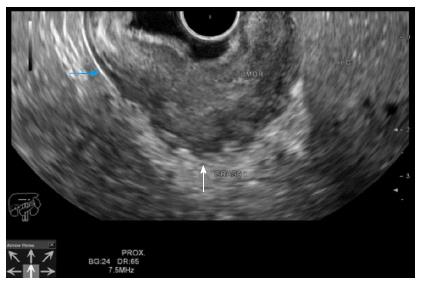
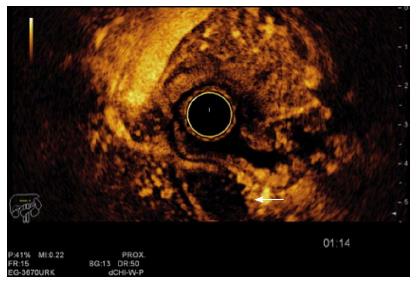
Thus, these imaging devices are mostly used to diagnose locally advanced lesions (T3-T4 or N+) or distant metastasis than early stages of GC. On the contrary, EUS is an accurate device for the loco-regional staging[51,52] (Figure 2). The employment of EUS in the preoperative stage of GC has shown to change the therapeutic management in 30% of cases, resulting in more limited surgical resections, especially in stages T1 and T3[53].
A recent meta-analysis by Mocellin et al[54] and the Cochrane Collaboration Group (2015) evaluated 66 articles (n = 7747) about GC staged with EUS. The aim was to evaluate EUS ability to separate patients with GC who would best benefit from surgery without preoperative radio-chemotherapy (T1-T2) from those with advanced tumors (T3-T4) who are likely to benefit from neoadjuvant therapy. They found EUS sensitivity and specificity to discriminate T1-T2 from T3-T4 lesions to be 86% and 90% respectively. A second analysis was made to evaluate EUS ability to discriminate between patients with superficial cancers (T1 from T2 and T1a from T1b), with the intention of identifying patients who would benefit from endoscopic resection rather than surgery. The sensitivity and specificity of EUS to distinguish T1 (early GC) from T2 (muscle-infiltrating) was 85% and 90% respectively. As for the capacity of EUS to distinguish between T1a (mucosal) vs T1b (submucosal), they showed that the sensitivity and specificity was 87% and 75% respectively. They concluded that EUS can distinguish between superficial (T1-T2) and advanced (T3-T4) primary tumors with a sensitivity and specificity greater than 85%. This performance is maintained for the discrimination between T1 and T2 superficial tumors. However, EUS diagnostic accuracy is lower when it comes to distinguishing between the different types of early tumors (T1a vs T1b)[54]. This conclusion correlates with Mocellin et al[55] previous results (2011) when they described that EUS can differentiate T1-2 from T3-4 GC with high accuracy (sensitivity of 86% and specificity of 91%). Cardoso et al[56] (2012) also showed that EUS seems to identify advanced T stage (T3 and T4) better than it identifies less advanced T stage or N stage, with a combined accuracy for T staging of 75%. Puli et al[57] (2008) evaluated 22 studies (n = 1896) and described the usefulness of EUS in GC. The sensitivity and specificity by stage were, 88.1% and 100% for T1, 82.3% and 95.6% for T2, 89.7% and 94.7% for T3, and 99.2% and 96.7% for T4. Incidentally, EUS for T stage detection was more accurate in advanced cancer than in early cancer. Kwee et al[58] (2008) showed in a systematic review (18 studies), the accuracy of EUS in differentiating mucosal (T1m) from deeper GC (> T1sm) and found that sensitivity and specificity of EUS in detecting cancerous extension beyond the mucosa ranged from 18.2% to 100% (median 87.8%) and from 34.7% to 100% (median 80.2%) respectively. They concluded that the studies showed too much heterogeneity and it is still unclear whether EUS can accurately differentiate between mucosal and deeper GC[58].
The accuracy of EUS for N staging has shown remarkable heterogeneity of results. Mocellin et al[54] described after the evaluation of 44 studies (n = 3573) an overall sensitivity and specificity of 83% and 67% respectively[54]. Cardoso et al[56] reported accuracy for N stage of 64%, sensitivity of 74%, and specificity of 80%. These results were due to the low possibility of detecting metastasized lymph nodes that are distant from the lesion[56]. Kwee et al[59] found that sensitivity and specificity of EUS varied from 16.7% to 95.3% (median 70.8%) and 48.4% to 100% (median, 84.6%). Puli et al[57] after the analysis of 22 studies (n = 1896) reported a sensitivity for N1 of 58.2% and N2 of 64.9%. The pooled sensitivity to diagnose distant metastasis was 73.2%.
There is a remarkable heterogeneity of the evidence currently available about the ability of EUS to differentiate T1a vs T1b tumors and to diagnose lymph node metastasis (N0 vs N+). Therefore, physicians should be cautious at the time of interpreting these results. Tumor features like size and location may affect diagnostic performance of EUS. A tumor size greater than 3 cm is associated with overstaging by EUS and decreases the diagnostic accuracy to 50%[60]. The cardia, the greater curve of upper body, the lesser curve at the incisura and the pyloric channel are the most challenging areas to examine[61].
Even though CT has proved useful for evaluating an abnormal gastric wall thickening, EUS, on the other hand, has shown itself to be superior for examining nodal involvement, extension and depth of tumor invasion[62]. The EUS diagnostic accuracy in gastric lymphoma is 91%-95% for T stage and 77%-83% for N stage[63,64]. The use of EUS-FNA combined with flow cytometry and immunohistochemistry can improve N staging accuracy substantially[65].
EUS has also shown a significant impact on treatment decisions. Gastric lymphoma confined to the mucosal and submucosal (T1) can simply be treated with H. pylori eradication therapy. However, if EUS shows deeper invasion, chemotherapy, radiation or surgical treatment may be necessary[66]. Moreover, EUS has proven to be useful for surveillance of recurrences at an early stage[62].
Accurate staging in rectal cancer (RC) is crucial for choosing the best multimodal therapy. Treatment decisions and prognosis depends on both T and N stage of the disease at the time of diagnosis[67]. In the absence of distant metastasis (M), EUS is the most accurate imaging modality for loco-regional staging (T and N stages) of rectal tumors[68]. Stage I disease includes early rectal lesions (T1-T2 N0 M0) (Table 3). While T1 lesions can benefit from endoscopic mucosal resection or transanal endoscopic microsurgery, T2 lesions need surgery[69,70]. Stage II disease with locally advanced cancer (T3-T4 N0 M0), or stage III with lymph node metastasis (T1-4 N1-2 M0) will benefit maximally and improve recurrence-free survival when neoadjuvant radio-chemotherapy is given[71-74]. Preoperative biopsies of rectal tumors may fail to diagnose an invasive carcinoma, with up to 24% false negative results. The preoperative use of EUS reduces the rate of missed carcinomas from 21% to 3%[75]. EUS compared to other imaging modalities (CT, PET/CT, MRI) is superior and more accurate in determining T stage (EUS: 87%, CT: 76% and MRI: 77%)[70,76-77]. In N stage situations, it is also superior, but the difference is less obvious and accuracy varies between studies (EUS 63%-85%, CT 56%-79% and MRI 57%-85%)[78-82]. Usually CT and PET/CT are used for distant metastasis diagnosis[82]. It is also reported that when CT was the original mode of investigation but a further EUS was done, in 31% of the cases the mode of treatment was changed because of the result[70]. The combination of CT and EUS seems to be the most cost-effective diagnostic strategy[83]. MRI has less accuracy in the T stage than EUS does, but provides a good definition of the circumferential resection margin (CRM). While EUS is more useful for staging early RC, MRI is indicated for staging advanced disease and defines CRM. Also, it can be used in the case of stenotic tumors, when EUS is less accurate. Thus, EUS and MRI are complementary and should be both used for preoperative staging[81,84].
| Primary tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ: Intraepithelial or invasion of lamina propria |
| T1 | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades through the muscularis propria into pericolorectal tissues |
| T4a | Tumor penetrates to the surface of the visceral peritoneum |
| T4b | Tumor directly invades or is adherent to other organs or structures |
| Regional lymph nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in 1-3 regional lymph nodes |
| N1a | Metastasis in 1 regional lymph node |
| N1b | Metastasis in 2-3 regional lymph nodes |
| N1c | Tumor deposit(s) in the subserosa, mesentery, or non-peritonealized pericolic or perirectal tissues without regional nodal metastasis |
| N2 | Metastasis in 4 or more regional lymph nodes |
| N2a | Metastasis in 4-6 regional lymph nodes |
| N2b | Metastasis in 7 or more regional lymph nodes |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Metastasis confined to one organ or site (for example, liver, lung, ovary, nonregional node) |
| M1b | Metastases in more than one organ/site or the peritoneum |
RC recurrence rates range from 20% to 50%, depending on how advanced the cancer is and if neoadjuvant therapy has been administered before surgery[85,86]. It has been proven that there is a significant reduction in tumor recurrence when patients undergo EUS staging compared to those who do not[87]. In addition to this, EUS can be used to evaluate the colorectal anastomosis during follow-up of patients operated for RC and confirm or rule out recurrence with 97% sensitivity, 100% specificity, 100% positive predictive value (PPV), 94% negative predictive value (NPV), and an overall accuracy of 98%[88,89]. One limitation that has been attributed to EUS is its difficulty in differentiating between post-operative benign lesions and recurring cancer in postoperative lesions. However, the use of EUS-guided FNA increases the specificity from 57% to 97%[85,86]. Thus, EUS has a key role in both preoperative staging and follow-up after surgery.
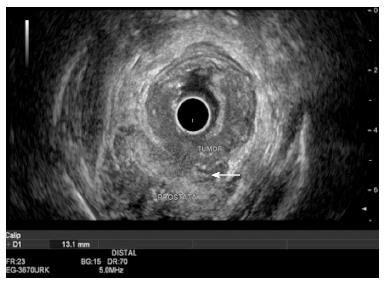
Over- or under-staging leads to changes in a patient’s treatment. Surgery instead of endoscopic resection and the use of chemoradiotherapy could be wrongly indicated when there is over-staging. On the other hand, under-staging with the lack of neoadjuvant indication could lead to an insufficient treatment. According to a recent review performed by Marone et al[90] (33 articles, n = 4976), EUS assesses the tumor penetration depth into the rectal wall with an overall accuracy for T stage of about 84%, ranging from 63% to 96%, while the reported accuracy of CT and MRI are 65%-75% and 75%-85%, respectively. They showed also that EUS accuracy for T stage is strictly related to the depth of infiltration, being lower for T2 stage than for early (T1) or advanced (T3-4) RC (T1: 88%, T2: 78.4%, T3: 85.4% and T4: 80.2%)[90]. Similarly, a meta-analysis (42 studies, n = 5039 patients) showed that EUS has an overall RC staging sensitivity of 81%-96% and specificity of 91%-98%, showing higher sensitivity for advanced RC (95%) than early cancer (88%). The pooled sensitivity and specificity by stage was for T1: 88% and 98%, T2: 81% and 96%, T3: 96% and 91% and T4: 95% and 98%, respectively. The authors concluded that EUS should be the imaging method of choice for the T staging of RC[91] (Figure 3). Superficial RC limited to the mucosa can be resected endoscopically. EUS has a high accuracy rate in differentiating T1 from T2 lesions, ranging from 81% to 95%, with an overstaging or understaging rate of 9%[92]. Puli et al[93] evaluated, in a meta-analysis (11 studies, n = 1791), the efficacy of preoperative EUS in staging patients with RC confined to the mucosa (T0) and found that sensitivity was 97% and specificity 96%. They concluded that EUS should be strongly considered for staging of early RCs[93].
EUS role in the determination of lymph node (LN) metastasis is less precise than T staging, with a mean accuracy of 74% (range 63%-85%)[90]. However, the accuracy is still better than others imaging modalities like CT (56%-79%) or MRI (57%-85%)[78-82]. Similarly, a meta-analysis including 35 articles showed that EUS has a sensitivity of 73% and specificity of 76% for N staging. This low EUS performance is related to the difficulty in evaluating distant metastatic LN that are out of EUS scanning, discriminating between inflammatory and metastatic LN and the tendency to overlook small metastatic LN compared to larger LN[94-98]. The presence of all malignant features (enlarged node ≥ 1 cm, hypoechoic appearance, round shape, and smooth border) is related to 100% of PPV for malignancy, however this situation is seen in less than 25% of cases[21]. It is known that there is a correlation between T stage and risk of LN involvement in patients with RC. The risk varies from 6%-11% for T1, 10%-35% for T2 and 26%-65% for T3-T4 RC[99]. Similarly, the EUS accuracy for N staging also depends on T staging and seems to be better for advanced disease (84% in T3 compared to 48% in T1). This is explained by the fact that in T1 lesions metastatic nodes are possibly small[98]. On the other hand, beside EUS limitations in N staging, EUS guided FNA can be used to balance and improve the accuracy from 75% to 87%[100]. EUS-FNA has a sensitivity, specificity, PPV and NPV of 89%, 79%, 89% and 79% respectively[97,101]. The fact that EUS-FNA has a moderate NPV (77%) for N staging means that LN metastases cannot be ruled out by a negative FNA[102]. Even though most perirectal nodes detected by EUS in patients with RC are metastatic, it is important to confirm this. EUS-FNA should be indicated when results change the therapeutic strategy. The presence or absence of LN metastasis in T1-T2 lesions change the stage of the patient from I to III and indicates the chemoradiotherapy strategy. EUS-FNA changes patient management in 19% of the cases[70,103].
EUS performance is operator-dependent and accuracy improves with experience. This fact explains the wide range of overall accuracy for T and N staging between studies (63% to 95%)[104,105]. A high inter-observer variability (61%-77%) has been described according to the experience of the operator, with overstaging values of 19% and understaging of 12%[104]. Also, EUS seems to be less accurate in restaging RC after neoadjuvant therapy (NAT), due to the limitations in differentiating inflammation, edema, necrosis and fibrosis from neoplastic infiltration, with the risk of overstaging and overtreatment[68,106,107]. EUS correctly predicts complete response to chemoradiation in 50%-63% of the cases. It has an overall accuracy for T stage of 48%, with 38% of overstaging and 14% of understaging[108,109]. Another limitation is that in 14% of RC there is a stricture that cannot be traversed by the echoendoscope, leading to an inaccurate T and N staging. The presence of a stricture decreases the EUS accuracy rate for T stage from 93% to 56%. When the T stages were analyzed separately, the accuracy was 76% for T1, 72% for T2, 91% for T3 and 67% for T4 stage. Moreover, there was an 11% of over-staging and 5% of under-staging errors[110]. Ultrasound catheter probes can be used to compensate this limitation. A meta-analysis (10 studies, n = 642) showed a high performance using ultrasound catheter probes for T and N staging. The pooled sensitivity and specificity were for T1: 91% and 98%, T2: 78% and 94%, T3-T4: 97% and 90%, respectively. The sensitivity and specificity for N staging were 63% and 82%, respectively[111]. Finally, the circumferential resection margin (CRM) is an important factor in predicting local recurrence. MRI has been described to have a better overall accuracy compared to EUS (92% vs 84%) with similar NPV (97%), especially in mid-rectum[112]. However, in low RC the accuracy in both modalities is similar (87%) with a NPV of 96%[113].
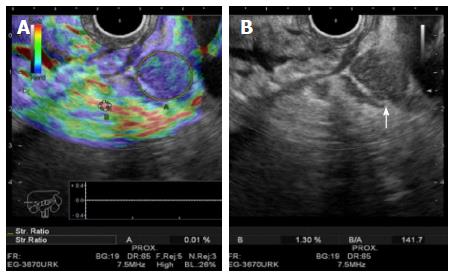
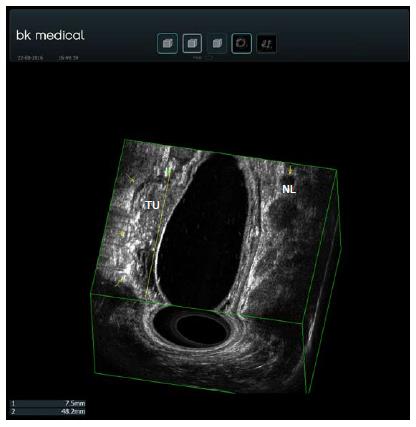
EUS elastography is a software application that can analyze the elastic properties of tissues (Figure 4). Harder tissue (usually malignant) appears blue which allows one to distinguish between adenocarcinomas and adenomas with high accuracy (94%)[114]. It seems that EUS elastography is better in RC staging than EUS alone especially for early cancers[115]. Contrast enhanced ultrasonography (CE-US) can be used to evaluate tumor vascularity and response to antiangiogenic treatment[116] (Figure 5). Computed parameters can be used to quantify tumor angiogenesis and measure vascularity changes after therapy[117]. Finally, 3D-EUS development allows spatial display of rectal and perirectal anatomy[112] (Figure 6). It improves accuracy for both T and N staging, better than EUS alone, especially in the middle third of the rectum[118]. Published data shows that its accuracy for N stage improves from 65% to 85% and for T stage is 97.1% for T1, 94.3% for T2, 95.7% for T3 and 98.5% for T4[119-121].
Despite improvements in EUS technology that allows a forward viewing, the EUS examination of the colon has proved to be less accurate for T and N staging (81% and 52.4% respectively)[122]. This decrease is due to the difficulty in evaluating the proximal colon segments and bowel movement[123]. Mini-probe EUS can be passed through the working channel of regular colonoscopes and can be used to evaluate lesions of the entire colon compensating for some of these limitations[124].
EUS is useful for assessing the involvement of anal sphincters in low rectal tumors and in the staging of anal squamous-cell carcinomas. Treatment decisions in anal cancer depends on sphincter invasion and EUS has an accuracy of 96%, sensitivity of 100%, specificity of 87% and NPV of 100% in evaluating it[125,126]. Clinical staging of anal cancer tends to under-diagnose sphincter invasion[127-129]. Most clinically classified T1-T2 patients will have T3 lesions under EUS evaluation[129]. Giovannini et al[130] confirm this in a prospective multicenter study and recommend that in T1-T2 N0 tumors, a transrectal EUS should be performed. EUS can be used also to determine multimodality therapy response[131]. A greater proportion of T1-T2 N0 lesions classified by EUS had a complete response to treatment than those classified by conventional clinical staging (94.5% vs 80%, respectively)[130]. The use of 3D-EUS in anal carcinoma seems to add some benefits in perirectal lymph node and tumor invasion detection, when compared to standard EUS, but further studies are needed[132].
Prognosis of patients with gastrointestinal cancer is strictly related to the stage of the disease at the time of diagnosis. Therefore, an accurate staging is crucial to decide the best treatment in each patient, because of the possibility of under-staging or over-staging, with subsequent mistreatments. CT scan, MRI, PET are the imaging methods that can give better information on distant disease. EUS has proven to be essential for loco-regional staging in pre-surgical evaluation. It reduces the number of unnecessary surgeries, reduces local recurrences, improves survival outcomes and guides physicians in the development of the most appropriate therapeutic strategy. It has excellent sensitivity and specificity in accurately diagnosing T and N cancer stages. FNA substantially improves EUS outcomes by enabling tissue sampling, especially for N staging. New technologies, like elastography, contrast-enhancement EUS, high-frequency probes and 3D technology are also improving EUS accuracy. On the other hand, physicians should be warned that EUS has some limitations. EUS has low accuracy in restaging RC after treatment due to the difficulty in differentiating inflammation and tissue fibrosis from residual cancer. There is also some heterogeneity in the evidence currently available about EUS results in diagnosing superficial tumors (T1a) and LN in some situations.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Ecuador
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Altonbary AY, Imagawa A, Samiullah S, Slomiany BL S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | DiMagno EP, Buxton JL, Regan PT, Hattery RR, Wilson DA, Suarez JR, Green PS. Ultrasonic endoscope. Lancet. 1980;1:629-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 208] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 2. | Sreenarasimhaiah J. The emerging role of endoscopic ultrasonography in cancer staging. Am J Med Sci. 2005;329:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Pungpapong S, Noh KW, Wallace MB. Endoscopic ultrasonography in the diagnosis and management of cancer. Expert Rev Mol Diagn. 2005;5:585-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Pfau PR, Chak A. Endoscopic ultrasonography. Endoscopy. 2002;34:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Annema JT, Versteegh MI, Veseliç M, Welker L, Mauad T, Sont JK, Willems LN, Rabe KF. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA. 2005;294:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Gan SI, Rajan E, Adler DG, Baron TH, Anderson MA, Cash BD, Davila RE, Dominitz JA, Harrison ME, Ikenberry SO. Role of EUS. Gastrointest Endosc. 2007;66:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Luo LN, He LJ, Gao XY, Huang XX, Shan HB, Luo GY, Li Y, Lin SY, Wang GB, Zhang R. Endoscopic Ultrasound for Preoperative Esophageal Squamous Cell Carcinoma: a Meta-Analysis. PLoS One. 2016;11:e0158373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Harewood GC, Kumar KS. Assessment of clinical impact of endoscopic ultrasound on esophageal cancer. J Gastroenterol Hepatol. 2004;19:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6465] [Article Influence: 431.0] [Reference Citation Analysis (0)] |
| 10. | Sgourakis G, Gockel I, Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol. 2013;19:1424-1437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Evans JA, Early DS, Chandraskhara V, Chathadi KV, Fanelli RD, Fisher DA, Foley KQ, Hwang JH, Jue TL, Pasha SF. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc. 2013;77:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Altorki NK, Lee PC, Liss Y, Meherally D, Korst RJ, Christos P, Mazumdar M, Port JL. Multifocal neoplasia and nodal metastases in T1 esophageal carcinoma: implications for endoscopic treatment. Ann Surg. 2008;247:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Oh YS, Early DS, Azar RR. Clinical applications of endoscopic ultrasound to oncology. Oncology. 2005;68:526-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Pfau PR, Perlman SB, Stanko P, Frick TJ, Gopal DV, Said A, Zhang Z, Weigel T. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc. 2007;65:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Dyer SM, Levison DB, Chen RY, Lord SJ, Blamey S. Systematic review of the impact of endoscopic ultrasound on the management of patients with esophageal cancer. Int J Technol Assess Health Care. 2008;24:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 17. | van Westreenen HL, Heeren PA, van Dullemen HM, van der Jagt EJ, Jager PL, Groen H, Plukker JT. Positron emission tomography with F-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J Gastrointest Surg. 2005;9:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 243] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (2)] |
| 19. | Thosani N, Singh H, Kapadia A, Ochi N, Lee JH, Ajani J, Swisher SG, Hofstetter WL, Guha S, Bhutani MS. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Vazquez-Sequeiros E, Wiersema MJ, Clain JE, Norton ID, Levy MJ, Romero Y, Salomao D, Dierkhising R, Zinsmeister AR. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology. 2003;125:1626-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Catalano MF, Sivak MV, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Kelly S, Harris KM, Berry E, Hutton J, Roderick P, Cullingworth J, Gathercole L, Smith MA. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 244] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: a prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am J Gastroenterol. 2004;99:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Eloubeidi MA, Wallace MB, Reed CE, Hadzijahic N, Lewin DN, Van Velse A, Leveen MB, Etemad B, Matsuda K, Patel RS. The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: a single-center experience. Gastrointest Endosc. 2001;54:714-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Marsman WA, van Wissen M, Bergman JJ, van Lanschot JJ, Obertop H, Tytgat GN, Fockens P. Outcome of patients with esophageal carcinoma and suspicious celiac lymph nodes as determined by endoscopic ultrasonography. Endoscopy. 2004;36:961-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Fernández-Sordo JO, Konda VJ, Chennat J, Madrigal-Hoyos E, Posner MC, Ferguson MK, Waxman I. Is Endoscopic Ultrasound (EUS) necessary in the pre-therapeutic assessment of Barrett’s esophagus with early neoplasia? J Gastrointest Oncol. 2012;3:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 27. | Menzel J, Hoepffner N, Nottberg H, Schulz C, Senninger N, Domschke W. Preoperative staging of esophageal carcinoma: miniprobe sonography versus conventional endoscopic ultrasound in a prospective histopathologically verified study. Endoscopy. 1999;31:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Murata Y, Suzuki S, Ohta M, Mitsunaga A, Hayashi K, Yoshida K, Ide H. Small ultrasonic probes for determination of the depth of superficial esophageal cancer. Gastrointest Endosc. 1996;44:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Isenberg G, Chak A, Canto MI, Levitan N, Clayman J, Pollack BJ, Sivak MV. Endoscopic ultrasound in restaging of esophageal cancer after neoadjuvant chemoradiation. Gastrointest Endosc. 1998;48:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 103] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Bang JY, Ramesh J, Hasan M, Navaneethan U, Holt BA, Hawes R, Varadarajulu S. Endoscopic ultrasonography is not required for staging malignant esophageal strictures that preclude the passage of a diagnostic gastroscope. Dig Endosc. 2016;28:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Qumseya BJ, Brown J, Abraham M, White D, Wolfsen H, Gupta N, Vennalaganti P, Sharma P, Wallace MB. Diagnostic performance of EUS in predicting advanced cancer among patients with Barrett’s esophagus and high-grade dysplasia/early adenocarcinoma: systematic review and meta-analysis. Gastrointest Endosc. 2015;81:865-874.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1060] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 33. | Bergeron EJ, Lin J, Chang AC, Orringer MB, Reddy RM. Endoscopic ultrasound is inadequate to determine which T1/T2 esophageal tumors are candidates for endoluminal therapies. J Thorac Cardiovasc Surg. 2014;147:765-771: Discussion 771-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Bulsiewicz WJ, Dellon ES, Rogers AJ, Pasricha S, Madanick RD, Grimm IS, Shaheen NJ. The impact of endoscopic ultrasound findings on clinical decision making in Barrett’s esophagus with high-grade dysplasia or early esophageal adenocarcinoma. Dis Esophagus. 2014;27:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Pouw RE, Heldoorn N, Alvarez Herrero L, ten Kate FJ, Visser M, Busch OR, van Berge Henegouwen MI, Krishnadath KK, Weusten BL, Fockens P. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest Endosc. 2011;73:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Sepesi B, Watson TJ, Zhou D, Polomsky M, Litle VR, Jones CE, Raymond DP, Hu R, Qiu X, Peters JH. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg. 2010;210:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Hölscher AH. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann Surg. 2011;254:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 38. | Leers JM, DeMeester SR, Oezcelik A, Klipfel N, Ayazi S, Abate E, Zehetner J, Lipham JC, Chan L, Hagen JA. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 39. | Nentwich MF, von Loga K, Reeh M, Uzunoglu FG, Marx A, Izbicki JR, Bogoevski D. Depth of submucosal tumor infiltration and its relevance in lymphatic metastasis formation for T1b squamous cell and adenocarcinomas of the esophagus. J Gastrointest Surg. 2014;18:242-249; discussion 249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11836] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 41. | Hirasawa K, Kokawa A, Oka H, Yahara S, Sasaki T, Nozawa A, Morimoto M, Numata K, Taguri M, Morita S. Risk assessment chart for curability of early gastric cancer with endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Kang KJ, Kim KM, Min BH, Lee JH, Kim JJ. Endoscopic submucosal dissection of early gastric cancer. Gut Liver. 2011;5:418-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 44. | Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;CD004064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 350] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 45. | Fairweather M, Jajoo K, Sainani N, Bertagnolli MM, Wang J. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol. 2015;111:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Kim JW, Shin SS, Heo SH, Lim HS, Lim NY, Park YK, Jeong YY, Kang HK. The role of three-dimensional multidetector CT gastrography in the preoperative imaging of stomach cancer: emphasis on detection and localization of the tumor. Korean J Radiol. 2015;16:80-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Hur J, Park MS, Lee JH, Lim JS, Yu JS, Hong YJ, Kim KW. Diagnostic accuracy of multidetector row computed tomography in T- and N staging of gastric cancer with histopathologic correlation. J Comput Assist Tomogr. 2006;30:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Seevaratnam R, Cardoso R, McGregor C, Lourenco L, Mahar A, Sutradhar R, Law C, Paszat L, Coburn N. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer. 2012;15 Suppl 1:S3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 49. | Kim EY, Lee WJ, Choi D, Lee SJ, Choi JY, Kim BT, Kim HS. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol. 2011;79:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Ha TK, Choi YY, Song SY, Kwon SJ. F18-fluorodeoxyglucose-positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer. J Korean Surg Soc. 2011;81:104-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Hargunani R, Maclachlan J, Kaniyur S, Power N, Pereira SP, Malhotra A. Cross-sectional imaging of gastric neoplasia. Clin Radiol. 2009;64:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Polkowski M. Endosonographic staging of upper intestinal malignancy. Best Pract Res Clin Gastroenterol. 2009;23:649-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Sandhu IS, Bhutani MS. Gastrointestinal endoscopic ultrasonography. Med Clin North Am. 2002;86:1289-1317, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;CD009944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 55. | Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc. 2011;73:1122-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 56. | Cardoso R, Coburn N, Seevaratnam R, Sutradhar R, Lourenco LG, Mahar A, Law C, Yong E, Tinmouth J. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S19-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 57. | Puli SR, Batapati Krishna Reddy J, Bechtold ML, Antillon MR, Ibdah JA. How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:4011-4019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Kwee RM, Kwee TC. The accuracy of endoscopic ultrasonography in differentiating mucosal from deeper gastric cancer. Am J Gastroenterol. 2008;103:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 60. | Kim JH, Song KS, Youn YH, Lee YC, Cheon JH, Song SY, Chung JB. Clinicopathologic factors influence accurate endosonographic assessment for early gastric cancer. Gastrointest Endosc. 2007;66:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 61. | Yoshimoto K. Clinical application of ultrasound 3 D imaging system in lesions of the gastrointestinal tract. Endoscopy. 1998;30 Suppl 1:A145-A148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol. 2003;98:975-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Palazzo L, Roseau G, Ruskone-Fourmestraux A, Rougier P, Chaussade S, Rambaud JC, Couturier D, Paolaggi JA. Endoscopic ultrasonography in the local staging of primary gastric lymphoma. Endoscopy. 1993;25:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Caletti G, Fusaroli P, Togliani T. EUS in MALT lymphoma. Gastrointest Endosc. 2002;56:S21-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, Wang KK, Clain JE, Wiersema MJ. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Sackmann M, Morgner A, Rudolph B, Neubauer A, Thiede C, Schulz H, Kraemer W, Boersch G, Rohde P, Seifert E. Regression of gastric MALT lymphoma after eradication of Helicobacter pylori is predicted by endosonographic staging. MALT Lymphoma Study Group. Gastroenterology. 1997;113:1087-1090. [PubMed] |
| 67. | Avallone A, Aloj L, Delrio P, Pecori B, Leone A, Tatangelo F, Perri F, Petrillo A, Scott N, Budillon A. Multidisciplinary approach to rectal cancer: are we ready for selective treatment strategies? Anticancer Agents Med Chem. 2013;13:852-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Cârtână ET, Gheonea DI, Săftoiu A. Advances in endoscopic ultrasound imaging of colorectal diseases. World J Gastroenterol. 2016;22:1756-1766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Lee P, Oyama K, Homer L, Sullivan E. Effects of endorectal ultrasonography in the surgical management of rectal adenomas and carcinomas. Am J Surg. 1999;177:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Harewood GC, Wiersema MJ, Nelson H, Maccarty RL, Olson JE, Clain JE, Ahlquist DA, Jondal ML. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1818] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 72. | Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Medical Research Council Rectal Cancer Working Party. Lancet. 1996;348:1605-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 242] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | Grann A, Feng C, Wong D, Saltz L, Paty PP, Guillem JG, Cohen AM, Minsky BD. Preoperative combined modality therapy for clinically resectable uT3 rectal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2001;49:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3120] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 75. | Worrell S, Horvath K, Blakemore T, Flum D. Endorectal ultrasound detection of focal carcinoma within rectal adenomas. Am J Surg. 2004;187:625-629; discussion 629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Savides TJ, Master SS. EUS in rectal cancer. Gastrointest Endosc. 2002;56:S12-S18. [PubMed] [DOI] [Full Text] |
| 77. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 721] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 78. | Fuchsjäger MH, Maier AG, Schima W, Zebedin E, Herbst F, Mittlböck M, Wrba F, Lechner GL. Comparison of transrectal sonography and double-contrast MR imaging when staging rectal cancer. AJR Am J Roentgenol. 2003;181:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Akasu T, Kondo H, Moriya Y, Sugihara K, Gotoda T, Fujita S, Muto T, Kakizoe T. Endorectal ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Ferri M, Laghi A, Mingazzini P, Iafrate F, Meli L, Ricci F, Passariello R, Ziparo V. Pre-operative assessment of extramural invasion and sphincteral involvement in rectal cancer by magnetic resonance imaging with phased-array coil. Colorectal Dis. 2005;7:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Skandarajah AR, Tjandra JJ. Preoperative loco-regional imaging in rectal cancer. ANZ J Surg. 2006;76:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Samee A, Selvasekar CR. Current trends in staging rectal cancer. World J Gastroenterol. 2011;17:828-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 83. | Harewood GC, Wiersema MJ. Cost-effectiveness of endoscopic ultrasonography in the evaluation of proximal rectal cancer. Am J Gastroenterol. 2002;97:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Ho ML, Liu J, Narra V. Magnetic resonance imaging of rectal cancer. Clin Colon Rectal Surg. 2008;21:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Hünerbein M, Totkas S, Moesta KT, Ulmer C, Handke T, Schlag PM. The role of transrectal ultrasound-guided biopsy in the postoperative follow-up of patients with rectal cancer. Surgery. 2001;129:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Löhnert MS, Doniec JM, Henne-Bruns D. Effectiveness of endoluminal sonography in the identification of occult local rectal cancer recurrences. Dis Colon Rectum. 2000;43:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 87. | Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, Heresbach D, Pujol B, Fernández-Esparrach G, Vazquez-Sequeiros E. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 89. | Fernández-Esparrach G, Alberghina N, Subtil JC, Vázquez-Sequeiros E, Florio V, Zozaya F, Araujo I, Ginès A. Endoscopic ultrasound-guided fine needle aspiration is highly accurate for the diagnosis of perirectal recurrence of colorectal cancer. Dis Colon Rectum. 2015;58:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Marone P, de Bellis M, D’Angelo V, Delrio P, Passananti V, Di Girolamo E, Rossi GB, Rega D, Tracey MC, Tempesta AM. Role of endoscopic ultrasonography in the loco-regional staging of patients with rectal cancer. World J Gastrointest Endosc. 2015;7:688-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 91. | Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 92. | Marone P, Petrulio F, de Bellis M, Battista Rossi G, Tempesta A. Role of endoscopic ultrasonography in the staging of rectal cancer: a retrospective study of 63 patients. J Clin Gastroenterol. 2000;30:420-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR. Can endoscopic ultrasound predict early rectal cancers that can be resected endoscopically? A meta-analysis and systematic review. Dig Dis Sci. 2010;55:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 224] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 95. | Gleeson FC, Clain JE, Papachristou GI, Rajan E, Topazian MD, Wang KK, Levy MJ. Prospective assessment of EUS criteria for lymphadenopathy associated with rectal cancer. Gastrointest Endosc. 2009;69:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 96. | Krajewski KM, Kane RA. Ultrasound staging of rectal cancer. Semin Ultrasound CT MR. 2008;29:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Wiersema MJ, Harewood GC. Endoscopic ultrasound for rectal cancer. Gastroenterol Clin North Am. 2002;31:1093-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Landmann RG, Wong WD, Hoepfl J, Shia J, Guillem JG, Temple LK, Paty PB, Weiser MR. Limitations of early rectal cancer nodal staging may explain failure after local excision. Dis Colon Rectum. 2007;50:1520-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Puli SR, Reddy JB, Bechtold ML, Choudhary A, Antillon MR, Brugge WR. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:1255-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 100. | Park HH, Nguyen PT, Tran Q, Chang KJ. Endoscopic ultrasound-guided fine needle aspiration in the staging of rectal cancer [abstract]. Gastrointest Endosc. 2000;51:AB171. [DOI] [Full Text] |
| 101. | Knight CS, Eloubeidi MA, Crowe R, Jhala NC, Jhala DN, Chhieng DC, Eltoum IA. Utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of colorectal carcinoma. Diagn Cytopathol. 2013;41:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Maleki Z, Erozan Y, Geddes S, Li QK. Endorectal ultrasound-guided fine-needle aspiration: a useful diagnostic tool for perirectal and intraluminal lesions. Acta Cytol. 2013;57:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Shami VM, Parmar KS, Waxman I. Clinical impact of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in the management of rectal carcinoma. Dis Colon Rectum. 2004;47:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Kauer WK, Prantl L, Dittler HJ, Siewert JR. The value of endosonographic rectal carcinoma staging in routine diagnostics: a 10-year analysis. Surg Endosc. 2004;18:1075-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Marusch F, Koch A, Schmidt U, Zippel R, Kuhn R, Wolff S, Pross M, Wierth A, Gastinger I, Lippert H. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 106. | Maier AG, Barton PP, Neuhold NR, Herbst F, Teleky BK, Lechner GL. Peritumoral tissue reaction at transrectal US as a possible cause of overstaging in rectal cancer: histopathologic correlation. Radiology. 1997;203:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Schizas AM, Williams AB, Meenan J. Endosonographic staging of lower intestinal malignancy. Best Pract Res Clin Gastroenterol. 2009;23:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 108. | Marone P, de Bellis M, Avallone A, Delrio P, di Nardo G, D’Angelo V, Tatangelo F, Pecori B, Di Girolamo E, Iaffaioli V. Accuracy of endoscopic ultrasound in staging and restaging patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Clin Res Hepatol Gastroenterol. 2011;35:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | Maor Y, Nadler M, Barshack I, Zmora O, Koller M, Kundel Y, Fidder H, Bar-Meir S, Avidan B. Endoscopic ultrasound staging of rectal cancer: diagnostic value before and following chemoradiation. J Gastroenterol Hepatol. 2006;21:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 110. | Vanagunas A, Lin DE, Stryker SJ. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am J Gastroenterol. 2004;99:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 111. | Gall TM, Markar SR, Jackson D, Haji A, Faiz O. Mini-probe ultrasonography for the staging of colon cancer: a systematic review and meta-analysis. Colorectal Dis. 2014;16:O1-O8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 112. | Kim MJ. Transrectal ultrasonography of anorectal diseases: advantages and disadvantages. Ultrasonography. 2015;34:19-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 113. | Granero-Castro P, Muñoz E, Frasson M, García-Granero A, Esclapez P, Campos S, Flor-Lorente B, Garcia-Granero E. Evaluation of mesorectal fascia in mid and low anterior rectal cancer using endorectal ultrasound is feasible and reliable: a comparison with MRI findings. Dis Colon Rectum. 2014;57:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 114. | Waage JE, Havre RF, Odegaard S, Leh S, Eide GE, Baatrup G. Endorectal elastography in the evaluation of rectal tumours. Colorectal Dis. 2011;13:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 115. | Waage JE, Leh S, Røsler C, Pfeffer F, Bach SP, Havre RF, Haldorsen IS, Ødegaard S, Baatrup G. Endorectal ultrasonography, strain elastography and MRI differentiation of rectal adenomas and adenocarcinomas. Colorectal Dis. 2015;17:124-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 116. | Tranquart F, Mercier L, Frinking P, Gaud E, Arditi M. Perfusion quantification in contrast-enhanced ultrasound (CEUS)--ready for research projects and routine clinical use. Ultraschall Med. 2012;33 Suppl 1:S31-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Zhuang H, Yang ZG, Chen HJ, Peng YL, Li L. Time-intensity curve parameters in colorectal tumours measured using double contrast-enhanced ultrasound: correlations with tumour angiogenesis. Colorectal Dis. 2012;14:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 118. | Kim JC, Kim HC, Yu CS, Han KR, Kim JR, Lee KH, Jang SJ, Lee SS, Ha HK. Efficacy of 3-dimensional endorectal ultrasonography compared with conventional ultrasonography and computed tomography in preoperative rectal cancer staging. Am J Surg. 2006;192:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 119. | Kolev NY, Tonev AY, Ignatov VL, Zlatarov AK, Bojkov VM, Kirilova TD, Encheva E, Ivanov K. The role of 3-D endorectal ultrasound in rectal cancer: our experience. Int Surg. 2014;99:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 120. | Vyslouzil K, Cwiertka K, Zboril P, Kucerova L, Starý L, Klementa I, Skalický P, Duda M. Endorectal sonography in rectal cancer staging and indication for local surgery. Hepatogastroenterology. 2007;54:1102-1106. [PubMed] |
| 121. | Kim JC, Cho YK, Kim SY, Park SK, Lee MG. Comparative study of three-dimensional and conventional endorectal ultrasonography used in rectal cancer staging. Surg Endosc. 2002;16:1280-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 122. | Kongkam P, Linlawan S, Aniwan S, Lakananurak N, Khemnark S, Sahakitrungruang C, Pattanaarun J, Khomvilai S, Wisedopas N, Ridtitid W. Forward-viewing radial-array echoendoscope for staging of colon cancer beyond the rectum. World J Gastroenterol. 2014;20:2681-2687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 123. | Bhutani MS, Nadella P. Utility of an upper echoendoscope for endoscopic ultrasonography of malignant and benign conditions of the sigmoid/left colon and the rectum. Am J Gastroenterol. 2001;96:3318-3322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 124. | Urban O, Kliment M, Fojtik P, Falt P, Orhalmi J, Vitek P, Holeczy P. High-frequency ultrasound probe sonography staging for colorectal neoplasia with superficial morphology: its utility and impact on patient management. Surg Endosc. 2011;25:3393-3399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 125. | Assenat E, Thézenas S, Samalin E, Bibeau F, Portales F, Azria D, Quenet F, Rouanet P, Saint Aubert B, Senesse P. The value of endoscopic rectal ultrasound in predicting the lateral clearance and outcome in patients with lower-third rectal adenocarcinoma. Endoscopy. 2007;39:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 126. | Senesse P, Khemissa F, Lemanski C, Masson B, Quenet F, Saint-Aubert B, Simony J, Ychou M, Dubois JB, Rouanet P. Contribution of endorectal ultrasonography in preoperative evaluation for very low rectal cancer. Gastroenterol Clin Biol. 2001;25:24-28. [PubMed] |
| 127. | Giovannini M, Seitz JF, Houvenaeghel G, Delpero JR, Rosello R, Gauthier A. Intrarectal and intravaginal echography in the evaluation of the extension and the monitoring of cancer of the anal canal. Presse Med. 1989;18:1439-1440. [PubMed] |
| 128. | Giovannini M, Seitz JF, Rosello R, Houvenaeghel G, Delpero JR, Gauthier A. The value of endo-anorectal echography in the evaluation of the loco-regional extension and the monitoring of cancers of the anal canal. Ann Gastroenterol Hepatol (Paris). 1990;26:3-4. [PubMed] |
| 129. | Giovannini M, Seitz JF, Sfedj D, Houvenaeghel G, Delpero JR. Transanorectal ultrasonography in the evaluation of extension and the monitoring of epidermoid cancers of the anus treated by radiation or chemotherapy. Gastroenterol Clin Biol. 1992;16:994-998. [PubMed] |
| 130. | Giovannini M, Bardou VJ, Barclay R, Palazzo L, Roseau G, Helbert T, Burtin P, Bouché O, Pujol B, Favre O. Anal carcinoma: prognostic value of endorectal ultrasound (ERUS). Results of a prospective multicenter study. Endoscopy. 2001;33:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 131. | Tarantino D, Bernstein MA. Endoanal ultrasound in the staging and management of squamous-cell carcinoma of the anal canal: potential implications of a new ultrasound staging system. Dis Colon Rectum. 2002;45:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | Christensen AF, Nielsen MB, Engelholm SA, Roed H, Svendsen LB, Christensen H. Three-dimensional anal endosonography may improve staging of anal cancer compared with two-dimensional endosonography. Dis Colon Rectum. 2004;47:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |