Published online Mar 16, 2017. doi: 10.4253/wjge.v9.i3.139
Peer-review started: May 3, 2016
First decision: May 17, 2016
Revised: December 6, 2016
Accepted: December 27, 2016
Article in press: December 28, 2016
Published online: March 16, 2017
Processing time: 319 Days and 21.8 Hours
Langerhans cell histiocytosis (LCH) is a rare syndrome characterized by unifocal, multifocal unisystem, or disseminated/multi-system disease that commonly involves the bone, skin, lymph nodes, pituitary, or sometimes lung (almost exclusively in smokers) causing a variety of symptoms from rashes and bone lesions to diabetes insipidus or pulmonary infiltrates. We present a previously unreported case of gastrointestinal LCH as well as a novel characteristic lesion affecting the colon of a young woman who presented with signs and symptoms mimicking acute on chronic appendicitis. Immunohistochemical analysis of appendectomy specimen and nodular specimens on colonoscopy demonstrated S-100, CD1a, and langerin reactivity. The patient underwent systemic chemotherapy with cytarabine and demonstrated excellent response to therapy.
Core tip: Langerhans cell histiocytosis (LCH) of the adult gastrointestinal tract can affect the appendix and present as mild inflammatory findings on exploratory laparascopy, or white-yellowish nodularities and polyps on lower endoscopy. Immunohistochemical staining of surgical specimens for CD1a, S-100, and langerin should be considered in gastrointestinal lesions that demonstrate histology concerning for histiocytosis in an attempt to decrease morbidity related to undiagnosed LCH.
- Citation: Karimzada MM, Matthews MN, French SW, DeUgarte D, Kim DY. Langerhans cell histiocytosis masquerading as acute appendicitis: Case report and review. World J Gastrointest Endosc 2017; 9(3): 139-144
- URL: https://www.wjgnet.com/1948-5190/full/v9/i3/139.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i3.139
Langerhans cell histiocytosis (LCH) is a rare syndrome characterized by unifocal, multifocal unisystem, or disseminated/multi-system disease that commonly involves the bone, skin, lymph nodes, pituitary, and sometimes lungs (almost exclusively in smokers) causing a variety of symptoms from rashes and bone lesions to diabetes insipidus or pulmonary infiltrates. The liver, spleen, or bone marrow may also be involved and are considered “risk organs” that signify a less favorable prognosis[1,2]. The gastrointestinal tract is rarely involved, and when it is present, it usually affects pediatric patients with severe multisystem disease[3]. LCH typically affects children under 10 years of age with European ancestry. Peak incidence is under 2 years of age with a 2:1 predilection for males[1,2].
There are case reports of patients presenting up to the eight decade of life and some evidence to suggest a higher incidence in patients of Hispanic descent when compared to whites[1,3-5]. An incidence of 1 out of 200000 is commonly cited for the pediatric population compared to 1-2 per 1 million in the adult population. Diagnosis is typically confirmed by CD1a and S-100 reactivity on immunohistochemical staining of biopsied lesions and diagnosis is typically confirmed by the finding of Birbeck granules on electron microscopy or langerin reactivity on histology[1]. Treatment consists of supportive care, surgery, chemotherapy (especially in multisystem involvement), radiotherapy, and sometimes hormone replacement therapy in cases of pituitary dysfunction. Recent research supports the notion that LCH is more align with a neoplastic process rather than a reactive one, with a proportion of lesions presenting with BRAF or MAP2K1 mutations. Such mutations may be of some value for risk stratification and prognostication, and immunotherapy with BRAF inhibitors have shown some promise in treatment[1,6]. Five-year mortality runs up to 20% with a greater risk of mortality in younger patients[2-4].
A 20-year-old G1P1 Hispanic female in previously good health with a history of pyelonephritis during pregnancy and eczema presented with 3 d of sharp, intermittent right lower quadrant pain that radiates towards the midline with movement. She vomited non-bloody, non-bilious emesis three times the day before presentation after eating and presented to our emergency department after having acute appendicitis ruled out at an outside hospital three days prior. Her pain responds to ibuprofen. She denies fevers, chills, diarrhea, urinary urgency or hesitancy, vaginal discharge or bleeding, or trauma. She engages in monogamous unprotected sex with a male partner and endorses mild dysuria. She denies alcohol, tobacco, or other drug use. She experienced a similar episode of pain a couple months prior to presentation. Her only medications are intramuscular depo provera for birth control and ibuprofen for her abdominal pain. She is afebrile with vitals within normal limits and body mass index of 19. Abdominal examination revealed present bowel sounds, mild tenderness to the right of midline near the right lower quadrant without guarding, McBurney’s point tenderness or Murphy’s sign. The remainder of the exam, including a pelvic exam, was unremarkable. Abdominal and pelvic causes of pain including acute appendicitis, nephrolithiasis, pregnancy, tuboovarian abscess, ovarian torsion, small bowel obstruction, functional intestinal obstruction, urinary tract infection, sexually transmitted infection, muscle strain were considered. Transvaginal and limited abdominal ultrasound performed at the outside hospital demonstrated small bilateral follicular cysts and no evidence of appendicitis or other tuboovarian processes. Urine studies were negative for bacteriuria, leukocyte esterase, nitrite, and both gonococcal and chlamydial RNA. Urine cultures acquired at that time were eventually negative for growth. Urine beta-hCG was negative. Wet mount unremarkable. Abdominal X-ray was unremarkable with nonspecific bowel gas pattern. At this point her signs/symptoms and lab testing were unremarkable for acute pathology other than perhaps abdominal pain secondary to follicular cysts. She was discharged in ambulatory, stable condition with instructions to present to pediatric acute follow-up clinic in 2-3 d and pain control with ibuprofen.
The next day she presented to the pediatric acute follow up clinic with severe and constant right lower quadrant pain that was no longer responsive to ibuprofen, and is exacerbated with any ambulation or lifting her child. She is afebrile with normal vitals. Abdominal exam is remarkable for right lower quadrant tenderness to palpation, guarding, and Rovsing’s sign with no rebound tenderness. The patient also clarifies that her right lower quadrant pain has been intermittent and ongoing for the last four months. Hemoglobin (13.1 mg/dL), white blood count (7100/μL), platelets (260000/μL), fibrinogen (303 mg/dL) were within normal with a blood differential notable for peripheral eosinophilia (18% eosinophils with 1300/μL absolute count). Inflammatory markers C-reactive protein (< 0.02 mg/dL) and erythrocyte sedimentation rate (8 mm/h) were normal. Amylase (82 U/L) and lipase (24 U/L) were within normal limits and pelvic as well as right upper quadrant ultrasounds both unremarkable for acute pathology. CT scan of the abdomen and pelvis is unremarkable, particularly demonstrating an air-filled appendix within normal limits and no fat stranding. She is admitted to the pediatrics ward and a gynecology consult determined that a gynecological problem is unlikely. Pediatric surgery was consulted and a diagnostic laparoscopy found normal liver, intestines, uterus, bilateral ovaries and fallopian tubes. There were anomalous adhesions of the cecum to the anterior abdominal wall and the appendix was mildly injected with some area of thickening and induration suspicious for chronic appendicitis, and thus resected.
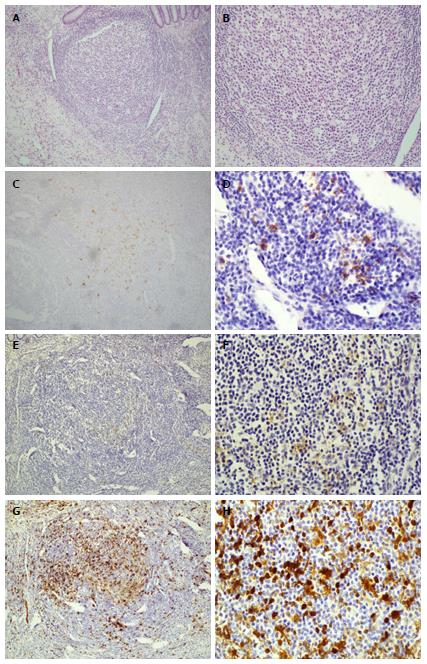
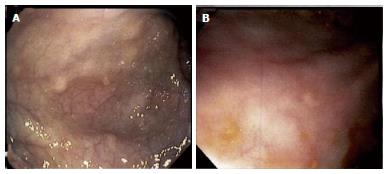
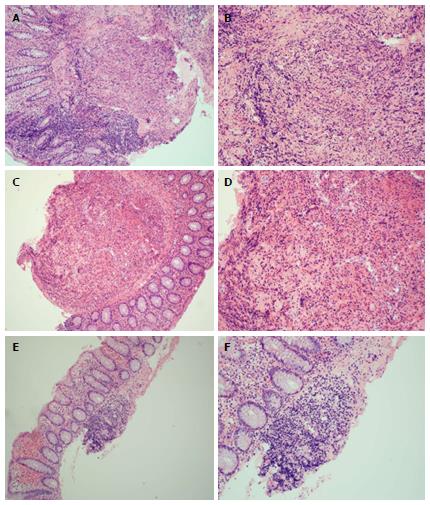
Her immediate post-op period was unremarkable and she was discharged with appropriate incisional tenderness on post-op day two. She presented to the emergency department on post-op day five complaining of right lower quadrant pain similar to that prior to surgery. She was readmitted and further imaging and workup including pelvic ultrasound as well as MRI of the brain and spine, and failed to show a cause of her pain. Her pain was managed with non-steroidal anti-inflammatory drugs, intravenous morphine, and Norco while in hospital. Surgical pathology of her appendix was grossly unremarkable, without ulceration or inflammation, yet demonstrated histiocytes in lymphoid aggregates on H and E stain and suspicious for LCH. This was confirmed with S-100, CD1a, and CD207 reactivity (Figure 1). Upper and lower endoscopy demonstrated whitish-yellowish polypoid nodules less than 5 mm in size at the luminal surface of the cecum, transverse, and descending colon (Figure 2). Microscopic evaluation demonstrated similar morphology to the appendix H and E, and immunohistochemical analysis demonstrated S-100, CD1a, CD207 (langerin) reactivity of these nodules (Figure 3) whereas biopsy samples of the esophagus, stomach, duodenum, and rectum failed to show histiocytosis. Bone marrow aspirates, bone scan, and MRI brain were negative for bone marrow, skeletal, and pituitary involvement, respectively.
Given the extensive involvement of her colon, she was diagnosed with unisystem, multifocal LCH that solely involves the colon. A port-a-cath was placed and the patient was started on cytarabine chemotherapy at 100 mg/m2 per day for 5 d. The patient was to undergo 12 cycles, each one month apart, over the course of 12 mo.
At the time of writing this report, the patient is status post six cycles of cytarabine with marked clinical improvement. Follow-up upper and lower endoscopies with biopsy after her third cycle of chemotherapy demonstrated decreased disease burden with residual foci of histiocytosis present in colon biopsies (particularly the ascending colon) and her abdominal pain completely resolved sometime after her fourth cycle of chemotherapy.
In general, gastrointestinal involvement is rare in both child and adult forms of LCH and there are few case reports in the literature documenting gastrointestinal disease in adult patients with LCH. Gastrointestinal involvement in childhood onset LCH typically occurs in systemic LCH and thus forewarns of a poor prognosis. In contrast, adult patients who are diagnosed with unisystem disease such as in this case are thought to have an excellent prognosis upwards of 90% survival at five years[6]. Adult patients who present with enteric-related signs and symptoms seem to have a predilection towards oral lesions such as palatal ulcers, and there is a small case series that demonstrates 12 patients with incidentally found LCH lesions on endoscopy that predominantly affected the colon (with 2 cases of cecal involvement) and described as polyps or ulcerative lesions[3,4,6,7]. There seem to be even fewer case reports of small bowel involvement[8,9]. There are none that report an acute presentation of right lower quadrant pain that presents with features similar to acute appendicitis[3,4,10].
The patient presented with a story concerning for acute on chronic appendicitis. Exploratory laparotomy was performed under this suspicion, and although the appendix was a focus of disease, the gross findings of mild inflammation could have possibly been dismissed as normal variation of anatomy. Additionally, she is a young Hispanic woman in contrast to the stereotypical infant/toddler male of European descent. It is worth mentioning that there is some evidence to suggest this notion may have to be dismissed in favor of one that denotes Hispanics and those who live in crowded counties or areas with a low education level may be at higher risk of LCH[1,3,5,11].
Only after tissue analysis demonstrated histological findings suspicious for LCH, was the diagnosis entertained, and further testing performed. It is typical for surgeons to routinely send appendectomy specimens for analysis, and some studies have stressed the importance of routine histological examination of appendectomy specimens in order to identify uncommon etiologies of appendiceal disease such as neoplasms or various infectious processes, however there is some evidence to suggest otherwise[3,4]. In this case, had a surgical specimen not been taken, the time to diagnosis would have likely been prolonged, leading to further patient discomfort, lack of appropriate treatment, and possible loss to follow up due to a combination of these two.
Her persistent right lower quadrant pain was not relieved by the appendectomy, but spontaneously resolved after a considerable amount of chemotherapy. Lacking evidence of musculoskeletal, neurological, or other abdominopelvic pathology, it may be reasonable to suggest that her pain was caused by the histiocytosis of her colon that seems to have affected the entirety of her colon.
Gastrointestinal histiocytosis in the adult may present with an appendicitis-like syndrome that may be harbinger of more extensive colonic involvement. Additionally, whitish-yellowish polypoid lesions on endoscopy should involve a differential diagnosis of gastrointestinal histiocytosis. In total, a combination of surgical specimens and endoscopy are crucial in diagnosing and evaluating the extent of gastrointestinal involvement of LCH in adults.
Three days of sharp, intermittent right lower quadrant pain exacerbated by movement accompanied by non-bloody, non-bilious vomiting.
Mutlifocal gastrointestinal adult langerhans cell histiocytosis (LCH).
Causes of right lower quadrant pain accompanied by nausea in reproductive age women include acute appendicitis, nephrolithiasis, pregnancy, tuboovarian abscess, ovarian torsion, small bowel obstruction, functional intestinal obstruction, urinary tract infection, sexually transmitted infection, muscle strain.
Labs notable for peripheral eosinophilia.
Unremarkable brain, skeletal, abdominal, and pelvic imaging except for subcentimeter polyps found throughout colon on lower endoscopy.
LCH of the appendix and colon confirmed by S-100, CD1a, and CD207 reactivity.
Twelve cycles of cytarabine therapy at 100 mg/m2 per day for five days with cycles one month apart.
Adult LCH is a rare entity, and there are few reports of bowel involvement that are either asymptomatic and found incidentally on endoscopy or a single report of small bowel hemorrhage secondary to the disease; importantly, there are no reports that presented like this case.
Surgical biopsy is an invaluable practice that can identify uncommon etiologies of appendicitis and may be of great value in guiding any further treatment; in the same vein, a practitioner must entertain common and rare diagnoses for a complete differential.
The authors have done a very good job of writing up this fascinating case.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Imashuku S, Mentes O, Parker W S- Editor: Qiu S L- Editor: Ji FF E- Editor: Wu HL
| 1. | Harmon CM, Brown N. Langerhans Cell Histiocytosis: A Clinicopathologic Review and Molecular Pathogenetic Update. Arch Pathol Lab Med. 2015;139:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Lipton JM. Langerhans Cell Histiocytosis. Merck Manual database online ([accessed. 2016;Merck Sharp & Dohme Corp, 2013. |
| 3. | Akbulut S, Tas M, Sogutcu N, Arikanoglu Z, Basbug M, Ulku A, Semur H, Yagmur Y. Unusual histopathological findings in appendectomy specimens: a retrospective analysis and literature review. World J Gastroenterol. 2011;17:1961-1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Swank HA, Eshuis EJ, Ubbink DT, Bemelman WA. Is routine histopathological examination of appendectomy specimens useful? A systematic review of the literature. Colorectal Dis. 2011;13:1214-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Roeb E, Etschmann B, Gattenlöhner S. Is it always Crohn’s disease in a patient with perianal fistulae and skip lesions in the colon? Gastroenterology. 2012;143:e7-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Aricò M, Girschikofsky M, Généreau T, Klersy C, McClain K, Grois N, Emile JF, Lukina E, De Juli E, Danesino C. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39:2341-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Singhi AD, Montgomery EA. Gastrointestinal tract langerhans cell histiocytosis: A clinicopathologic study of 12 patients. Am J Surg Pathol. 2011;35:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Pops MA, Campbell T. Gastrointestinal bleeding from ileal ulcerations in histiocytosis X. Arch Intern Med. 1968;122:271-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Lee-Elliott C, Alexander J, Gould A, Talbot R, Snook JA. Langerhan’s cell histiocytosis complicating small bowel Crohn’s disease. Gut. 1996;38:296-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yashoda-Devi B, Rakesh N, Agarwal M. Langerhans cell histiocytosis with oral manifestations: a rare and unusual case report. J Clin Exp Dent. 2012;4:e252-e255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Ribeiro KB, Degar B, Antoneli CB, Rollins B, Rodriguez-Galindo C. Ethnicity, race, and socioeconomic status influence incidence of Langerhans cell histiocytosis. Pediatr Blood Cancer. 2015;62:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |