Published online Mar 16, 2017. doi: 10.4253/wjge.v9.i3.105
Peer-review started: September 4, 2016
First decision: September 29, 2016
Revised: October 18, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: March 16, 2017
Processing time: 192 Days and 18.8 Hours
Obesity is a growing problem in developed countries, and surgery is the most effective treatment in terms of weight loss and improving medical comorbidity in a high proportion of obese patients. Despite the advances in surgical techniques, some patients still develop acute and late postoperative complications, and an endoscopic evaluation is often required for diagnosis. Moreover, the high morbidity related to surgical reintervention, the important enhancement of endoscopic procedures and technological innovations introduced in endoscopic equipment have made the endoscopic approach a minimally-invasive alternative to surgery, and, in many cases, a suitable first-line treatment of bariatric surgery complications. There is now evidence in the literature supporting endoscopic management for some of these complications, such as gastrointestinal bleeding, stomal and marginal ulcers, stomal stenosis, leaks and fistulas or pancreatobiliary disorders. However, endoscopic treatment in this setting is not standardized, and there is no consensus on its optimal timing. In this article, we aim to analyze the secondary complications of the most expanded techniques of bariatric surgery with special emphasis on those where more solid evidence exists in favor of the endoscopic treatment. Based on a thorough review of the literature, we evaluated the performance and safety of different endoscopic options for every type of complication, highlighting the most recent innovations and including comparative data with surgical alternatives whenever feasible.
Core tip: In developed countries obesity is a prevalent and rising problem. Bariatric surgery is the most effective treatment to obtain sustainable weight loss but postoperative complications may be serious and challenging to treat. The minimally-invasive character of endoscopic treatment has led endoscopic management of bariatric complications to become a suitable alternative to surgery. In this article, we discuss the indications of endoscopic treatment after bariatric surgery and the available endoscopic techniques.
- Citation: Souto-Rodríguez R, Alvarez-Sánchez MV. Endoluminal solutions to bariatric surgery complications: A review with a focus on technical aspects and results. World J Gastrointest Endosc 2017; 9(3): 105-126
- URL: https://www.wjgnet.com/1948-5190/full/v9/i3/105.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i3.105
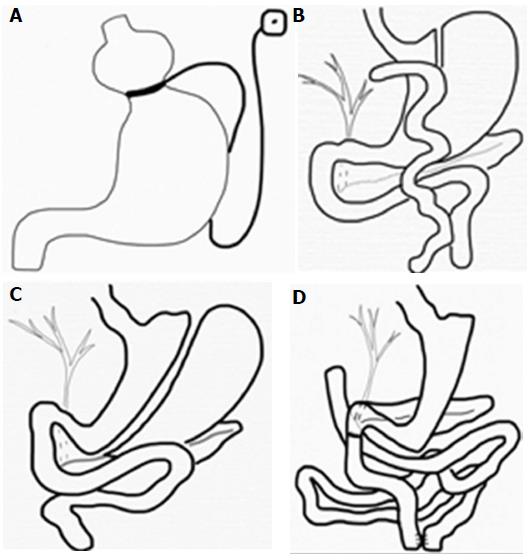
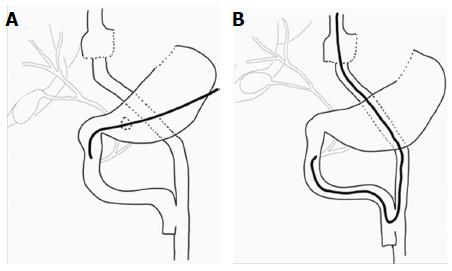
Obesity is a major health problem with significant morbidity and mortality. An estimated 3.4 million deaths worldwide were caused by obesity in 2010, and its prevalence rose by 27.5% for adults between 1980 and 2013[1]. Due to the far superior results of surgical treatment compared with obesity medical therapy, the American Society for Metabolic and Bariatric Surgery stated a grade A recommendation for bariatric surgery for patients with a body mass index (BMI) ≥ 40 kg/m2 or those with a BMI ≥ 35 kg/m2 and comorbid conditions who have failed to respond to prior medical intervention[2]. The most frequent bariatric operations performed worldwide are Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) (Figure 1). Although Gastric Roux-en-Y bypass (RYGB) is the most effective bariatric surgery, it is technically demanding, and, therefore, SG has been increasingly performed due to its simplicity. Laparoscopic adjustable gastric banding (LAGB) is now an outdated procedure despite its safety profile because of its poor long-term results. Other bariatric techniques, such as biliopancreatic diversion with or without duodenal switch (BD/DS) and jejunoileal bypass, have been abandoned due to severe metabolic complications[3].
Weight-loss surgery has been associated with an important reduction in obesity-related medical complications, such as diabetes mellitus and other cardiovascular risk factors[4]. The increased use of laparoscopy and improvements of surgical techniques have led to a significant decrease in surgical complications with a current mortality lower than 1% in centers of excellence. However, postoperative complications are still common, and minimally invasive endoscopic treatments have gained popularity to avoid surgical reinterventions.
In the literature, up to 30% of patients present symptoms in the perioperative setting requiring endoscopic evaluation[5]. The most common symptoms are abdominal pain, nausea, vomiting, dysphagia and gastrointestinal bleeding. These symptoms are predictive of pathologic findings at endoscopy, and, interestingly, this predictive potential depends on the time elapsed from surgery. In the first 6 mo following surgery, 85% of upper endoscopic explorations had, at least, one abnormal finding vs 47% after 6 mo. In addition, time elapsed from surgery also predicts the type of complication; in the first 6 mo after the surgery, the incidence of staple-line dehiscence is higher whereas stomal ulcerations or strictures are less probable[5]. Optimal management should be individualized because surgical reconstruction may make endoscopic access and resolution difficult or even impossible. In addition to these local complications directly related to the surgical procedure, other long-term metabolic consequences secondary to the rapid loss of weight, such as gallstones or hepatobiliary disease, may further complicate the clinical scenario and may also require endoscopic management[5].
Because the majority of symptomatic patients are endoscopically evaluated, the gastroenterologists must be familiar with post-surgical anatomy and complications, and their endoscopic management. In this review, we will briefly describe the most frequent complications related to every type of bariatric surgical technique and the most recent and remarkable results about the endoscopic procedures with proven effectiveness.
Gastrointestinal (GI) bleeding after bariatric surgery can occur in the first 30 d following surgery as an early complication, or afterwards as a delayed one. Early bleeding usually presents within the first 48 h in 1%-5% of patients after RYGB[6]. SG presents a variable rate of bleeding between 0% and 8%[7] whereas LAGB is almost never complicated with bleeding (0.1%)[8]. The risk of iatrogenic perforations at the surgical site along with the self-limited character in most cases are the reasons why minor bleeding is usually managed conservatively with fluid resuscitation or blood transfusion and proton pump inhibitors. In cases of severe bleeding, endoscopic exploration is mandatory, or even surgical intervention when no blood exteriorization is observed and after CT diagnosis of extraluminal bleeding[9,10]. Late bleeding is usually secondary to anastomotic ulceration and is almost never extraluminal.
Endoscopy must be performed with minimal insufflation with CO2 if possible. Upper endoscopy is able to reach the gastrojejunal anastomosis but a balloon-assisted enteroscopy may be required to access the excluded gastric remnant, which carries a risk of perforation due to the immature anastomoses and the traction forces during enteroscopy. In this situation, if a skilled endoscopist is not available, surgical intervention should be the first option.
After bariatric surgery, the most common bleeding is at the staple-line of the anastomotic gastrojejunostomy in RYGB but it can occur anywhere in the gastrointestinal tract. There are few studies published on this subject but endoscopic management of bleeding at the gastrojejunal anastomosis has been successfully managed with the standard hemostatic modalities without more complications than in contexts other than bariatric surgery[11]. Injection of epinephrine or polidocanol[12], thermal methods[13] and endoclips have been used with success[14]. Nevertheless, mechanical methods, especially endoscopic clips, should be favored. Thermal techniques must be avoided at the staple-line and the anastomosis as for other surgical settings to prevent tissue injury. Hemostatic powder might be another option although no case has been reported thus far.
Ulceration is a late complication that typically occurs 1 to 6 mo after surgery. In patients with epigastric pain after RYGB, ulceration at the gastrojejunal anastomosis is the most frequent finding during endoscopy and is also one of the most common complications after RYGB appearing in up to 16% of patients[15]. Other symptoms at diagnosis are nausea, vomiting, and bleeding, either overt or occult. Ulcerations on the gastric side of the anastomosis are referred to as stomal ulcers and those on the jejunal side as marginal ulcers[16].
The cause of stomal ulcers is known to be ischemia whereas the mechanism for marginal ulcers is not well understood. Local ischemia, acidic gastric secretions, NSAID use, alcohol intake, smoking, or a foreign body, such as non-absorbable suture material, have been suggested as plausible causes[17]. After resecting the duodenum, there is no longer a pH buffering function, and acidic secretions are poorly tolerated at the jejunum; however, refractory ulcers should raise the suspicion of a gastrogastric fistula causing continuous acid exposure. The role of Helicobacter pylori (H. pylori) is controversial in this setting, and there is no consensus regarding preoperative screening for H. pylori and subsequent eradication therapy in patients undergoing bariatric surgery[18]. However, some authors have suggested that H. pylori-related mucosal damage before surgery may result in postoperative ulceration even after eradication. The efficacy of proton pump inhibitors (PPI) in preventing marginal ulcers is controversial[19]. After ulcer development, most authors advocate indefinite PPI treatment but if aspiration of gastric pouch fluid reveals a non-acidic pH, then acid suppression is less effective and sucralfate may be the treatment of choice[20].
The endoscopic intervention is especially indicated when a foreign body is suspected to be the underlying cause. Embedded sutures or staples can promote an inflammatory reaction exposed to the irritating lumen environment; thus, large amounts of sutures should be removed even in asymptomatic patients[21]. A double channel endoscope may be useful to introduce grasping or rat tooth forceps, loop cutters, endoscopic scissors or argon plasma coagulation probes. In some series that use these endoscopic accessories to manage foreign bodies, over 70% of patients reported clinical improvement[22]. Endoscopy should be repeated at 8 wk to confirm ulcer resolution. In cases of unhealed or recurrent ulcers, a gastrogastric fistula or staple-line dehiscence should be investigated.
A particular type of ulceration is related to gastric band erosion, which occurs in 1%-4% of patients 1 or 2 years after LAGB[23]. Traditionally, surgical removal performing another bariatric surgical reconstruction during the same procedure was the classical approach. Nevertheless, endoscopic extraction of gastric bands is feasible and is especially useful in patients not suitable for a new bariatric intervention. The procedure usually involves several steps. First, partially migrated bands must become more accessible from the gastric cavity and this is achieved by placement of a fully covered self-expanding metal stent (FCSEMS) inducing mucosal necrosis[24,25]. Next, the band must be transected before band removal transorally. Although different endoscopic procedures have been described to cut the band, such as band transection using argon plasma, neodymium-YAG laser or endoscopic scissors, the easiest method is the wire-cutting technique. In this technique, a guide-wire is passed through the band and the distal end of the wire is caught and pulled back on the other extremity forming a loop. Then, the band transection is accomplished using a mechanical lithotripter. Before band extraction, the subcutaneous port must be disconnected. The technical success range from 80% to 100%, and failures are mainly related to adherences between perigastric tissues and the gastric band[25-28].
Stenoses are usually secondary to stricture development although they may be due to a malfunction of prosthetic devices. Although LAGB and SG may be complicated with stenosis, this complication is more commonly observed after RYGB and more often located at the gastrojejunal anastomosis. Less frequent locations are the jejunojejunal anastomosis and sites of passage through the mesocolon or intestinal adhesions. Stomal stenosis occurs in as many as 3% to 28% of patients who have undergone RYGB[29-32] and are defined as stomas that are smaller than 10 mm in diameter or as stomas that prevent the passage of the standard gastroscope[33]. The clinical presentation consists of dysphagia, nausea, vomiting and early satiety without abdominal pain. They develop gradually; therefore, they are usually late complications presenting several weeks after the surgical intervention. Medical factors, such as the use of NSAIDs, smoking or alcohol intake, can promote stenosis development. Moreover, surgical factors, including the method for anastomosis creation and mechanical tension or ischemia at the anastomosis, are also associated. Anastomoses performed with a circular stapler resulted in a higher stricture rate than those hand-sewn or performed with a linear stapler[34].
The most common endoscopic approach is dilation with a through-the-scope balloon (TTS balloon). Although the use of fluoroscopy is advisable to avoid entry into the blind limb in cases of non-traversable stenosis with the gastroscope, balloon dilation without fluoroscopy guidance has been reported to be safe. In a series of 22 patients with a stomal stenosis, balloon dilation was performed without fluoroscopy and achieved a success rate of 100% without any perforation[35]. Based on these results, the authors concluded that fluoroscopy is not always required for positioning the balloon and recommended the use of fluoroscopy liberally in difficult cases.
Balloon dilation to 15 mm in the first session has been shown to be safe although several dilations, every 2 or 3 wk with balloon diameters gradually increased from 12 to 20 mm, are often needed to achieve resolution and to prevent perforation and weight regain secondary to the loss of volume restriction[36]. Overall, the most recent studies in the literature have reported a success rate higher than 90% with very few complications (Table 1)[35-46]. It is important to note that in one study reporting on balloon dilation in 72 gastrojejunal strictures after RYGB, late strictures (> 90 d after RYGB) were found to have an inferior rate of response to endoscopic dilation (61% vs 98%) and often required revisional surgery[46]. When balloon dilation has failed to succeed, endoscopic incision using a needle knife papillotome prior to balloon dilation can be tried[47]. Suture material at the site of the stenosis can also hinder full balloon expansion and its removal may be necessary before the procedure[48]. In addition, some authors have reported satisfactory results with injection of a saline solution or steroids in the stenosis after balloon dilation. This may prevent restenosis by disrupting the scar tissue; however, the real efficacy and mechanism of action are not well known[40].
| Ref. | No. patients | Time interval to stricture diagnosis (d) | No. of sessions | Success rate (%) | Balloon diameter | Complication rate (%) | Perforation rate (%) |
| Barba et al[36] | 24 | 28-270 | 1-3 | 100 | 8-13 mm | 0 | 0 |
| Go et al[37] | 38 | 53.9 (21-168) | 1-6 | 95 | 12-16 mm | 3 1 pneumothorax and pneumomediastinum | 3 |
| Rossi et al[38] | 38 | - | 1-3 | 100 | - | 0 | 0 |
| Carrodeguas et al[39] | 94 | 52.7 (20-154) | 1-4 | 99 | - | 2.1 Perforations | 2.1 |
| Catalano et al[40] | 26 | 63 (28-63) | 1-7 | 96.2 | 8-15 mm | 3.8 Surgical revision for recurrent stenosis | 0 |
| Peifer et al[41] | 43 | 49.7 (24-197) | 1-3 | 93 | 9-20 mm | 0.5 Surgical revision for recurrent stenosis | 0 |
| Caro et al[42] | 111 | 56 (3-237) | 1-4 | 100 | 6-18 mm | 2.7 2 contained perforations 1 esophageal hematoma | 1.8 |
| Ukleja et al[43] | 61 | 60 (30-180 ) | 1-5 | 100 | 6-18 mm | 4.9 3 perforations | 2.21 |
| Mathew et al[44] | 58 | 66.2 (12-365) | 1-7 | 100 | 6-20 mm | 3.2 Perforations | 3.2 |
| Da Costa et al[45] | 105 | 90 (30-270) | 1-4 | 100 | 6-20 mm | 3.8 1 hemorrhage 3 perforations | 1.81 |
| Espinel et al[35] | 22 | 126 (26-768) | 1-4 | 100 | 12-20 mm | 4.5 Small tear | 0 |
| Yimcharoen et al[46] | 72 | 46 < 90 25 > 90 | 1-15 | 84.7 98% < 90 d 61% > 90 d | 8-18 mm | 1.3 1 perforation, pneumoperitoneum and death | 1.3 |
The most feared complication is perforation, which occurs in 3%-5% of patients after balloon dilation[37]. Early detection of this complication is crucial. Perforation may be managed by stent insertion or surgical repair. In addition to perforation, another risk of dilation is weight regain related to the loss of volume restriction. Nevertheless, some of the published studies have analyzed the impact of dilation on the decrease in weight-loss rate. The weight loss at baseline and during the follow-up did not differ from that of patients without stricture[41].
Savary-Gilliard bougie dilation (Wilson-Cook Medical Inc, Winston-Salem, NC) is another method to dilate anastomotic strictures. Although few patients with post-bariatric stenoses have undergone this technique, it seems to be highly effective and safe. Two studies have demonstrated a 100% efficacy without serious complications[49,50].
Esophageal FCSEMS have also been used to treat chronic gastrojejunostomy strictures with a 12.5% successful response[51]. In this study, all of the patients had a stricture at the gastrojejunal anastomosis after RYGB but one had a stricture at the duodeno-ileal anastomosis following BPD/DS. The poor response was associated with stent migration.
The prevalence of symptomatic stenosis following laparoscopic sleeve gastrectomy (LSG) is between 0.1% and 3.9%[52]. In patients with LSG, functional stenosis may occur at the angularis incisura or the gastroesophageal junction and endoscopic treatment seems to play a smaller role based on the lower efficacy rates. In a study of 16 patients who underwent TTS balloon dilation under fluoroscopic or endoscopic guidance, the efficacy was only 44% after 1 to 3 sessions[53]. Repeated sessions of endoscopic dilation should be indicated only in patients with some symptomatic relief following the first session because these strictures are more likely due to a fibrotic reaction. In contrast, endoscopic dilation has no efficacy when twisting rotation of the sleeve is the cause of functional stenosis and these patients should be managed surgically[53,54]. There are some reports on the use of 30 mm achalasia balloons to treat SG strictures with 71% to 100% resolution rates[52]. These higher rates of resolution are overshadowed by the higher risk of perforation secondary to the more rigid achalasia balloons.
More recently, new self-expanding metallic stents have been manufactured to treat leaks after SG: Megastents (Taewoong Medical Industries, South Korea) and Hanaro (MITech, Seoul, South Korea). These stents are longer than conventional SEMS positioned from the distal esophagus to the duodenal bulb bypassing the entire gastric sleeve. Although they are intended to reduce the migration rate while sealing a leak after SG, they also confer radial strength useful to dilate a possible stenosis at the same time[55]. Nevertheless, studies using these stents have only reported results of leak closure.
Mechanisms of stenosis after gastric banding include fibrotic reaction, band rotation and adhesions with pouch angulation. Dilation is only effective in cases of fibrotic stenosis. If endoscopic dilation is not successful after one session, another endoscopic session should not be performed and surgical options must be considered including conversion to RYGB[56].
Leaks are defined as the exit of luminal contents due to a discontinuity of the tissue apposition at the surgical anastomosis, whereas fistulas are abnormal passageways usually between two hollow viscera or communicating to the skin and they result from chronic healing of local inflammation caused by leaks. The incidence of staple-line or anastomotic leaks varies depending on the type of bariatric surgery, with studies reporting 2% to 5% for laparoscopic RYGB, 1.6% to 2.6% after open RYGB and 0.6% to 7% in patients following a sleeve gastrectomy[29,30,57-59]. Because LAGB does not involve transection of the stomach, the reported incidence of leakage is very low, ranging from 0% to 0.5%[60-62]. Leaks are the most dreadful complication and after pulmonary embolism they represent the second most common complication leading to death after bariatric surgery[63]. Although several patient-related conditions, such as superobesity, age above 55 years, male sex, and a personal history of diabetes mellitus, sleep apnea, hypertension or previous surgery, are thought to predispose patients to leaks[64], early postoperative leaks within the three first days after surgery are usually due to a technical error, such as anastomotic tension, stapler malfunction and suture or staple-line seepage[65]. However, leaks presenting later in the perioperative setting after RYGB are usually due to ischemia at the staple-line or anastomosis and they appear with decreasing frequency at the gastrojejunostomy, gastric pouch and jejunojejunal anastomosis[66-68]. High pressure in the gastric sleeve due to stenosis at the incisura, pyloric dysfunction and twisted or atonic sleeve is the most common cause of leaks several days after LGS but ischemia may also be responsible following ligation of the short gastric arteries. In this setting, most leaks occur at the angle of His where the highest pressures are present[69-71]. The uncommon cases of leak after LAGB are mainly due to ischemia secondary to band slippage or migration[72]. However, the rate of leaks is increased after revision bariatric procedures for gastric band failures and it has been reported to be as high as 18%[73,74]. This occurs more often when the revision surgery after LAGB is accomplished to treat band complications, such as band erosion, slippage and migration, than after a revision procedure for weigh regain. Factors contributing to this increased risk of leaks are the fibrotic and inflammatory tissue surrounding the band and secondary adhesions to the neighboring structures like the pancreas[73,74]. The incidence of fistulas is not well known although some authors have reported that 14% of patients with anastomotic leaks will develop fistulas[67]. The most common sites are gastrogastric and gastrocutaneous. Gastrogastric fistula is a specific complication of RYGB consisting of an organized communication between the gastric pouch and the gastric remnant. It was one of most common complications in the past but currently, after generalizing the transection of the stomach, the incidence of gastrogastric fistulas has decreased to 1.2%-6%[75-77].
Clinical presentation of leaks is variable, ranging from asymptomatic patients to sepsis-related multiorgan failure. Early suspicion of a leak must be raised in cases of any deviation from the normal postoperative course with tachycardia being the most sensitive indicator of a leak[67,78]. Chronic fistulas show more indolent presentation. Abdominal discomfort and heartburn due to acid flow into the pouch and weight regain are the most common symptoms in patients who present a gastrogastric fistula. The most sensitive method to diagnose leaks is computed tomography with oral, water-soluble contrast[79]. Nevertheless, leaks may be discovered by routine upper gastrointestinal studies performed systematically by some authors during the first three days after surgery, or by oral administration of methylene blue when drains are still in place[80,81]. Endoscopic diagnosis is also feasible, combining a bubble test (drain immersion during endoscopic insufflation) with the administration of contrast with methylene blue into the drain while keeping fluoroscopic and endoscopic view looking for a leak[82].
The treatment strategy depends on the clinical condition of patients and the time of presentation, and relies on the following three mainstays: Medical support, drainage of leaked material and repair of the wall defect. Therapeutic medical measures involve suppression of the oral intake, parenteral nutrition or distal enteral feeding, and broad-spectrum intravenous antibiotics. For hemodynamically unstable patients with generalized peritonitis or sepsis, surgical drainage and cleansing of the peritoneal cavity is mandatory and a feeding jejunostomy should be performed at the same time to allow enteral nutrition. Otherwise, conservative management based on medical therapy and external drainage is a convenient approach that has been reported to achieve leak resolution in 75% of early, asymptomatic and small-volume leaks[83]. However, some authors prefer surgical repair in these early small leaks[84]. In stable patients with larger leaks or leaks presenting late in the postoperative course, where conservative management keeps the patient stable but does not succeed in stopping the leak, further treatment should be considered. Nevertheless, primary surgical repair is associated with high rates of recurrence and other reinterventions, such as gastrectomy, sleeve conversion to gastric bypass or fistulojejunostomy, which have high morbidity; therefore, the management of these patients has shifted from surgery to less invasive endoscopic therapy[67,85].
Endoscopic treatment is used very often as an adjuvant therapy to surgery but the management of post-bariatric leaks may be accomplished entirely by endoscopic means. The endoscopic approach usually involves a stepped and multimodal procedure as has been previously described[86].
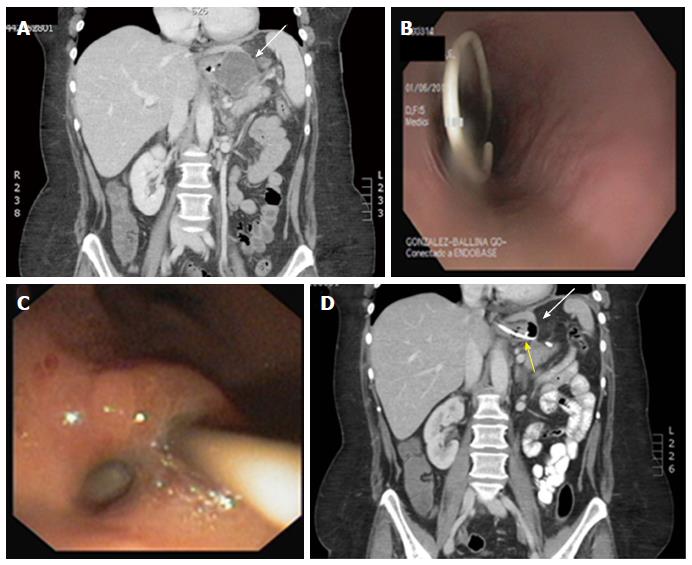
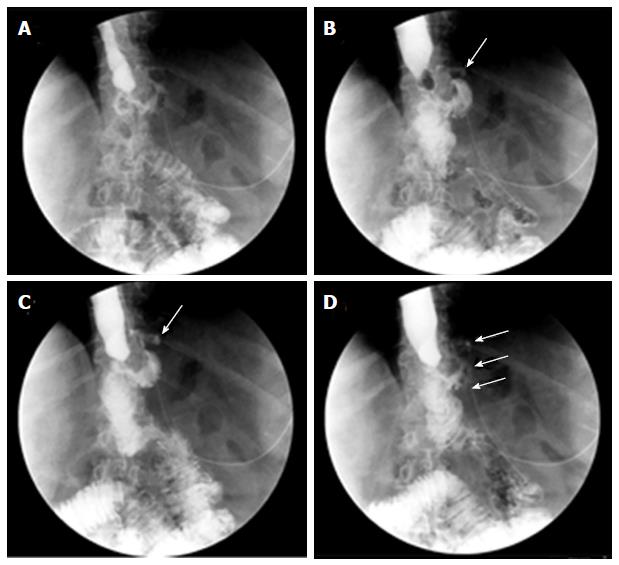
Before any attempt to close the wall defect, the first step is to guarantee the appropriate drainage of the leaked content. For early leaks, this is commonly accomplished by surgical drains maintained in the postoperative period but if drains are no longer in place, percutaneous drains should be placed either surgically or by interventional radiology whenever accessible. Nevertheless, in patients with delayed leakage or when surgical cleansing is not required or for those whose collections are not radiologically accessible, the endoscopic internal drainage (EID) into the digestive lumen might be the first option for well-circumscribed collections[87]. In addition when combined with surgical cleansing in patients presenting with severe sepsis, EID allows early removal of surgical drainage preventing chronic fistula tract formation[88]. EID consists of trans-fistulary insertion under fluoroscopy guidance of one or two 7 or 8.5 Fr double-pigtail plastic stents, or one double-pigtail stent and one nasocystic catheter in cases of large collections requiring lavage to eliminate pus and debris[87] (Figure 2). Double-pigtail stents keep the leak orifice open favoring the passage of fluid content into the digestive lumen with progressive reduction in the collection size until it eventually becomes a virtual cavity. Meanwhile, a foreign body reaction in the edges of the leak is triggered by plastic stents promoting the reepithelialization over the stent and the fistula closure, resulting in an all-in-one procedure without the need of further treatment. A residual small cavity like a pseudodiverticulum is common at the end of the process without any clinical repercussion[88]. In some cases, the leak orifice is not clearly identified or the communicating tract between the digestive lumen and the fluid collection is complex, then, internal drainage may be accomplished by EUS-guided drainage, such as for pseudocysts or other postsurgical collections[87-90]. In addition to stenting, debridement may also be needed in cases of infected collections containing necrotic tissue. Endoscopic necrosectomy is also feasible, similar to treatment of organized pancreatic necrosis[86,90,91].
Although EID is now most commonly used as a first-line approach, it has also been reported as a bridge to other endoscopic methods and as a salvage treatment when other endoscopic techniques have failed[87-89]. In the largest series reporting on EID, double pigtails were delivered as a first line approach in 66 patients[88]. Among them, 42 had a surgical drainage placed close to the leak. The 78% of patients were cured by EID after a mean of 58 d. Oral diet was reassumed following the confirmation of collection reduction in a CT performed three days after pigtail insertion. In this study a protocol with systematic endoscopic review every 4-6 wk was followed. At each session stent exchange was performed until fistula healing was achieved to avoid stent obstruction and to stimulate tissue granulation by the traumatism induced by the stent on the fistula edges. In a more recent study on 33 patients with fluid collections after SG or RYGB (in 19 patients after previous unsuccessful endoscopic treatment), internal drainage achieved 78.8% clinical success[87]. After confirming biological and clinical improvement, this approach allowed early oral re-feeding in the first 24-48 h following stent insertion without any negative impact on the final results. No standardized protocol to remove the stents was observed and the decision was left to the discretion of the endoscopist and decided on an individual basis, although the stent retrieval was planned at least 4 mo after complete clinical resolution. However, in most patients successfully treated by internal drainage, no other endoscopic procedures were required because stents often migrated spontaneously. In addition to the high efficacy, EID is burdened with a low complication rate. In the first study 6 stenoses, as well as two stenoses in the second series, were observed, probably related to granulation tissue induced by the pigtails, and successfully treated either with achalasia balloon dilation or FCSEMS[87,88]. In the second study, two other complications were also reported[87]. One patient presented bleeding from the leak orifice with internal stent migration that was endoscopically treated by endoscopic coagulation and endoscopic retrieval of the migrated stent. The other patient developed a splenic hematoma that required surgical treatment. The authors argued that the proximity of the pigtails to the spleen parenchyma might explain this serious complication. Despite the high efficacy of EID, some patients in both studies did not respond to EID after median stent dwell duration of 58 d (range 10-206) and 115 d (range 23-773) respectively, and other treatments were required. The appropriate time interval for stent exchanging and the optimal timing to consider EID failure in leak healing and to proceed with other surgical or endoscopic treatments remain to be defined.
Methods other than pigtail stenting for internal drainage have been described. To drain the leaked content, stricturotomy, more recently named as septotomy, has been successfully tried in patients with postoperative leaks after SG or DS[92,93]. This procedure derives from the endoscopic treatment for Zencker diverticulum and involves cutting the septum between the perigastric cavity and the gastric pouch using argon plasma coagulation or a needle-knife. In a prospective study, 27 patients were treated with stricturotomy and after 1 to 6 endoscopic sessions, all of the patients had their fistulas closed. More than half of the patients in this study presented stenosis at the angularis incisura, and they were all endoscopically dilated. This result highlights that besides drainage it is of paramount importance to detect and treat predisposing factors that can perpetuate the leak. Therefore, in combination with achalasia balloon dilation of post-LSG stenosis, septotomy has become an appealing alternative to treat chronic leaks connected to a not drained cavity after LSG. This procedure allows decreasing intragastric pressure, rerouting the gastric content and the internal drainage of the cavity by cutting the staple-line interposed between the cavity and the gastric lumen[92,93]. Anecdotal experience with internal drainage using a vacuum-assisted closure system is now available[94,95]. This device consists of a sponge endoscopically placed inside the cavity and connected by a transnasal tube to external continuous vacuum suction[96]. The sponge must be changed every 2-4 d and adapted as the fistula cavity reduces in size until it eventually seals. The sponge results in granulation tissue and the vacuum helps to extract the fluid content, improves blood flow and promotes leak closure. Nine patients in the post-bariatric surgery context have been treated with this device recently. The rescue rate was 89% after an average of 50 d and 10.3 procedures per patient. One patient required surgery and in two others, a complementary over-the-scope clip was placed[95].
Some patients have their leaks closed after conservative management or internal drainage but in other cases the leak persists. For these patients and for those with larger leaks at presentation who will predictably fail to respond to the above measures, further intervention is required. Therefore, once drainage is ensured, the next step is aimed at treating the wall defect. There are two different endoscopic strategies to proceed, either to primarily close the leak orifice or to exclude it by diverting the enteric content, and the choice mostly depends on the size of the wall defect. Both strategies may be complementary usually in a sequential manner or an adjuvant therapy to surgical drainage.
Wall defect exclusion: The endoscopic technique to divert the gastrointestinal stream is stent placement. Endoscopically placed self-expandable endoscopic stents (SEES) offer several advantages. They decrease the intraluminal pressure, which is one of the pathophysiologic factors of leaks after SG, prevent or decrease peritoneal contamination by excluding the wall defect from esophagogastric secretions and thus, promote healing of the leak and permit oral nutrition to be resumed[51,97]. This is the endoscopic treatment for leaks after bariatric surgery where most evidence is available. Table 2 shows the largest studies in the literature reporting on stent treatment of leaks after bariatric surgery[98-111]. In one meta-analysis on 7 studies published in 2011, fistula closure was achieved in 88%. The rate of successful removal of the stent after leak closure was 92% and stent migration, noted in 17% of cases, was the most common complication[64]. However, the largest studies have been published after this meta-analysis and the rates of leak closure and complications range from 65% to 100% and 14% to 86%, respectively, with migration being the most frequent complication with rates of 5%-67%[98-111]. It is noteworthy that figures of success in most studies have included not only primary success following stenting but also success after combining stenting with other complementary endoscopic techniques. Moreover, these studies have pooled patients with different bariatric surgeries, mostly RYGB and SG. Additionally, the timing of stenting after surgery is heterogeneous, the size of the wall defect has rarely been reported and different stents have been used. All of these issues are important factors influencing rates of success and migration. Due to anatomical reasons, lower rates of leak closure are achieved in SG because the area to cover is larger, it is more difficult to obtain close apposition between the stent and the wall defect and tissue hyperplasia increasing the water tightness is less common[99]. It has also been advised that management of postbariatric surgery leaks must be guided by the size of the wall defect as it has been shown that leaks larger than 1 cm take longer to heal after stenting or even fail to seal with only stent placement[112]. In addition, the time interval between leak development and endoscopic stenting is a proven factor that impacts outcomes. The fibrotic transformation of leaks into chronic fistulas over time significantly decreases the probability of leak closure after stent placement[51,105,107]. Heterogeneity regarding all of these clinical factors may explain, at least in part, the large differences in rates of success across the studies.
| Ref. | Nº patients | Bariatric procedure | Time interval to stent | Type of stent | Success rate (%) | Complications rate1 | Migration rate (%) | Nº of procedures | Time to fistula closure |
| Salinas et al[98] | 17 | RYGB | 1-3 wk | PCSEMS | 94 | 41% 1 migration, 2 mucosal tears, 4 stent obstruction by food | 6 | 1.2 (1-2) | 3.2 ± 1.2 mo |
| Eisendrath et al[99] | 21 | RYGB/ LGS/DS | 31 d (14-199) | PCSEMS | 81 | 14% 1 migration, 2 dysphagia due to tissue hyperplasia | 5 | 1-6 | NA |
| Eubanks et al[100] | 13 | RYGB/LGS | Acute leaks (11) Chronic leaks (2) | FCSEMS/FCSEPS | 77 | 70% (not specified) | NA | NA | Acute leaks - 33 d Chronic leaks - 45 d |
| Blackmon et al[101] | 10 | RYGB/LGS | NA | FCSEMS/PCSEMS | 100 | NA | NA | NA | NA |
| Leenders et al[102] | 11 | RYGB/LSG | 8 d (1-33) | FCSEMS | 73 | 50% 3 disintegrated stent, 2 migration | 17 | 2 (1-4) | 16 wk (5-63) |
| El Mourad et al[103] | 47 | RYGB/LSG/DS | 10.5 d (1-74) | PCSEMS | 87 | 30% 1 mucosal stripping, 1 perforation, 1 dysphagia, 1 stricture, 1 bleeding 1 stent angulation, 7 migration | 15 | NA | 44 d (3-90) |
| Orive-Calzada et al[104] | 11 | LSG | NA | FCSEMS | 73 | NA | NA | NA | NA |
| Alazmi et al[105] | 17 | LSG | < 1 wk (10) 1 wk-1 mo (6) > 1 mo (1) | PCSEMS | 76 | 36% 2 bleeding, 3 dysphagia, 1 migration | 6 | NA | 42 d (28-84) |
| Quezada et al[106] | 29 | RYGB/SG | 8 d (0-104) | CSEMS | 96.5 | 41% 10 migration, 1 stent fracture, 1 opening of blind end of alimentary limb | 34 | NA | 82 d (2-352) |
| Murino et al[107] | 91 | RYGB/LSG | 25 d (2-308) | PCSEMS | 81 | 22% 5 bleeding, 2 perforation (1 death), 7 migration, 13 esophageal stricture | 8 | 1 (1-7) | NA |
| Fishman et al[108] | 26 | LSG | < 1 wk (1) 1-6 wk (17) 7-12 wk (5) > 12 wk (3) | FCSEMS2 | 65 | 46% 4 severe stent intolerance (stent removal) 1 severe bleeding, 7 migration | 27 | NA | NA |
| Southwell et al[109] | 21 | LSG | < 1 wk (6) 1-6 wk (12) 7-12 wk (1) > 12 wk (2) | FCSEMS2/PCSEMS | 95 | 86% 10 migration,, 2 esophageal strictures 1 leak due to erosion by the stent 5 severe intolerance (stent removal) | 48 | 5 (2-13) | 75 d (9-187) |
| Van Wezenbeek et al[110] | 12 | RYGB/LSG | 8 d (0-24) | FCSEMS2 | 75 | 75% 7 migration, 1 perforation 1 perforation secondary to migration | 67 | 2.4 (1-3) | 38 d (28-49) |
| Shehab et al[111] | 22 | RYGB/LSG | 11 d (3-30) | FCSEMS2 | 82 | 41% 1 perforation, 1 esophageal stricture, 4 migrations, 2 bleeding (1 death), 1 stent intolerance (removal) | 18 | 2.8 (2-5) | 6.8 wk (2-14) |
SEES, along with sepsis treatment, have become an attractive alternative due to the high rates of leak closure, its minimally invasive placement, the early restoration of oral nutrition and faster recovery with shorter hospital stays. Although there is no consensus on the optimal timing of stenting, most experts suggest early application after diagnosis[113]. In general, stents are well tolerated. Minor symptoms, such as nausea, vomiting and abdominal discomfort, are common and usually transient, but severe stent intolerance has been reported, leading to early stent removal. The main drawback of SEES is the high rate of migration. Other than migration, most complications are conservatively managed and not severe; however, severe bleeding and perforations have been observed. They are serious complications that have resulted in death in a few cases and in many occasions are associated with stent migration[107,111]. One explanation for the high migration rate is that SEES have been designed to treat benign and malignant esophageal strictures. In the esophagus, close contact between the stent and the mucosa is feasible whereas in a gastric pouch the friction between the stent and the mucosa is not enough to keep the stent in place, predisposing the stent to migrate. This is especially true for stents placed across the gastrojejunal anastomosis in RYGB[100]. Many attempts to decrease the risk of migration and its consequences have been made concerning the type and design of SEES and different methods to anchor the stents. SEES are currently made either of metal (SEMS) or polyester (SEPS). Metal stents are said to have a higher friction coefficient but, in practice, migration rates seem to be similar; however, metal stents are easier to insert due to the small caliber and higher flexibility of the delivery system[100]. There are two types of metal stents used in post-bariatric complications, partially (PCSEMS) and fully covered SEES (FCSEMS), and there is controversy about which is the better option. The silicon coating completely covering the FCSEMS is intended to easily remove the stent but this advantage is overshadowed by the higher trend toward migration. In contrast, the uncovered ends of PCSEMS induce tissue hyperplasia that helps to hold the stent in place and increase the water tightness of the stent, favoring leak closure. Nevertheless, tissue ingrowth into the stent makes stent removal difficult and increases the risk of bleeding, mucosal stripping and perforation. In the absence of randomized prospective trials comparing both stents, the choice relies on the preferences of the endoscopist. To prevent migration, an increasingly expanded practice at present is to use PCSEMS and if significant tissue in growth occurs a second SEPS is inserted to pressure necrosis of the hyperplastic tissue; then, both stents can be safely removed after several days[107]. Ablation of the hyperplastic tissue using argon plasma coagulation is another option and also, anatomic constrictions can improve stent fixation by using overlapping stents (one through another) bridging the esophageal junction and the pylorus.
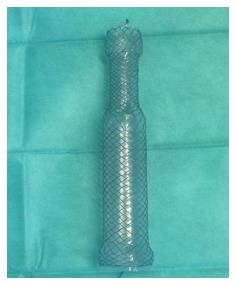
The following two FCSEMS have been recently designed to be adapted to the sleeve anatomy: ECBB Hanarostent® (MI-tech, Seoul, South Korea) (Figure 3) and Megastent® (Taewoong, Seoul, South Korea). They have a larger diameter to ensure optimal adherence to the sleeve wall and are longer, up to 24 cm, to allow the proximal end to be placed in the distal esophagus and the distal end in the duodenal bulb to decrease migration. Whether these stents reduce the rate of migration is not clear because few cases have been reported and migration has been observed in 18% to 67%[108-111]. However, one highlighted advantage is that these stents are always retrievable endoscopically because their larger sizes prevent them to migrate far distally in contrast with conventional stents[111]. Two problems have been observed with these stents. The first one is the worse tolerability to the stent requiring stent removal in some cases, with common pain, heartburn and vomiting due to biliary reflux, and the second problem is the decubitus lesion in the duodenal bulb caused by the distal edge[108,114]. Another type of stent is uncovered biodegradable stents. They tend to migrate less than FCSEMS and do not need extraction. However, the severity of tissue hyperplasia and the time to complete degradation cannot currently be accurately predicted[115]. Therefore, degradation before the leak closure is possible. Anecdotal experience with biodegradable stents has been published in three cases with satisfactory results[116].
A further attempt to prevent stent migration is endoscopic stent fixation. Through-the-endoscope clips are of limited value[86,117,118] and limited experience with the new endoscopic suturing devices to anchor the stents shows variable results[119,120].
Despite the above efforts, stent migration is an unsolved problem and the longer stents remain in place, the higher the probability of migration. For this reason, most experts recommend removing the stent after 6-8 wk, enough time to allow the leak closure and to avoid developing excessive tissue hyperplasia. Until then, weekly scheduled re-evaluations are highly advised because detection of incipient stent dislodgement may allow easy endoscopic stent repositioning or removal. In cases of stent dislodgement or persistent leakage at scheduled controls, additional SEES can be placed, covering the proximal and distal ends, to obtain better anchoring and to seal the leak. In fact, repeated endoscopic procedures are often necessary ranging from 1 to 7 in reported studies[98-111].
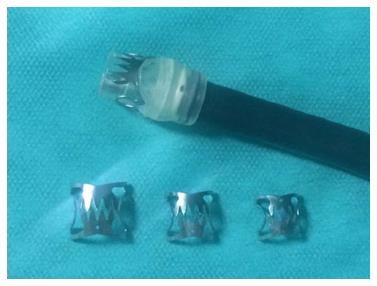
Endoscopic closure of wall defects: Several techniques are available to try directly closing leaks instead of covering the leak opening, such as clipping, endoscopic suturing, or obstructing the fistula with fibrin sealants or plugs. Limited data about the use of through-the-scope clips in this indication fail to show any advantage of these clips in post-bariatric leaks. Preliminary experience with a new clipping device made of nitinol and loaded at the tip of the endoscope, over-the-scope clip (OTSC) (Ovesco Endoscopy, Tübingen, Germany) seems promising (Figure 4)[111,121-123]. This clip allows full thickness apposition in wall defects smaller than 3 cm. The OTSC in post-bariatric leaks has been used almost exclusively in SG leaks. Before placing the OTSC, appropriate drainage of leaked material is required as for SEES and it is strongly advised to de-epithelialize the fistula edges either with a cytology brush or with argon plasma coagulation to promote granulation tissue. In two studies reporting on 18 and 26 patients with SG leaks, leak closure was possible in 81% and 89%[122,123]. However, several patients in both studies also received a SEMS, resulting in 61% and 62% of primary success. In one of these studies, most failures occurred in leaks at the level of the antrum, which are well known difficult-to-treat fistulas. The procedure was safe without serious complications; only one patient developed one gastric stricture caused by the OTSC, which was successfully treated with a colonic SEMS and one tearing of the fistula edges occurred in another patient during the procedure[122]. Nevertheless, several endoscopic sessions may be needed ranging from 2 to 7 in one study and failures to correctly place the OTSC may be challenging[123].
The endoscopic injection of two sealant materials, fibrin glue and cyanoacrylate, has been used to occlude the leak orifice. The mechanism of action of fibrin glue is two-fold as follows: Mechanical occlusion by rapid formation of a clot and promotion of wound healing by inducing fibroblast proliferation. Cyanocrilate is a synthetic glue that rapidly solidifies when in contact with weak bases, forming a cast[124]. Some cases have reported using glue injection as the sole therapy to occlude post-bariatric leaks, and frequently multiple applications were needed[125-128]. Because these fistulas were occluded early in the postoperative course where the simple watchful waiting strategy could have been enough, the efficacy of glue sealants as the primary treatment has been questioned[121]. More often, this modality of treatment has been used as an adjunct to SEES (Figures 5 and 6). Accordingly, in one study reporting on percutaneous treatment of gastrocutaneous fistula after LSG in 10 patients, glue injection was only effective when performed after endoscopic stent placement[129].
For refractory gastrocutaneous fistulas after bariatric surgery, successful treatment using a biomaterial plug has also been described. First developed to treat anorectal fistulas, cone-shaped plugs manufactured with Surgisis, an acellular matrix extracted from the porcine small intestine submucosa, have been used to heal postsurgical fistulas in different contexts[130,131]. For gastrocutaneous fistulas, these plugs are inserted through the cutaneous orifice up to the gastric lumen by a rendezvous procedure via endoscopic and percutaneous approaches. Before inserting the plug, the fistulous tract is abraded using a modified pusher provided with multiple barbs over a guide wire. The Surgisis material stimulates the proliferation of fibroblasts in the wound area without triggering a foreign body response. Two studies have presented the results with fistula plugs in patients with post-bariatric gastrocutaneous fistulas refractory either to at least 4 wk of conservative management or to previous endoscopic or surgical treatments[132,133]. In both studies, fistula closure was achieved in 80% without complications using plugs as the sole endoscopic treatment or as an adjunct to SEMS.
Finally, the use of novel endoscopic suturing systems in post-bariatric leaks is still experimental. The StomaphyX™ system (EndoGastric Solutions, Inc., Redmond, WA, United States) and the OverStitch system (Apollo Endosurgery, Austin, Texas, United States) were reported to be successfully used in two bariatric fistulas each[134,135]. Initial experience with the EndoCinch system (CR Bard, Murray Hill, NJ) showed success but not long-lasting efficacy to close gastrogastric fistulas following RYGB. Among 95 fistulas, 90 closed after suturing but reopening was observed in 65% secondary to absorbable sutures after an average of 177 d[136].
As described above, the management of post-bariatric leaks is challenging and requires a multidisciplinary approach. Frequently, endoscopic treatment is an adjunct to surgery although a complete endoscopic approach is also feasible[86]. In both cases, endoscopic treatment involves not only repeated endoscopic procedures but also a combination of different endoscopic modalities of treatment. As a result, there is an important heterogeneity in the management of these patients through the literature and the individual evaluation of each endoscopic modality is very difficult. Accordingly, one recent study has evaluated the overall efficacy of interventional endoscopy in this setting involving different techniques[137]. Among 110 included patients with post-LSG leaks, 74% healed with endoscopic treatment. In chronic fistulas after 6 mo of management, the success rate decreased to 52.4%. The most common endoscopic techniques were stenting, clip placement and glue application. A mean of 4.7 endoscopic procedures and 2.5 endoscopic techniques per patient were needed. The key role of endoscopic treatment relied on stenting, and morbidity in this study was also related to stent placement, mainly migration, impaction and ulceration, digestive perforation and incarceration secondary to tissue hyperplasia. In the multivariate analysis, the authors found the following four predictive factors of healing following endoscopic treatment: The time interval between LSG and fistula diagnosis < 3 d, time interval between fistula diagnosis and first endoscopy < 21 d, no history of gastric banding and a small fistula.
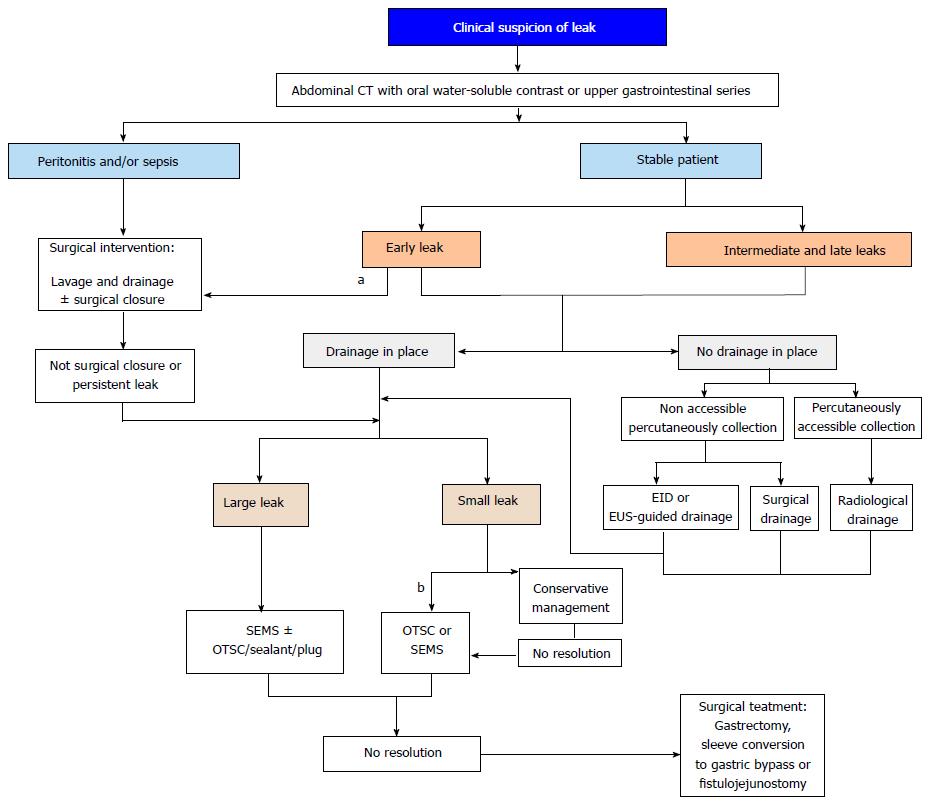
In the last years, EID with double pigtail stents is gaining popularity as a first line endoscopic treatment for early and intermediate leaks after bariatric surgery[87,88]. The abovementioned shortcomings of SEMS have led some experts to carefully reevaluate their place in the treatment of post-bariatric leaks and to consider EID as an attractive alternative with fewer complications and similar success rate[88]. In chronic fistulas the endoscopic treatment should be always offered before considering radical surgery. However, the results of endoluminal therapy, including SEMS, in chronic leaks after bariatric surgery are far from optimal[51]. Recently, promising results with the septotomy procedure in the management of chronic leaks connected to a not drained cavity have led some experts to favor this technique against SEMS[92,93]. Yet, endoscopic treatment in the field of post-bariatric leaks has not been standardized and there is no consensus about the optimal timing and combination of endoscopic procedures. For this purpose, prospective randomized studies are eagerly awaited. Until then, and based on available evidence at present, we propose an algorithm of treatment for leaks after bariatric surgery (Figure 7).
Physiologic changes, such as hypersaturation of bile with cholesterol secondary to rapid weight loss as it occurs after bariatric surgery, induce a lithogenic state[138,139]. Further complicating the scenario, cholecystectomy is not systematically performed during laparoscopic techniques of bariatric surgery. The decreased gallbladder emptying after RYGB also contributes to gallstone formation[140,141]. Subsequently, cholelithiasis is diagnosed in up to 50% of patients following RYGB and therefore, complications secondary to gallstones, such as choledocolithiasis, are common[141]. Whereas performing endoscopic retrograde cholangiography (ERCP) in patients with LAGB or SG is straightforward because conventional transoral ERCP is feasible, this is not the case for RYGB. Accessing the biliary tree after RYGB is challenging and involves crossing the gastrojejunostomy, going down the Roux limb to arrive at the jejunojejunal anastomosis and up through the biliopancreatic limb to reach the papilla. The long length of the small bowel that the endoscope must traverse and the sharp angulation at the jejunojejunal anastomosis are the main constraints that render ERCP in patients with RYGB very difficult, even impossible[142].
In the attempt to overcome these limitations, alternative routes have been proposed (Figure 8). The most important one is laparoscopy-assisted ERCP. After the first laparoscopic gastrostomy was created intentionally to perform ERCP in 2002, experience in the procedure has accumulated and it is now a widely accepted technique to perform ERCP in postbariatric RYGB (Table 3)[143-154].
| Ref. | No. ERCP1 | Success of CBD cannulation (%) | ERCP Findings | Operative time (min) | Complications related to ERCP | Complications related to laparoscopic transgastric access |
| Ceppa et al[144] | 5 | 80 (4/5) | 2 BDS/2 CBD stones/1 CBD sludge | NA | None | None |
| Patel et al[145] | 6 | 100 | 4 BPS/1 CBD stones/1 normal | NA | None | None |
| Roberts et al[146] | 5 | 100 | 2 BPS/2 SOD/1 CBD stone | 64-93 | None | None |
| Gutierrez et al[147] | 23 | 100 | 3 CBC stone/1 PC/2 N/9 SOD/5 BPS/1 cholecystitis/1 BPS + SOD/1BSP+ PS | 200 (98-138) | 1 postERCP pancreatitis | 17% 2 leak after g-tube removal/1 converted to open/1 gastrostomy site infection |
| Lopes et al[148] | 9 | 89 (8/9) | 3 BPS/1 CBD stone/2 N/2 SOD | 89 (41-245) | 2 postERCP pancreatitis | 11% 1 pneumotorax |
| Bertin et al[149] | 22 | 100 (20/20)2 | 18 SOD/4 Recurrent pancreatitis | 226 | 1 retroperitoneal perforation | 5% 1 hematoma of the abdominal wall |
| Richardson et al[150] | 11 | 100 | 7 CBD stone/2 BPS/1 SOD/1 CP | NA | None | None |
| Saleem et al[151] | 15 | 100 | 5 BPS/2 CBD stone/3 CBD sludge/1 PD/1 SOD/1 BPS + SOD/1 BPS + CBD stenosis/1 biliary leak | NA | None | None |
| Schreiner et al[152] | 24 | 100 | 20 BPS/3 CBD stones/1 PC | 172 | 1 postERCP pancreatitis | 8% 1 enterocutaneous fistula |
| Falcão et al[153] | 23 | 100 | 17 CBD stone/6 BPS 17 CBD stone/1 CBD sludge/1 BSP | 93 | 1 postERCP pancreatitis | None |
| Snauwaert et al[154] | 23 | 100 | 1 N/1 CBD transection | NA | None | 9% 2 converted to open |
During LA-ERCP, the standard laparoscopy is performed and once the pneumoperitoneum has been established, a gastrostomy is created on the greater curve of the excluded stomach. Then, a 15-mm trocar is placed into the gastrostomy through the abdominal wall at the upper left quadrant. A conventional duodenoscope is finally passed through the trocar into the gastric remnant and progressed through the pylorus into the duodenum. A review of the literature yields a rate of successful ERCP of 80% to 100% although most studies reported a 100% success rate. Complications related to the laparoscopic transgastric access were observed in 0% to 17% of cases. The length of the entire procedure was variable and reported operative times ranged from 41 to 245 min. However, the ERCP time was not reported in the majority of studies and in many cases, the surgical procedure included cholecystectomy and/or closure of internal hernias found during laparoscopic exploration.
Technological advances in endoscopy have made access of the biliary tree in the conventional transoral route feasible. Endoscopically-assisted ERCP options include double or single balloon assisted ERCP (BEA-ERCP), spiral overtube-assisted ERCP, percutaneous transgastric ERCP and endoscopic ultrasound-directed transgastric ERCP. Double and single balloon enteroscopes, initially conceived for deeper access to the small bowel, are useful for performing ERCP in patients with complex postsurgical anatomy with similar success rates[155-157]. Several studies have evaluated the performance of both enteroscopes in ERCP in patients with Roux-en-Y anatomy. Reaching the blind end was possible in 69%-100% of patients and in these cases ERCP was satisfactorily accomplished in 78%-100%[152,157-166].
However, the majority of patients in these had a non-bariatric RYGB where the Roux limb is usually shorter. One study directly compared the performance of LA-ERCP and BEA-ERCP[152]. In this study, a total of 32 patients with bariatric RYGB underwent BEA-ERCP, either with a single or double balloon, and 24 underwent LA-ERCP. Identification of papilla was possible in 72% and cannulation and therapeutic success was achieved in 59% of patients. The impossibility of reaching the papilla was responsible for the majority of failures; in fact, a limb length (length of the Roux limb plus distance from the Treitz ligament to the jejunojejunal anastomosis) less than 150 cm was the only factor significantly associated with therapeutic success. One case of mild pancreatitis was the only complication observed in this study. Nevertheless, LA-ERCP was found to be superior with rates of papilla identification, cannulation and therapeutic success of 100% each (P = 0.005, P < 0.001, P < 0.001, respectively) without more complications (one mild pancreatitis and one enterocutaneous fistula). Although the mean procedure time was shorter in the BEA-ERCP group (106 min vs 172 min, P < 0.001), the endoscopic time was longer (106 min vs 75 min, P = 0.006) reflecting the higher complexity of the endoscopic procedure with BEA-ERCP. One reason why BEA-ERCP compares poorly with LA-ERCP is the specific challenges that BEA-ERCP with forward view endoscopes must face. In addition to the long length of the small bowel that must be traversed, it is more difficult to obtain a frontal view of the papilla making the cannulation of a native papilla difficult. Once the papilla is cannulated, the lack of an elevator and the long working channel with limited diameter render therapeutic procedures challenging. Most conventional accessories used in ERCP are shorter than the enteroscope working channel. Although this has been overcome with new short-type enteroscopes with 152 cm length (Fujifilm, Osaka, Japan), the diameter is still limited to allow the insertion of certain devices.
Spiral enteroscopy is a deep enteroscopy technique that uses an overtube with a spiral at the distal end (Endo-Ease Discovery SB overtube, Spirus Medical, Stoughton, MA, United States). While clockwise rotation is applied to the overtube, the spiral transforms the rotational energy into linear force to fold the small bowel on the endoscope allowing for enteroscope advancement[167,168]. Although few series have reported on spiral overtube-assisted ERCP, this technique seems to perform similarly to double or single balloon-assisted ERCP but no specific data are available in patients with bariatric RYGB[157,169-175].
Another route to try ERCP in RYGB is through a gastrostomy approach. In two different studies, 28 and 44 patients respectively underwent ERCP through laparoscopic or open surgical gastrostomy at the gastric remnant[147,171]. In the first study, the ERCP was performed on the same day of the gastrostomy procedure after tacking the stomach to the abdominal wall, whereas in the second study, ERCP was deferred 4-6 wk to allow for gastrostomy tract maturity. After completing ERCP on the same day of gastrostomy creation, a gastrostomy tube was placed to allow for tract maturation before its removal no sooner than 4 wk. In cases of a mature tract, the gastrostomy tube was either completely removed or replaced when further ERCP procedures were thought to be needed. In these two studies, ERCP was successful in 100% and 97% of patients, and complications were observed in 18 and 14.5%, respectively. Most complications were related to the gastrostomy. When compared with BEA-ERCP, ERCP via gastrostomy was more successful but its morbidity was also significantly higher[171]. Percutaneous-assisted transprosthetic endoscopy therapy is a new technique that allows for single-session ERCP via retrograde percutaneous endoscopic gastrostomy[172,173]. Gastrostomy is created by using a single or double balloon enteroscope to access the excluded stomach, followed by placement of a fully-covered self-expandable metal stent. Conventional ERCP is then performed with a standard side-viewing endoscope through the previously dilated stent. Another alternative is the endoscopic ultrasound directed transgastric ERCP (EDGE)[174]. This technique uses endoscopic ultrasound guidance to perform the gastrostomy, identifying the gastric remnant from the gastric pouch. One week later, transgastric ERCP is performed after dilatation of the gastrostomy tract. To avoid a two stage procedure, a variation of the last technique (internal EDGE) consists of performing a EUS-guided gastrogastric fistula[175]. Then, a fully-covered tissue apposition stent is placed to keep the fistula open and to avoid leakage. The stent is dilated and a conventional duodenoscope is passed through the stent into the gastric remnant and ERCP is performed in an anterograde fashion. All of these innovative techniques have shown promising outcomes in some patients and are appealing due to their minimally invasive character. However, experience is still very limited.
Despite having several options for accessing the biliary tree in patients with RYGB instead of conventional ERCP, either surgically or endoscopically assisted, if cholecystectomy has not been performed previously, LA-ERCP at the time of laparoscopic cholecystectomy seems to be the most suitable approach. Otherwise, attempting enteroscopy-assisted ERCP (EA-ERCP) first, either with a balloon enteroscope or a spiral overtube, seems reasonable because these techniques are less invasive. In cases of EA-ERCP failure, continuing with LA-ERCP is a cost-saving strategy[152]. There is less experience with other techniques although placing a gastrostomy tube may be practical when repeated ERCP procedures are expected.
As obesity becomes more prevalent, weight loss treatment, particularly bariatric surgery, is becoming a more established therapy. Some complications of bariatric surgery, such as leaks, are severe and potentially fatal and their treatment is challenging. Technological enhancements in endoscopy have led to endoscopic management of post-bariatric complications gaining popularity, either as a first-line treatment or as complementary therapy to surgery. In some cases, several endoscopic modalities are available, and, although reported results are promising, evidence in the literature is often weak and is almost entirely from retrospective and small case series. There is a need for standardization and guidelines to assist physicians to address these complications. For this purpose, larger and prospective trials are needed to clearly define the place and optimal timing of endoscopic treatment and determine whether one endoscopic option is superior to surgery or other endoscopic modalities.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Eizaguirre E, Ishaq S, Rabago L S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | American Society for Metabolic and Bariatric Surgery. Obesity in America (Fact sheet). asmbs.org. 2013;([accessed 2016 Aug 22]) Available from: https://asmbs.org/wp/uploads/2014/05/Obesity-in-America.pdf. |
| 3. | Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1004] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 4. | Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5073] [Cited by in RCA: 4706] [Article Influence: 224.1] [Reference Citation Analysis (1)] |
| 5. | Huang CS, Forse RA, Jacobson BC, Farraye FA. Endoscopic findings and their clinical correlations in patients with symptoms after gastric bypass surgery. Gastrointest Endosc. 2003;58:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Ferreira LE, Song LM, Baron TH. Management of acute postoperative hemorrhage in the bariatric patient. Gastrointest Endosc Clin N Am. 2011;21:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Khoursheed M, Al-Bader I, Mouzannar A, Ashraf A, Bahzad Y, Al-Haddad A, Sayed A, Fingerhut A. Postoperative Bleeding and Leakage After Sleeve Gastrectomy: a Single-Center Experience. Obes Surg. 2016;26:2944-2951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Rao AD, Ramalingam G. Exsanguinating hemorrhage following gastric erosion after laparoscopic adjustable gastric banding. Obes Surg. 2006;16:1675-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Heneghan HM, Meron-Eldar S, Yenumula P, Rogula T, Brethauer SA, Schauer PR. Incidence and management of bleeding complications after gastric bypass surgery in the morbidly obese. Surg Obes Relat Dis. 2012;8:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Nguyen NT, Rivers R, Wolfe BM. Early gastrointestinal hemorrhage after laparoscopic gastric bypass. Obes Surg. 2003;13:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Spaw AT, Husted JD. Bleeding after laparoscopic gastric bypass: Case report and literature review. Surg Obes Relat Dis. 2005;1:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Fernández-Esparrach G, Bordas JM, Pellisé M, Gimeno-García AZ, Lacy A, Delgado S, Cárdenas A, Ginès A, Sendino O, Momblán D. Endoscopic management of early GI hemorrhage after laparoscopic gastric bypass. Gastrointest Endosc. 2008;67:552-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Jamil LH, Krause KR, Chengelis DL, Jury RP, Jackson CM, Cannon ME, Duffy MC. Endoscopic management of early upper gastrointestinal hemorrhage following laparoscopic Roux-en-Y gastric bypass. Am J Gastroenterol. 2008;103:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Tang SJ, Rivas H, Tang L, Lara LF, Sreenarasimhaiah J, Rockey DC. Endoscopic hemostasis using endoclip in early gastrointestinal hemorrhage after gastric bypass surgery. Obes Surg. 2007;17:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Dallal RM, Bailey LA. Ulcer disease after gastric bypass surgery. Surg Obes Relat Dis. 2006;2:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Azagury DE, Abu Dayyeh BK, Greenwalt IT, Thompson CC. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43:950-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Csendes A, Burgos AM, Altuve J, Bonacic S. Incidence of marginal ulcer 1 month and 1 to 2 years after gastric bypass: a prospective consecutive endoscopic evaluation of 442 patients with morbid obesity. Obes Surg. 2009;19:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Rawlins L, Rawlins MP, Brown CC, Schumacher DL. Effect of Helicobacter pylori on marginal ulcer and stomal stenosis after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Rasmussen JJ, Fuller W, Ali MR. Marginal ulceration after laparoscopic gastric bypass: an analysis of predisposing factors in 260 patients. Surg Endosc. 2007;21:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Kaplan LM. Gastrointestinal management of the bariatric surgery patient. Gastroenterol Clin North Am. 2005;34:105-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Frezza EE, Herbert H, Ford R, Wachtel MS. Endoscopic suture removal at gastrojejunal anastomosis after Roux-en-Y gastric bypass to prevent marginal ulceration. Surg Obes Relat Dis. 2007;3:619-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Ryou M, Mogabgab O, Lautz DB, Thompson CC. Endoscopic foreign body removal for treatment of chronic abdominal pain in patients after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Weiner R, Blanco-Engert R, Weiner S, Matkowitz R, Schaefer L, Pomhoff I. Outcome after laparoscopic adjustable gastric banding - 8 years experience. Obes Surg. 2003;13:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Hookey LC, Mehdi A, Le Moine O, Devière J. Removal of a gastroplasty ring. Gastrointest Endosc. 2005;61:594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Blero D, Eisendrath P, Vandermeeren A, Closset J, Mehdi A, Le Moine O, Devière J. Endoscopic removal of dysfunctioning bands or rings after restrictive bariatric procedures. Gastrointest Endosc. 2010;71:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Mozzi E, Lattuada E, Zappa MA, Granelli P, De Ruberto F, Armocida A, Roviaro G. Treatment of band erosion: feasibility and safety of endoscopic band removal. Surg Endosc. 2011;25:3918-3922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Chisholm J, Kitan N, Toouli J, Kow L. Gastric band erosion in 63 cases: endoscopic removal and rebanding evaluated. Obes Surg. 2011;21:1676-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Neto MP, Ramos AC, Campos JM, Murakami AH, Falcão M, Moura EH, Evangelista LF, Escalona A, Zundel N. Endoscopic removal of eroded adjustable gastric band: lessons learned after 5 years and 78 cases. Surg Obes Relat Dis. 2010;6:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Sanyal AJ, Sugerman HJ, Kellum JM, Engle KM, Wolfe L. Stomal complications of gastric bypass: incidence and outcome of therapy. Am J Gastroenterol. 1992;87:1165-1169. [PubMed] |
| 30. | Blachar A, Federle MP. Gastrointestinal complications of laparoscopic roux-en-Y gastric bypass surgery in patients who are morbidly obese: findings on radiography and CT. AJR Am J Roentgenol. 2002;179:1437-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Nguyen NT, Stevens CM, Wolfe BM. Incidence and outcome of anastomotic stricture after laparoscopic gastric bypass. J Gastrointest Surg. 2003;7:997-1003; discussion 1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Goitein D, Papasavas PK, Gagné D, Ahmad S, Caushaj PF. Gastrojejunal strictures following laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2005;19:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Csendes A, Burgos AM, Burdiles P. Incidence of anastomotic strictures after gastric bypass: a prospective consecutive routine endoscopic study 1 month and 17 months after surgery in 441 patients with morbid obesity. Obes Surg. 2009;19:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Gonzalez R, Lin E, Venkatesh KR, Bowers SP, Smith CD. Gastrojejunostomy during laparoscopic gastric bypass: analysis of 3 techniques. Arch Surg. 2003;138:181-184. [PubMed] |
| 35. | Espinel J, De-la-Cruz JL, Pinedo E, Canga J, De-la-Cruz F. Stenosis in laparoscopic gastric bypass: management by endoscopic dilation without fluoroscopic guidance. Rev Esp Enferm Dig. 2011;103:508-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Barba CA, Butensky MS, Lorenzo M, Newman R. Endoscopic dilation of gastroesophageal anastomosis stricture after gastric bypass. Surg Endosc. 2003;17:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Go MR, Muscarella P, Needleman BJ, Cook CH, Melvin WS. Endoscopic management of stomal stenosis after Roux-en-Y gastric bypass. Surg Endosc. 2004;18:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Rossi TR, Dynda DI, Estes NC, Marshall JS. Stricture dilation after laparoscopic Roux-en-Y gastric bypass. Am J Surg. 2005;189:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Carrodeguas L, Szomstein S, Zundel N, Lo Menzo E, Rosenthal R. Gastrojejunal anastomotic strictures following laparoscopic Roux-en-Y gastric bypass surgery: analysis of 1291 patients. Surg Obes Relat Dis. 2006;2:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Catalano MF, Chua TY, Rudic G. Endoscopic balloon dilation of stomal stenosis following gastric bypass. Obes Surg. 2007;17:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Peifer KJ, Shiels AJ, Azar R, Rivera RE, Eagon JC, Jonnalagadda S. Successful endoscopic management of gastrojejunal anastomotic strictures after Roux-en-Y gastric bypass. Gastrointest Endosc. 2007;66:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Caro L, Sánchez C, Rodríguez P, Bosch J. Endoscopic balloon dilation of anastomotic strictures occurring after laparoscopic gastric bypass for morbid obesity. Dig Dis. 2008;26:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Ukleja A, Afonso BB, Pimentel R, Szomstein S, Rosenthal R. Outcome of endoscopic balloon dilation of strictures after laparoscopic gastric bypass. Surg Endosc. 2008;22:1746-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Mathew A, Veliuona MA, DePalma FJ, Cooney RN. Gastrojejunal stricture after gastric bypass and efficacy of endoscopic intervention. Dig Dis Sci. 2009;54:1971-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Da Costa M, Mata A, Espinós J, Vila V, Roca JM, Turró J, Ballesta C. Endoscopic dilation of gastrojejunal anastomotic strictures after laparoscopic gastric bypass. Predictors of initial failure. Obes Surg. 2011;21:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Yimcharoen P, Heneghan H, Chand B, Talarico JA, Tariq N, Kroh M, Brethauer SA. Successful management of gastrojejunal strictures after gastric bypass: is timing important? Surg Obes Relat Dis. 2012;8:151-157. [PubMed] |
| 47. | Lee JK, Van Dam J, Morton JM, Curet M, Banerjee S. Endoscopy is accurate, safe, and effective in the assessment and management of complications following gastric bypass surgery. Am J Gastroenterol. 2009;104:575-582; quiz 583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 48. | Thompson CC, Carr-Locke D. Novel endoscopic approaches to common bariatric postoperative complications. ASGE Video Forum: Digestive Disease Week 2004; . |
| 49. | Fernández-Esparrach G, Bordas JM, Llach J, Lacy A, Delgado S, Vidal J, Cárdenas A, Pellisé M, Ginès A, Sendino O. Endoscopic dilation with Savary-Gilliard bougies of stomal strictures after laparosocopic gastric bypass in morbidly obese patients. Obes Surg. 2008;18:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Escalona A, Devaud N, Boza C, Pérez G, Fernández J, Ibáñez L, Guzmán S. Gastrojejunal anastomotic stricture after Roux-en-Y gastric bypass: ambulatory management with the Savary-Gilliard dilator. Surg Endosc. 2007;21:765-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Puig CA, Waked TM, Baron TH, Wong Kee Song LM, Gutierrez J, Sarr MG. The role of endoscopic stents in the management of chronic anastomotic and staple line leaks and chronic strictures after bariatric surgery. Surg Obes Relat Dis. 2014;10:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Ogra R, Kini GP. Evolving endoscopic management options for symptomatic stenosis post-laparoscopic sleeve gastrectomy for morbid obesity: experience at a large bariatric surgery unit in New Zealand. Obes Surg. 2015;25:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Shnell M, Fishman S, Eldar S, Goitein D, Santo E. Balloon dilatation for symptomatic gastric sleeve stricture. Gastrointest Endosc. 2014;79:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Iannelli A, Martini F, Schneck AS, Gugenheim J. Twisted gastric sleeve. Surgery. 2015;157:163-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Galloro G, Magno L, Musella M, Manta R, Zullo A, Forestieri P. A novel dedicated endoscopic stent for staple-line leaks after laparoscopic sleeve gastrectomy: a case series. Surg Obes Relat Dis. 2014;10:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Hocking MP, Bennett RS, Rout WR, Woodward ER. Pouch outlet obstruction following vertical ring gastroplasty for morbid obesity. Am J Surg. 1990;160:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | Podnos YD, Jimenez JC, Wilson SE, Stevens CM, Nguyen NT. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg. 2003;138:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 447] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 58. | Fuks D, Verhaeghe P, Brehant O, Sabbagh C, Dumont F, Riboulot M, Delcenserie R, Regimbeau JM. Results of laparoscopic sleeve gastrectomy: a prospective study in 135 patients with morbid obesity. Surgery. 2009;145:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 59. | Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26:1509-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 437] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 60. | Weiner R, Blanco-Engert R, Weiner S. Outcome after laparoscopic adjustable gastric banding 8 year experience. Obes Surg. 2003;13:427-434. |
| 61. | Favretti F, Cadiere GB, Segato G. Laparoscopic banding: selection and technique in 830 patients. Obes Surg. 2002;12:385-390. |
| 62. | Chevallier JM, Zinzindohoue F, Douard R. Complications after laparoscopic adjustable gastric banding for morbid obesity: experience with 1,000 patients over 7 years. Obes Surg. 2004;14:407-414. |
| 63. | Morino M, Toppino M, Forestieri P, Angrisani L, Allaix ME, Scopinaro N. Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Ann Surg. 2007;246:1002-1007; discussion 1007-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 64. | Puli SR, Spofford IS, Thompson CC. Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 65. | Fernandez AZ, DeMaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador J, Sugerman HJ. Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc. 2004;18:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 242] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 66. | Ballesta C, Berindoague R, Cabrera M, Palau M, Gonzales M. Management of anastomotic leaks after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 67. | Gonzalez R, Sarr MG, Smith CD, Baghai M, Kendrick M, Szomstein S, Rosenthal R, Murr MM. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J Am Coll Surg. 2007;204:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | Spyropoulos C, Argentou MI, Petsas T, Thomopoulos K, Kehagias I, Kalfarentzos F. Management of gastrointestinal leaks after surgery for clinically severe obesity. Surg Obes Relat Dis. 2012;8:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Deitel M, Gagner M, Erickson AL, Crosby RD. Third International Summit: Current status of sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 70. | Casella G, Soricelli E, Rizzello M, Trentino P, Fiocca F, Fantini A, Salvatori FM, Basso N. Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 71. | de Aretxabala X, Leon J, Wiedmaier G, Turu I, Ovalle C, Maluenda F, Gonzalez C, Humphrey J, Hurtado M, Benavides C. Gastric leak after sleeve gastrectomy: analysis of its management. Obes Surg. 2011;21:1232-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 72. | Carucci LR, Turner MA, Szucs RA. Adjustable laparoscopic gastric banding for morbid obesity: imaging assessment and complications. Radiol Clin North Am. 2007;45:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Acholonu E, McBean E, Court I, Bellorin O, Szomstein S, Rosenthal RJ. Safety and short-term outcomes of laparoscopic sleeve gastrectomy as a revisional approach for failed laparoscopic adjustable gastric banding in the treatment of morbid obesity. Obes Surg. 2009;19:1612-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Berende CA, de Zoete JP, Smulders JF, Nienhuijs SW. Laparoscopic sleeve gastrectomy feasible for bariatric revision surgery. Obes Surg. 2012;22:330-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 75. | Carucci LR, Conklin RC, Turner MA. Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of leak into excluded stomach with upper gastrointestinal examination. Radiology. 2008;248:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Tucker ON, Szomstein S, Rosenthal RJ. Surgical management of gastro-gastric fistula after divided laparoscopic Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg. 2007;11:1673-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Carrodeguas L, Szomstein S, Soto F, Whipple O, Simpfendorfer C, Gonzalvo JP, Villares A, Zundel N, Rosenthal R. Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis. 2005;1:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 78. | Carucci LR, Turner MA, Conklin RC, DeMaria EJ, Kellum JM, Sugerman HJ. Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of postoperative extraluminal leaks with upper gastrointestinal series. Radiology. 2006;238:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Lyass S, Khalili TM, Cunneen S, Fujita F, Otsuka K, Chopra R, Lahmann B, Lublin M, Furman G, Phillips EH. Radiological studies after laparoscopic Roux-en-Y gastric bypass: routine or selective? Am Surg. 2004;70:918-921. [PubMed] |
| 80. | Doraiswamy A, Rasmussen JJ, Pierce J, Fuller W, Ali MR. The utility of routine postoperative upper GI series following laparoscopic gastric bypass. Surg Endosc. 2007;21:2159-2162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Gonzalez R, Nelson LG, Gallagher SF, Murr MM. Anastomotic leaks after laparoscopic gastric bypass. Obes Surg. 2004;14:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Kumar N, Thompson CC. Endoscopic management of complications after gastrointestinal weight loss surgery. Clin Gastroenterol Hepatol. 2013;11:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Thodiyil PA, Yenumula P, Rogula T, Gorecki P, Fahoum B, Gourash W, Ramanathan R, Mattar SG, Shinde D, Arena VC. Selective nonoperative management of leaks after gastric bypass: lessons learned from 2675 consecutive patients. Ann Surg. 2008;248:782-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Jurowich C, Thalheimer A, Seyfried F, Fein M, Bender G, Germer CT, Wichelmann C. Gastric leakage after sleeve gastrectomy-clinical presentation and therapeutic options. Langenbecks Arch Surg. 2011;396:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | Csendes A, Braghetto I, León P, Burgos AM. Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. J Gastrointest Surg. 2010;14:1343-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 86. | Bège T, Emungania O, Vitton V, Ah-Soune P, Nocca D, Noël P, Bradjanian S, Berdah SV, Brunet C, Grimaud JC. An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc. 2011;73:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 87. | Bouchard S, Eisendrath P, Toussaint E, Le Moine O, Lemmers A, Arvanitakis M, Devière J. Trans-fistulary endoscopic drainage for post-bariatric abdominal collections communicating with the upper gastrointestinal tract. Endoscopy. 2016;48:809-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Donatelli G, Dumont JL, Cereatti F, Ferretti S, Vergeau BM, Tuszynski T, Pourcher G, Tranchart H, Mariani P, Meduri A. Treatment of Leaks Following Sleeve Gastrectomy by Endoscopic Internal Drainage (EID). Obes Surg. 2015;25:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 89. | Pequignot A, Fuks D, Verhaeghe P, Dhahri A, Brehant O, Bartoli E, Delcenserie R, Yzet T, Regimbeau JM. Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes Surg. 2012;22:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 90. | Gupta T, Lemmers A, Tan D, Ibrahim M, Le Moine O, Devière J. EUS-guided transmural drainage of postoperative collections. Gastrointest Endosc. 2012;76:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Lemmers A, Tan DM, Ibrahim M, Loi P, De Backer D, Closset J, Devière J, Le Moine O. Transluminal or Percutaneous Endoscopic Drainage and Debridement of Abscesses After Bariatric Surgery: a Case Series. Obes Surg. 2015;25:2190-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 92. | Baretta G, Campos J, Correia S, Alhinho H, Marchesini JB, Lima JH, Neto MG. Bariatric postoperative fistula: a life-saving endoscopic procedure. Surg Endosc. 2015;29:1714-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 93. | Campos JM, Ferreira FC, Teixeira AF, Lima JS, Moon RC, D’Assunção MA, Neto MG. Septotomy and Balloon Dilation to Treat Chronic Leak After Sleeve Gastrectomy: Technical Principles. Obes Surg. 2016;26:1992-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | Seyfried F, Reimer S, Miras AD, Kenn W, Germer CT, Scheurlen M, Jurowich C. Successful treatment of a gastric leak after bariatric surgery using endoluminal vacuum therapy. Endoscopy. 2013;45 Suppl 2 UCTN:E267-E268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis. 2016;12:1278-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 96. | Holle G, Riedel K, von Gregory H, Gazyakan E, Raab N, Germann G. Vacuum-assisted closure therapy. Current status and basic research. Unfallchirurg. 2007;110:490-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, Matter I, Alfici R, Mahajna A, Waksman I. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 98. | Salinas A, Baptista A, Santiago E, Antor M, Salinas H. Self-expandable metal stents to treat gastric leaks. Surg Obes Relat Dis. 2006;2:570-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 99. | Eisendrath P, Cremer M, Himpens J, Cadière GB, Le Moine O, Devière J. Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy. 2007;39:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 100. | Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, Miedema BW, Scott JS. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935-938; discussion 938-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 101. | Blackmon SH, Santora R, Schwarz P, Barroso A, Dunkin BJ. Utility of removable esophageal covered self-expanding metal stents for leak and fistula management. Ann Thorac Surg. 2010;89:931-936; discussion 936-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 102. | Leenders BJ, Stronkhorst A, Smulders FJ, Nieuwenhuijzen GA, Gilissen LP. Removable and repositionable covered metal self-expandable stents for leaks after upper gastrointestinal surgery: experiences in a tertiary referral hospital. Surg Endosc. 2013;27:2751-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 103. | El Mourad H, Himpens J, Verhofstadt J. Stent treatment for fistula after obesity surgery: results in 47 consecutive patients. Surg Endosc. 2013;27:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 104. | Orive-Calzada A, Calderón-García Á, Bernal-Martínez A, Díaz-Roca AB, Barrio-Beraza I, Cabriada-Nuño JL, Orive-Cura VM. Closure of benign leaks, perforations, and fistulas with temporary placement of fully covered metal stents: a retrospective analysis. Surg Laparosc Endosc Percutan Tech. 2014;24:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 105. | Alazmi W, Al-Sabah S, Ali DA, Almazeedi S. Treating sleeve gastrectomy leak with endoscopic stenting: the Kuwaiti experience and review of recent literature. Surg Endosc. 2014;28:3425-3428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Quezada N, Maiz C, Daroch D, Funke R, Sharp A, Boza C, Pimentel F. Effect of Early Use of Covered Self-Expandable Endoscopic Stent on the Treatment of Postoperative Stapler Line Leaks. Obes Surg. 2015;25:1816-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 107. | Murino A, Arvanitakis M, Le Moine O, Blero D, Devière J, Eisendrath P. Effectiveness of Endoscopic Management Using Self-Expandable Metal Stents in a Large Cohort of Patients with Post-bariatric Leaks. Obes Surg. 2015;25:1569-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 108. | Fishman S, Shnell M, Gluck N, Meirsdorf S, Abu-Abeid S, Santo E. Use of sleeve-customized self-expandable metal stents for the treatment of staple-line leakage after laparoscopic sleeve gastrectomy. Gastrointest Endosc. 2015;81:1291-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 109. | Southwell T, Lim TH, Ogra R. Endoscopic Therapy for Treatment of Staple Line Leaks Post-Laparoscopic Sleeve Gastrectomy (LSG): Experience from a Large Bariatric Surgery Centre in New Zealand. Obes Surg. 2016;26:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 110. | van Wezenbeek MR, de Milliano MM, Nienhuijs SW, Friederich P, Gilissen LP. A Specifically Designed Stent for Anastomotic Leaks after Bariatric Surgery: Experiences in a Tertiary Referral Hospital. Obes Surg. 2016;26:1875-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 111. | Shehab HM, Hakky SM, Gawdat KA. An Endoscopic Strategy Combining Mega Stents and Over-The-Scope Clips for the Management of Post-Bariatric Surgery Leaks and Fistulas (with video). Obes Surg. 2016;26:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 112. | Nedelcu M, Manos T, Cotirlet A, Noel P, Gagner M. Outcome of leaks after sleeve gastrectomy based on a new algorithm adressing leak size and gastric stenosis. Obes Surg. 2015;25:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 113. | Simon F, Siciliano I, Gillet A, Castel B, Coffin B, Msika S. Gastric leak after laparoscopic sleeve gastrectomy: early covered self-expandable stent reduces healing time. Obes Surg. 2013;23:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 114. | Galloro G, Ruggiero S, Russo T, Telesca DA, Musella M, Milone M, Manta R. Staple-line leak after sleve gastrectomy in obese patients: A hot topic in bariatric surgery. World J Gastrointest Endosc. 2015;7:843-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Lorenzo-Zúñiga V, Moreno-de-Vega V, Marín I, Boix J. Biodegradable stents in gastrointestinal endoscopy. World J Gastroenterol. 2014;20:2212-2217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 116. | Aras A, Oran E, Bozkurt MA, Halicioglu I, Alis H. Successful treatment of post-sleeve gastric leak with an uncovered biodegradable stent. Acta Endosc. 2014;44:382-384. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 117. | Segalin A, Bonavina L, Bona D, Chella B. Endoscopic clipping: a helpful tool for positioning self-expanding esophageal stents. Endoscopy. 1995;27:348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 118. | Kato H, Fukuchi M, Miyazaki T, Manda R, Faried A, Takita J, Nakajima M, Sohda M, Fukai Y, Masuda N. Endoscopic clips prevent self-expandable metallic stent migration. Hepatogastroenterology. 2007;54:1388-1390. [PubMed] |
| 119. | Fujii LL, Bonin EA, Baron TH, Gostout CJ, Wong Kee Song LM. Utility of an endoscopic suturing system for prevention of covered luminal stent migration in the upper GI tract. Gastrointest Endosc. 2013;78:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 120. | Sharaiha RZ, Kumta NA, Doukides TP, Eguia V, Gonda TA, Widmer JL, Turner BG, Poneros JM, Gaidhane M, Kahaleh M. Esophageal Stenting With Sutures: Time to Redefine Our Standards? J Clin Gastroenterol. 2015;49:e57-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 121. | Surace M, Mercky P, Demarquay JF, Gonzalez JM, Dumas R, Ah-Soune P, Vitton V, Grimaud J, Barthet M. Endoscopic management of GI fistulae with the over-the-scope clip system (with video). Gastrointest Endosc. 2011;74:1416-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 122. | Mercky P, Gonzalez JM, Aimore Bonin E, Emungania O, Brunet J, Grimaud JC, Barthet M. Usefulness of over-the-scope clipping system for closing digestive fistulas. Dig Endosc. 2015;27:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 123. | Keren D, Eyal O, Sroka G, Rainis T, Raziel A, Sakran N, Goitein D, Matter I. Over-the-Scope Clip (OTSC) System for Sleeve Gastrectomy Leaks. Obes Surg. 2015;25:1358-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 124. | Bhat YM, Banerjee S, Barth BA, Chauhan SS, Gottlieb KT, Konda V, Maple JT, Murad FM, Pfau PR, Pleskow DK. Tissue adhesives: cyanoacrylate glue and fibrin sealant. Gastrointest Endosc. 2013;78:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 125. | Papavramidis ST, Eleftheriadis EE, Apostolidis DN, Kotzampassi KE. Endoscopic fibrin sealing of high-output non-healing gastrocutaneous fistulas after vertical gastroplasty in morbidly obese patients. Obes Surg. 2001;11:766-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 126. | Papavramidis ST, Eleftheriadis EE, Papavramidis TS, Kotzampassi KE, Gamvros OG. Endoscopic management of gastrocutaneous fistula after bariatric surgery by using a fibrin sealant. Gastrointest Endosc. 2004;59:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 127. | Kowalski C, Kastuar S, Mehta V, Brolin RE. Endoscopic injection of fibrin sealant in repair of gastrojejunostomy leak after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2007;3:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 128. | Victorzon M, Victorzon S, Peromaa-Haavisto P. Fibrin glue and stents in the treatment of gastrojejunal leaks after laparoscopic gastric bypass: a case series and review of the literature. Obes Surg. 2013;23:1692-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 129. | Vilallonga R, Himpens J, Bosch B, van de Vrande S, Bafort J. Role of Percutaneous Glue Treatment After Persisting Leak After Laparoscopic Sleeve Gastrectomy. Obes Surg. 2016;26:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 130. | Champagne BJ, O’Connor LM, Ferguson M, Orangio GR, Schertzer ME, Armstrong DN. Efficacy of anal fistula plug in closure of cryptoglandular fistulas: long-term follow-up. Dis Colon Rectum. 2006;49:1817-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 131. | Johnson EK, Gaw JU, Armstrong DN. Efficacy of anal fistula plug vs. fibrin glue in closure of anorectal fistulas. Dis Colon Rectum. 2006;49:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 132. | Toussaint E, Eisendrath P, Kwan V, Dugardeyn S, Devière J, Le Moine O. Endoscopic treatment of postoperative enterocutaneous fistulas after bariatric surgery with the use of a fistula plug: report of five cases. Endoscopy. 2009;41:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 133. | Maluf-Filho F, Hondo F, Halwan B, de Lima MS, Giordano-Nappi JH, Sakai P. Endoscopic treatment of Roux-en-Y gastric bypass-related gastrocutaneous fistulas using a novel biomaterial. Surg Endosc. 2009;23:1541-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 134. | Overcash WT. Natural orifice surgery (NOS) using StomaphyX for repair of gastric leaks after bariatric revisions. Obes Surg. 2008;18:882-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Cai JX, Khashab MA, Okolo PI, Kalloo AN, Kumbhari V. Full-thickness endoscopic suturing of staple-line leaks following laparoscopic sleeve gastrectomy. Endoscopy. 2014;46 Suppl 1 UCTN:E623-E624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Fernandez-Esparrach G, Lautz DB, Thompson CC. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: a less-invasive approach. Surg Obes Relat Dis. 2010;6:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 137. | Christophorou D, Valats JC, Funakoshi N, Duflos C, Picot MC, Vedrenne B, Prat F, Bulois P, Branche J, Decoster S. Endoscopic treatment of fistula after sleeve gastrectomy: results of a multicenter retrospective study. Endoscopy. 2015;47:988-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 138. | Carey MC. Pathogenesis of gallstones. Am J Surg. 1993;165:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 139. | Iglézias Brandão de Oliveira C, Adami Chaim E, da Silva BB. Impact of rapid weight reduction on risk of cholelithiasis after bariatric surgery. Obes Surg. 2003;13:625-628. [PubMed] |
| 140. | Bastouly M, Arasaki CH, Ferreira JB, Zanoto A, Borges FG, Del Grande JC. Early changes in postprandial gallbladder emptying in morbidly obese patients undergoing Roux-en-Y gastric bypass: correlation with the occurrence of biliary sludge and gallstones. Obes Surg. 2009;19:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 141. | Shiffman ML, Sugerman HJ, Kellum JM, Brewer WH, Moore EW. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86:1000-1005. [PubMed] |
| 142. | Wright BE, Cass OW, Freeman ML. ERCP in patients with long-limb Roux-en-Y gastrojejunostomy and intact papilla. Gastrointest Endosc. 2002;56:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 196] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 143. | Peters M, Papasavas PK, Caushaj PF, Kania RJ, Gagné DJ. Laparoscopic transgastric endoscopic retrograde cholangiopancreatography for benign common bile duct stricture after Roux-en-Y gastric bypass. Surg Endosc. 2002;16:1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 144. | Ceppa FA, Gagné DJ, Papasavas PK, Caushaj PF. Laparoscopic transgastric endoscopy after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2007;3:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 145. | Patel JA, Patel NA, Shinde T, Uchal M, Dhawan MK, Kulkarni A, Colella JJ. Endoscopic retrograde cholangiopancreatography after laparoscopic Roux-en-Y gastric bypass: a case series and review of the literature. Am Surg. 2008;74:689-993; discussion 693-694. [PubMed] |
| 146. | Roberts KE, Panait L, Duffy AJ, Jamidar PA, Bell RL. Laparoscopic-assisted transgastric endoscopy: current indications and future implications. JSLS. 2008;12:30-36. [PubMed] |
| 147. | Gutierrez JM, Lederer H, Krook JC, Kinney TP, Freeman ML, Jensen EH. Surgical gastrostomy for pancreatobiliary and duodenal access following Roux en Y gastric bypass. J Gastrointest Surg. 2009;13:2170-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 148. | Lopes TL, Clements RH, Wilcox CM. Laparoscopy-assisted ERCP: experience of a high-volume bariatric surgery center (with video). Gastrointest Endosc. 2009;70:1254-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 149. | Bertin PM, Singh K, Arregui ME. Laparoscopic transgastric endoscopic retrograde cholangiopancreatography (ERCP) after gastric bypass: case series and a description of technique. Surg Endosc. 2011;25:2592-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 150. | Richardson JF, Lee JG, Smith BR, Nguyen B, Pham KP, Nguyen NT. Laparoscopic transgastric endoscopy after Roux-en-Y gastric bypass: case series and review of the literature. Am Surg. 2012;78:1182-1186. [PubMed] |
| 151. | Saleem A, Levy MJ, Petersen BT, Que FG, Baron TH. Laparoscopic assisted ERCP in Roux-en-Y gastric bypass (RYGB) surgery patients. J Gastrointest Surg. 2012;16:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 152. | Schreiner MA, Chang L, Gluck M, Irani S, Gan SI, Brandabur JJ, Thirlby R, Moonka R, Kozarek RA, Ross AS. Laparoscopy-assisted versus balloon enteroscopy-assisted ERCP in bariatric post-Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 153. | Falcão M, Campos JM, Galvão Neto M, Ramos A, Secchi T, Alves E, Franca E, Maluf-Filho F, Ferraz A. Transgastric endoscopic retrograde cholangiopancreatography for the management of biliary tract disease after Roux-en-Y gastric bypass treatment for obesity. Obes Surg. 2012;22:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 154. | Snauwaert C, Laukens P, Dillemans B, Himpens J, De Looze D, Deprez PH, Badaoui A. Laparoscopy-assisted transgastric endoscopic retrograde cholangiopancreatography in bariatric Roux-en-Y gastric bypass patients. Endosc Int Open. 2015;3:E458-E463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 155. | Koornstra JJ. Double balloon enteroscopy for endoscopic retrograde cholangiopancreaticography after Roux-en-Y reconstruction: case series and review of the literature. Neth J Med. 2008;66:275-279. [PubMed] |
| 156. | Mönkemüller K, Fry LC, Bellutti M, Neumann H, Malfertheiner P. ERCP with the double balloon enteroscope in patients with Roux-en-Y anastomosis. Surg Endosc. 2009;23:1961-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 157. | Shah RJ, Smolkin M, Yen R, Ross A, Kozarek RA, Howell DA, Bakis G, Jonnalagadda SS, Al-Lehibi AA, Hardy A. A multicenter, U.S. experience of single-balloon, double-balloon, and rotational overtube-assisted enteroscopy ERCP in patients with surgically altered pancreaticobiliary anatomy (with video). Gastrointest Endosc. 2013;77:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 158. | Shimatani M, Matsushita M, Takaoka M, Koyabu M, Ikeura T, Kato K, Fukui T, Uchida K, Okazaki K. Effective “short” double-balloon enteroscope for diagnostic and therapeutic ERCP in patients with altered gastrointestinal anatomy: a large case series. Endoscopy. 2009;41:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 159. | Siddiqui AA, Chaaya A, Shelton C, Marmion J, Kowalski TE, Loren DE, Heller SJ, Haluszka O, Adler DG, Tokar JL. Utility of the short double-balloon enteroscope to perform pancreaticobiliary interventions in patients with surgically altered anatomy in a US multicenter study. Dig Dis Sci. 2013;58:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 160. | Osoegawa T, Motomura Y, Akahoshi K, Higuchi N, Tanaka Y, Hisano T, Itaba S, Gibo J, Yamada M, Kubokawa M. Improved techniques for double-balloon-enteroscopy-assisted endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2012;18:6843-6849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 161. | Saleem A, Baron TH, Gostout CJ, Topazian MD, Levy MJ, Petersen BT, Wong Kee Song LM. Endoscopic retrograde cholangiopancreatography using a single-balloon enteroscope in patients with altered Roux-en-Y anatomy. Endoscopy. 2010;42:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 162. | Azeem N, Tabibian JH, Baron TH, Orhurhu V, Rosen CB, Petersen BT, Gostout CJ, Topazian MD, Levy MJ. Use of a single-balloon enteroscope compared with variable-stiffness colonoscopes for endoscopic retrograde cholangiography in liver transplant patients with Roux-en-Y biliary anastomosis. Gastrointest Endosc. 2013;77:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 163. | Itokawa F, Itoi T, Ishii K, Sofuni A, Moriyasu F. Single- and double-balloon enteroscopy-assisted endoscopic retrograde cholangiopancreatography in patients with Roux-en-Y plus hepaticojejunostomy anastomosis and Whipple resection. Dig Endosc. 2014;26 Suppl 2:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 164. | Kawamura T, Uno K, Suzuki A, Mandai K, Nakase K, Tanaka K, Yasuda K. Clinical usefulness of a short-type, prototype single-balloon enteroscope for endoscopic retrograde cholangiopancreatography in patients with altered gastrointestinal anatomy: preliminary experiences. Dig Endosc. 2015;27:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 165. | Yamauchi H, Kida M, Okuwaki K, Miyazawa S, Iwai T, Takezawa M, Kikuchi H, Watanabe M, Imaizumi H, Koizumi W. Short-type single balloon enteroscope for endoscopic retrograde cholangiopancreatography with altered gastrointestinal anatomy. World J Gastroenterol. 2013;19:1728-1735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 166. | Iwai T, Kida M, Yamauchi H, Imaizumi H, Koizumi W. Short-type and conventional single-balloon enteroscopes for endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy: single-center experience. Dig Endosc. 2014;26 Suppl 2:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 167. | Akerman PA, Haniff M. Spiral enteroscopy: prime time or for the happy few? Best Pract Res Clin Gastroenterol. 2012;26:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 168. | Lennon AM, Kapoor S, Khashab M, Corless E, Amateau S, Dunbar K, Chandrasekhara V, Singh V, Okolo PI. Spiral assisted ERCP is equivalent to single balloon assisted ERCP in patients with Roux-en-Y anatomy. Dig Dis Sci. 2012;57:1391-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 169. | Wagh MS, Draganov PV. Prospective evaluation of spiral overtube-assisted ERCP in patients with surgically altered anatomy. Gastrointest Endosc. 2012;76:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 170. | Skinner M, Popa D, Neumann H, Wilcox CM, Mönkemüller K. ERCP with the overtube-assisted enteroscopy technique: a systematic review. Endoscopy. 2014;46:560-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 171. | Choi EK, Chiorean MV, Coté GA, El Hajj II, Ballard D, Fogel EL, Watkins JL, McHenry L, Sherman S, Lehman GA. ERCP via gastrostomy vs. double balloon enteroscopy in patients with prior bariatric Roux-en-Y gastric bypass surgery. Surg Endosc. 2013;27:2894-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 172. | Law R, Wong Kee Song LM, Petersen BT, Baron TH. Single-session ERCP in patients with previous Roux-en-Y gastric bypass using percutaneous-assisted transprosthetic endoscopic therapy: a case series. Endoscopy. 2013;45:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 173. | Saxena P, Azola A, Kumbhari V, Kalloo AN, Khashab MA. Percutaneous through-the-stent assisted ERCP in patients with Roux-en-Y gastric bypass. Gastrointest Endosc. 2014;80:163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 174. | Kedia P, Kumta NA, Widmer J, Sundararajan S, Cerefice M, Gaidhane M, Sharaiha R, Kahaleh M. Endoscopic ultrasound-directed transgastric ERCP (EDGE) for Roux-en-Y anatomy: a novel technique. Endoscopy. 2015;47:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 175. | Kedia P, Sharaiha RZ, Kumta NA, Kahaleh M. Internal EUS-directed transgastric ERCP (EDGE): game over. Gastroenterology. 2014;147:566-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |