Published online Dec 16, 2017. doi: 10.4253/wjge.v9.i12.583
Peer-review started: August 4, 2017
First decision: September 1, 2017
Revised: September 4, 2017
Accepted: October 16, 2017
Article in press: October 17, 2017
Published online: December 16, 2017
Processing time: 128 Days and 1.1 Hours
Duodenal gastrointestinal stromal tumors (GISTs) are extremely rare disease entities, and the extraluminal type is difficult to diagnose. These tumors have been misdiagnosed as pancreatic tumors; hence, pancreaticoduodenectomy has been performed, although partial duodenectomy can be performed if accurately diagnosed. Developing a diagnostic methodology including endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA) has allowed us to diagnose the tumor directly through the duodenum. Here, we present a case of a 50-year-old woman with a 27-mm diameter tumor in the pancreatic uncus on computed tomography scan. EUS showed a well-defined hypoechoic mass in the pancreatic uncus that connected to the duodenal proper muscular layer and was followed by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). Histological examination showed spindle-shaped tumor cells positively stained for c-kit. Based on these findings, the tumor was finally diagnosed as a duodenal GIST of the extraluminal type, and the patient underwent successful mass resection with partial resection of the duodenum. This case suggests that EUS and EUS-FNA are effective for diagnosing the extraluminal type of duodenal GISTs, which is difficult to differentiate from pancreatic head tumor, and for performing the correct surgical procedure.
Core tip: Duodenal gastrointestinal stromal tumors are extremely rare disease entities, and the extraluminal type is difficult to diagnose. Therefore, these tumors have been misdiagnosed as pancreatic tumors; hence, pancreaticoduodenectomy has been performed, although partial duodenectomy can be performed if accurately diagnosed. Recent advances in developing endoscopic ultrasonography and endoscopic ultrasound-guided fine-needle aspiration are helpful for accurate diagnosis of the tumors located in the area and effective for performing the correct surgical procedure.
- Citation: Hayashi K, Kamimura K, Hosaka K, Ikarashi S, Kohisa J, Takahashi K, Tominaga K, Mizuno K, Hashimoto S, Yokoyama J, Yamagiwa S, Takizawa K, Wakai T, Umezu H, Terai S. Endoscopic ultrasound-guided fine-needle aspiration for diagnosing a rare extraluminal duodenal gastrointestinal tumor. World J Gastrointest Endosc 2017; 9(12): 583-589
- URL: https://www.wjgnet.com/1948-5190/full/v9/i12/583.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i12.583
Gastrointestinal stromal tumors (GISTs) are a group of mesenchymal tumors in the gastrointestinal tract that arise from the interstitial cells of Cajal[1]. These tumors contribute to about 1%-3% of all gastrointestinal malignancies and are frequently found in the stomach (60%-70%). Duodenal GISTs are very rare, with 5% rate of occurrence[2]. They are thought to be caused by a mutation in the c-kit gene and alpha-type platelet-derived growth factor receptor gene in the intestinal cells of Cajal or their precursors[3]. Due to its rarity and the complex anatomy of the pancreaticoduodenal region, it is extremely difficult to differentially diagnose duodenal GISTs from pancreatic tumors, especially when it is extraluminal. Because misdiagnosis may lead to an inaccurate choice of surgical procedure, we report our case of extraluminal-type duodenal GISTs correctly diagnosed with endoscopic ultrasonography (EUS) and EUS-guided fine-needle aspiration (EUS-FNA) followed by successful resection of the tumor. To date, the usefulness of these modalities in diagnosing the tumor has not been reported. This case suggests that EUS and EUS-FNA are effective for diagnosing extraluminal type of duodenal GISTs and for performing the correct surgical procedure.
A 50-year-old Japanese woman was found to have a pancreatic head tumor by abdominal ultrasonography on a health checkup and was referred to our hospital for further examination. She was in good physical condition, no evidence of melena, and had no remarkable history. The results of her initial physical examination were as follows: Body temperature, 37.0 °C blood pressure, 127/78 mmHg; pulse rate, 74 bpm, regular; a flat and soft abdomen without pain or tenderness; and no palpable masses.
Blood tests performed on admission revealed a slight elevated inflammatory response with a white blood cell count of 11370/μL and C-reactive protein level of 0.33 mg/dL. Other laboratory findings were normal including a red blood cell count of 326 × 104/μL and hemoglobin of 13.7 g/dL, indicating no existence of anemia. Tumor markers including carbohydrate antigen 19-9, carcinoembryonic antigen, DUPAN, SPan-1, and soluble interleukin-2 receptor levels were within normal limits.
An abdominal dynamic contrast-enhanced computed tomography (CT) showed a 27-mm diameter tumor in the pancreatic uncus, which was well defined and enhanced starting from the arterial to the venous phase, exhibiting the greatest enhancement in the arterial phase (Figure 1). Magnetic resonance imaging revealed the mass to be hypointense on T1-weighed imaging and slightly hyperintense on T2-weighed imaging. The contrast enhancement study showed a similar pattern on CT suggesting the diagnosis of duodenal GIST or pancreatic head neuroendocrine tumor (NET). Therefore, endoscopic examination was performed for the further diagnosis.
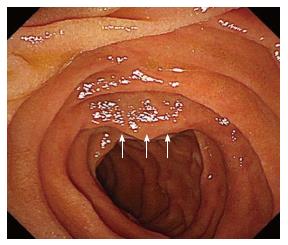
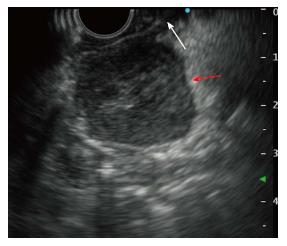
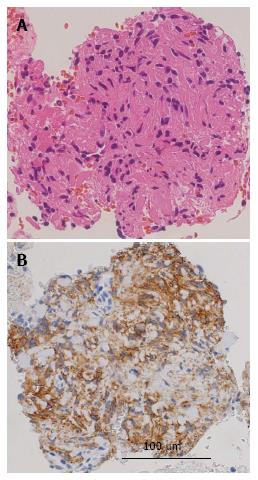
Upper gastroendoscopy showed a slightly elevated lesion located in the inferior angle of the duodenum with normal overlying mucosa detected on upper gastrointestinal endoscopy (Figure 2). EUS showed a well-defined hypoechoic mass placed close to the pancreatic uncus; however, the tumor was clearly revealed to be connected to the muscularis propria layer of the duodenum (Figure 3). Based on the EUS findings, duodenal GIST or pancreatic NET was suspected and EUS-FNA was performed for a definitive diagnosis. Histological examination revealed that the tumor was mainly composed of spindle-shaped cells (Figure 4). Immunohistochemistry (IHC) showed that the tumor cells were positive for c-kit, CD34, and S-100, but negative for desmin (Figure 4). Based on these results, the tumor was diagnosed as the extraluminal type of duodenal GIST.
The patient underwent mass resection of the tumor with partial resection of the second part of the duodenum. The tumor showed extraluminal growth and protruded into the pancreas but did not infiltrate the pancreatic parenchyma, consistent with the EUS findings. In addition, there was no ascites and no peritoneal dissemination.
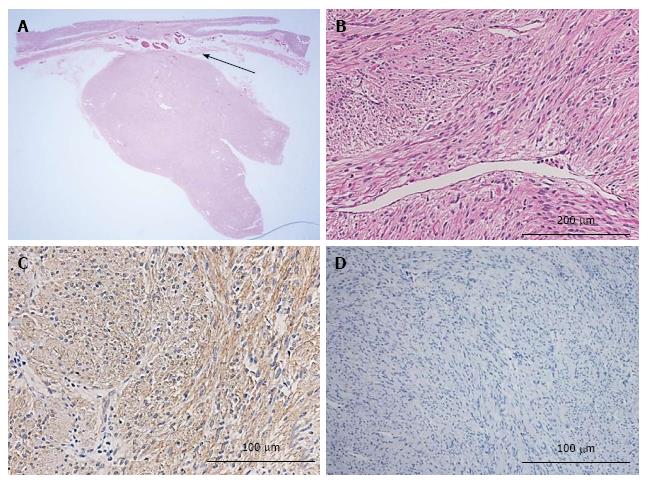
Histopathology of the resected tumor showed a mesenchymal, sharply margined tumor of 30 mm × 22 mm × 22 mm size, consisting of spindle cells without necrosis. Mitosis was detected in 2/50 high-power fields (HPFs). The tumor cells were positive for c-kit, and MIB-1 labeling index (Ki-67 stain) was < 1% (Figure 5).
No postoperative recurrence has been observed to date, and the patient did not require adjuvant chemotherapy for 2 years.
GISTs are the most common mesenchymal tumors in the gastrointestinal tract, contributing about 1%-3% of all gastrointestinal malignancies[1]. GISTs develop most frequently in the stomach (60%-70%), followed by the jejunum and ileum (20%-25%), duodenum (5%), colon and rectum (5%), and esophagus (< 5%)[3]. Miettinen et al[4] reported that duodenal GISTs most frequently involved the second portion of the duodenum, followed by the third, fourth, and first portions. They also reported that a majority of duodenal GISTs show submucosal tumor with a centrally ulcerated umbilication[4]; therefore, duodenal GISTs present with gastrointestinal bleeding, epigastric pain, a palpable mass, and intestinal obstruction[4].
In our case, the tumor exhibited exclusive extraluminal growth into the pancreatic head, and there was a slightly elevated lesion without ulceration in the inferior angle of the duodenum; this atypical finding made it difficult to distinguish it from a pancreatic NET[5]. Because the lesion without ulceration is difficult to diagnose by forcep-based biopsy on normal mucosa[6], EUS and EUS-FNA are helpful for its diagnosis. For EUS, it is important to determine whether there is a connection with gastrointestinal wall because it is the most accurate test to distinguish the layer where a lesion is located. The accuracy of the diagnosis was < 50% when using only EUS[7]. The sensitivity of EUS-FNA cytology was 84.4% for GISTs located in the stomach but poor for lesions located in the duodenum[8].Table 1 summarizes the cases of duodenal gastrointestinal tumors diagnosed with endoscopic ultrasound-guided fine-needle aspiration. Only a few reports show the usefulness of EUS-FNA for the diagnosis of duodenal GIST, especially when it is extraluminal type. Based on Skandalakis classification, among 11 cases reported, only 3 cases were extraluminal type and 2 showed mixed type. Ueda et al[9] reported that they diagnosed intra- and extraluminal growth type duodenal GIST by EUS-FNA. As summarized, while all case showed somewhat level of submucosal elevation, no ulcer was complicated in the lesion and EUS showed clear hypo-echoic mass in nine cases among 11 cases (Table 1). In addition, the connection to the proper muscle layer was shown in nine cases and FNA tissues have successfully performed to determine the histological analyses.
| Ref. | Age (yr) | Gender | Location in duodenum | Size (mm) | Endoscopic findings | EUS findings | Immunohistochemistry | Skandalakis classification | Treatment | Adjuvant chemo-therapy | Clinical course | Follow up period (yr) | ||||||||
| SMT | Central depression | Ulcerative lesion | well-demar-cated | Internal echogram | Cystic change | Connected to proper muscles | CD117 | CD34 | S-100 | MIB-1 labeling index | ||||||||||
| 9 | 72 | F | 3rd | 26 | + | - | - | + | hypo | - | + | + | + | N.A. | < 1% | Mixed | Partial duodenectomy | - | No recurrence | N.A. |
| 16 | 62 | F | 2nd | 40 | + | - | - | + | hypo | - | + | + | + | - | 0.60% | Eodoluminal | Partial duodenectomy | - | No recurrence | 6 |
| 16 | 69 | M | 1st | 15 | + | + | - | + | iso | - | + | + | + | N.A. | 0.50% | Eodoluminal | No surgery, Follow up | - | SD | 5 |
| 16 | 76 | M | 2nd | 35 | + | + | - | - | hetero | + | + | + | - | - | 0.70% | Eodoluminal | No surgery, Follow up | - | SD | 3 |
| 17 | 50s | F | 2nd | 35 | + | - | - | + | N.A. | - | N.A. | + | ± | + | < 5% | N.A. | Partial duodenectomy | - | N.A. | NA |
| 18 | 85 | F | 2nd | 30 | + | - | - | + | hypo | - | + | + | ± | - | N.A. | Eodoluminal | No surgery, Follow up | - | SD | 1.6 |
| 19 | 50s | F | 3rd | 25 | + | + | - | + | hypo | - | + | + | + | - | 2% | Eodoluminal | Partial duodenectomy | - | No recurrence | 1.3 |
| 19 | 30s | M | 3rd | 20 | ± | - | - | + | Aypo | - | + | + | + | - | 3% | Extraluminal | Partial duodenectomy | - | No recurrence | 1.2 |
| 20 | 75 | M | 3rd | 60 | + | - | - | + | hypo | - | - | + | - | - | 2% | Extraluminal | Subtotal stomach-preserving Pancreaticoduodenectomy | + | No recurrence | 1 |
| 21 | 51 | M | 2nd | 27.5 | + | - | - | + | hypo | - | + | + | + | N.A. | N.A. | Mixed | Surgery (no detail available) | N.A. | N.A. | N.A. |
| Our case | 50 | F | 2nd | 30 | ± | - | - | + | hypo | - | + | + | - | - | < 1% | Extraluminal | Partial duodenectomy | - | No recurrence | 2 |
In the reported case, EUS revealed the connection of the tumor and the muscularis propria layer (the fourth EUS layer). EUS-FNA showed that the tumor was composed of spindle-shaped cells, which were positive for c-kit, CD34, and S-100, but not for desmin, reported as a typical IHC result of GIST[10]. An accurate diagnosis helped determine the surgical procedure. Therefore, our case was successfully treated as reported[11].
Prognostic factors are very important for both assessing recurrence risk and the choice of adjuvant and neoadjuvant therapy[12]. The recently proposed "modified National Institutes of Health (NIH) classification" is defined by four factors: Number, size, location, and rupture of mitoses. This classification may offer advantages in the selection of patients who may require adjuvant therapy[13]. All GISTs that occurred in the intestines had more than a moderate possibility of metastasis when they were > 5 cm or had > 5 mitoses/50 HPFs. In tumors < 5 cm with a mitotic count < 5/50 HPFs, the intestinal GISTs had a low probability of metastasis[14].
Patients with duodenal GISTs classified as intermediate or high risk for tumor relapse should be treated with 400 mg imatinib daily for 3 years and there is no benefit for patients classified at low risk. As summarized in Table 1, other than 2 cases with no follow up data are available after the surgical treatment, no recurrence after the surgical treatment was confirmed in all other 6 cases for whom the surgery was performed. While other 3 cases showed stable disease with no surgical treatment because of low risk. Our patient was low risk according to the NIH consensus criteria for risk satisfaction of GISTs and has been followed without adjuvant chemotherapy.
After completed tumor resection, follow-up care should be every 3-6 mo, including clinical examination and CT scans of the abdomen and pelvis once a year for 5 years[15]. Our patient has been doing well with no tumor recurrence for 2 years since her surgery and will continue strict CT follow-up.
In summary, we have described a rare extraluminal growth type of duodenal GIST and showed the usefulness of EUS-FNA. This report will aid physicians in diagnosing rare duodenal tumors and contribute to determining the appropriate therapeutic strategy.
The authors present a case of a 50-year-old woman with a 27-mm diameter tumor in the pancreatic uncus on computed tomography scan. Endoscopic ultrasound (EUS) showed a well-defined hypoechoic mass in the pancreatic uncus that connected to the duodenal proper muscular layer and was followed by EUS-guided fine-needle aspiration (EUS-FNA). Histological analysis showed spindle-shaped tumor cells positively stained for c-kit. Therefore, the tumor was diagnosed as a duodenal gastrointestinal stromal tumors (GISTs) of the extraluminal type, and the patient underwent successful mass resection with partial resection of the duodenum.
A mass in the pancreatic uncus that connected to the duodenal proper muscular layer.
Pancreatic cancer; gastrointestinal stromal tumors; neuroendocrine tumor.
Laboratory data showed a slight elevated inflammatory response with a white blood cell count of 11370/μL and C-reactive protein level of 0.33 mg/dL. Tumor markers including carbohydrate antigen 19-9, carcinoembryonic antigen, DUPAN, SPan-1, and soluble interleukin-2 receptor levels were within normal limits.
EUS showed a well-defined hypoechoic mass in the pancreatic uncus that connected to the duodenal proper muscular layer. Magnetic resonance imaging revealed the mass to be hypointense on T1-weighed imaging and slightly hyperintense on T2-weighed imaging. The imaging studies suggested the diagnosis of duodenal GIST or pancreatic head neuroendocrine tumor (NET).
IHC showed that the tumor cells were positive for c-kit, CD34, and S-100, but negative for desmin. Based on these results, the tumor was diagnosed as the extraluminal type of duodenal GIST.
The patient underwent successful mass resection with partial resection of the duodenum.
GISTs: Gastrointestinal stromal tumors; NET: Neuroendocrine tumor; EUS:Endoscopic ultrasonography; FNA: Fine-needle aspiration.
This case suggests that EUS and EUS-FNA are effective for diagnosing the extraluminal type of duodenal GISTs, which is difficult to differentiate from pancreatic head tumor, and for performing the correct surgical procedure.
This is a well written case.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Huang CM, Yang F S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [PubMed] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [PubMed] |
| 3. | Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456:111-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625-641. [PubMed] |
| 5. | Raman SP, Hruban RH, Cameron JL, Wolfgang CL, Fishman EK. Pancreatic imaging mimics: part 2, pancreatic neuroendocrine tumors and their mimics. AJR Am J Roentgenol. 2012;199:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Yang F, Jin C, Du Z, Subedi S, Jiang Y, Li J, Di Y, Zhou Z, Tang F, Fu D. Duodenal gastrointestinal stromal tumor: clinicopathological characteristics, surgical outcomes, long term survival and predictors for adverse outcomes. Am J Surg. 2013;206:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Ueda K, Hijioka M, Lee L, Igarashi H, Niina Y, Osoegawa T, Nakamura K, Takahashi S, Aishima S, Ohtsuka T. A synchronous pancreatic neuroendocrine tumor and duodenal gastrointestinal stromal tumor. Intern Med. 2014;53:2483-2488. [PubMed] |
| 10. | Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol. 2011;104:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Vasile D, Iancu G, Iancu RC, Simion G, Ciuluvică RC. Duodenal gastrointestinal stromal tumor presenting as pancreatic head mass - a case report. Rom J Morphol Embryol. 2017;58:255-259. [PubMed] |
| 12. | Chung JC, Chu CW, Cho GS, Shin EJ, Lim CW, Kim HC, Song OP. Management and outcome of gastrointestinal stromal tumors of the duodenum. J Gastrointest Surg. 2010;14:880-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Colombo C, Ronellenfitsch U, Yuxin Z, Rutkowski P, Miceli R, Bylina E, Hohenberger P, Raut CP, Gronchi A. Clinical, pathological and surgical characteristics of duodenal gastrointestinal stromal tumor and their influence on survival: a multi-center study. Ann Surg Oncol. 2012;19:3361-3367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Zhong Y, Deng M, Liu B, Chen C, Li M, Xu R. Primary gastrointestinal stromal tumors: Current advances in diagnostic biomarkers, prognostic factors and management of its duodenal location. Intractable Rare Dis Res. 2013;2:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Joensuu H, Rutkowski P, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Braconi C, Bordoni A, Magnusson MK. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol. 2015;33:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Yagishita A, Matsubayashi H, Kakushima N, Tanaka M, Takizawa K, Yamaguchi Y, Ono H. Gastrointestinal stromal tumors of the duodenum: a report of four cases. Clin J Gastroenterol. 2011;4:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Sakata K, Nishimura T, Okada T, Nakamura M. [Local resection and jejunal patch duodeno-plasty for the duodenal gastrointestinal stromal tumor--a case report]. Gan To Kagaku Ryoho. 2009;36:2348-2350. [PubMed] |
| 18. | Minoda Y, Itaba S, Kaku T, Makihara K, Matsuoka J, Murao H, Hamada T, Nakamura K. [Synchronous gastrointestinal stromal tumors of the rectum and duodenum: a case report]. Nihon Shokakibyo Gakkai Zasshi. 2015;112:1991-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Inoue T, Okumura F, Fukusada S, Kachi K, Anbe K, Nishie H, Nishi Y, Mizushima T, Sano H. [Two cases of distal duodenal gastrointestinal stromal tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy]. Nihon Shokakibyo Gakkai Zasshi. 2013;110:2112-2118. [PubMed] |
| 20. | Yoshio K, Kitamura S, Okanobu H, Fukuda S, Nishida T. A Case of gastrointestinal stromal tumor. Jap J Clin Exp Med. 2016;9:1243-1246. |
| 21. | Castro-Poças FM, Araújo TP, Silva JD, Lopes CA, M Saraiva M. Duodenal gastrointestinal stromal tumor and endoscopic ultrasound. Rev Esp Enferm Dig. 2015;107:759-760. [PubMed] |