Published online Dec 16, 2017. doi: 10.4253/wjge.v9.i12.571
Peer-review started: May 17, 2017
First decision: June 16, 2017
Revised: August 28, 2017
Accepted: September 14, 2017
Article in press: September 15, 2017
Published online: December 16, 2017
Processing time: 208 Days and 12.5 Hours
To investigate technical feasibility, outcomes and adverse events of the lumen-apposing metal stent (LAMS) for benign gastrointestinal (GI) tract strictures.
Between July 2015 and January 2017, patients undergoing treatment by LAMS for benign GI strictures at three tertiary referral centers were included in this study. Primary outcomes included technical success, short-term clinical success, long-term clinical success, and adverse events. Short-term clinical success was defined as symptom resolution at 30 d after stent placement. Long-term clinical success was defined by symptom resolution at 60 d in patients who continued to have indwelling stent, or continued symptom resolution at 30 d after elective stent removal.
A total of 21 patients (mean age 62.6 years, 47.6% males) underwent placement of LAMS for benign GI strictures. A 15 mm × 10 mm LAMS was placed in 16 patients, a 10 mm × 10 mm LAMS was placed in 2 patients, and a 16 mm × 30 mm LAMS was placed in 3 patients. Technical success was obtained in all cases. Short-term clinical success was achieved in 19 out of 21 cases (90.5%), and long-term clinical success was achieved in 12 out of 18 (66.7%). Mean (range) stent indwell time was 107.2 (28-370) d. After a mean (range) dwell time of 104.3 (28-306) d, 9 LAMSs were removed due to the following complications: ulceration at stent site (n = 1), angulation (n = 2), migration (n = 4) and stricture overgrowth (n = 2). Migration occurred in 4 cases (19.0%), and it was associated with stricture resolution in one case. Median (range) follow-up period was 119 (31-422) d.
Utilization of LAMS for benign strictures has shown to be technically feasible and safe, but adverse events highlight the need for further study of its indications.
Core tip: Treatment of benign short gastrointestinal (GI) tract strictures has primarily involved endoscopic balloon dilation, intralesional steroid injection and the conventional fully-covered metal stent. The lumen-apposing metal stent (LAMS), which has been used to drain pancreatic fluid collections, may serve as a more effective alternative. This study measures technical feasibility and potential short and long-term effectiveness of LAMS for benign GI strictures at three tertiary referral centers. Although results are promising, complications include angulation, stricture overgrowth and ulceration at stent site. These highlight the need for further study to better specify which patients should receive LAMS and how to minimize burden of complications.
- Citation: Santos-Fernandez J, Paiji C, Shakhatreh M, Becerro-Gonzalez I, Sanchez-Ocana R, Yeaton P, Samarasena J, Perez-Miranda M. Lumen-apposing metal stents for benign gastrointestinal tract strictures: An international multicenter experience. World J Gastrointest Endosc 2017; 9(12): 571-578
- URL: https://www.wjgnet.com/1948-5190/full/v9/i12/571.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i12.571
Benign etiologies of gastrointestinal (GI) strictures include ulcers, caustic ingestion, post-operative anastomotic states and inflammation[1]. The management of benign GI strictures has typically entailed endoscopic balloon dilation (EBD). However, EBD often does not provide definitive treatment and carries risks of bleeding and perforation[2,3]. Intralesional steroid injections may serve as an adjunct to endoscopic dilation that leads to increased efficacy of dilation and decreased number of total dilations[4]. Conventional fully-covered self expandable metal stent (cSEMS) has offered an alternative for therapy in cases that are refractory to EBD and steroid injections[5,6]. The cSEMS holds advantages over uncovered and partially-covered stents due to the relative ease of deployment and retrieval[7]. Furthermore, cSEMS may provide a gradual and continuous dilation of the stenotic segment. However, the use of cSEMS has demonstrated high rates of migration that may occur early in the period after stent placement, which may ultimately compromise long-term clinical success. Endoscopic suturing of cSEMS to tissue has been a recent advance that has mitigated this issue of migration[8], but this procedure is expensive and can be technically challenging[9].
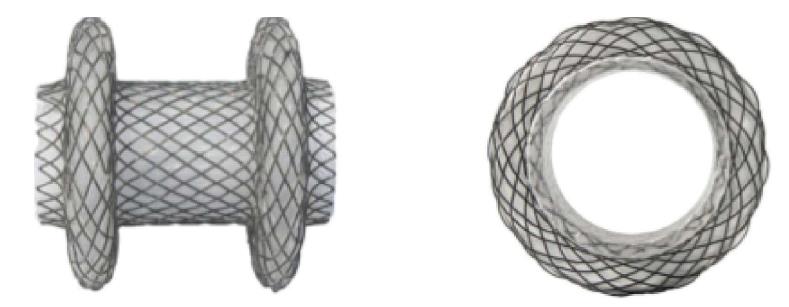
Recently, lumen-apposing metal stents (LAMS) have become widely available for drainage of pancreatic fluid collections that exhibit lumen-apposing and dual anchoring capabilities[10]. These design features allow for robust pseudocyst drainage and the passage of an endoscope with a lower risk of stent migration due to anchoring. The AXIOS™ stents (Boston Scientific, Marlborough, United States) are 10 mm in saddle length, 10 mm or 15 mm in diameter, and with flanges that are 21 mm or 24 mm in diameter.
The NAGI™ stent (Taewoong Medical Co., Ltd., Ilsan, South Korea) is another type of LAMS that has been feasible in treating both pancreatic pseudocysts and walled-off necrosis. The presence of flared edges allows for its dual-anchoring capabilities, and the inclusion of a retrieval string also facilitates the removal of the stent. These LAMS are 10-30 mm in length, 10-16 mm in diameter, and contain flared edges 20 mm in diameter. Overall, the dimensions of the AXIOS™ and NAGI™ stents allow for potential use in treating short-length strictures that are less than or equal to 10 mm with a low risk of migration due to the anchoring flanges.
Prior studies have thus far supported LAMS as a potentially safe and effective measure to treat benign GI strictures (Table 1). Majumder et al[11] demonstrated that in a group of 5 patients, the placement of AXIOS™ stent led to successful resolution of symptoms with no stent-related adverse events during a median follow-up period of 120 d. Irani and colleagues found that in a group of 25 patients with benign GI strictures refractory to standard therapies, the placement of AXIOS™ stent led to resolution of symptoms at 6 mo in 60.0% of cases[12]. Yang et al[13] demonstrated in a group of 30 patients, an indwelling AXIOS™ stent led to resolution of symptoms in 90.0% of cases, and 82.6% continued to have improved symptoms after LAMS removal. The data remains limited, and the prior studies solely involve the AXIOS™ stent. We describe the feasibility, safety and efficacy of treating benign GI strictures with two types of LAMS in an international multicenter setting.
| Majumder et al[11] (2015) | Irani et al[12] (2016) | Yang et al[13] (2017) | |
| Total cases | 5 | 25 | 30 |
| Age | 47.4 (mean) | 54 yr (median) | 51.6 (mean) |
| Females | 4 (80.0) | 18 (72.0) | 19 (63.3) |
| Underwent prior endoscopic dilation | 3 (60.0) | 20 (80.0) | 27 (90.0) |
| Prior cSEMS | 1 (20.0) | 1 (4.0) | 8 (29.6) |
| LAMS used | |||
| AXIOS 15 mm × 10 mm | 5 (100.0) | 25 (100.0) | 29 (96.7) |
| AXIOS 10 mm × 10 mm | 0 | 3 (12.0)1 | 1 (3.3) |
| Technical success | Not described | 25 (100)2 | 29 (96.7)3 |
| Clinical success | Not described | ||
| Short-term | 15 (60)4 | 27 (90.0)5 | |
| Long-term | 19 (82.6)6 | ||
| Migration | 0 | 2 (7.0) | 2 (8.0) |
| Median stent dwell time (range) | Not described | 92 d (3-273, median) | Not described |
| Median follow-up, d (range) | 120 (84-140) | 301 (62-681) | 100 (60-139) |
Between July 2015 and January 2017, patients who had undergone treatment by LAMS for benign GI strictures at three tertiary referral centers were identified. All cases were reviewed for demographic information, clinical presentation, initial diagnosis, anatomic location and prior endoscopic therapies. Inclusion criteria were patients with benign strictures that were not amenable to placement of cSEMS or had failed prior endoscopic therapies.
Primary outcomes evaluated included technical success, short-term clinical success, long-term clinical success, and adverse events. Technical success was defined by appropriate stent placement across the stricture verified endoscopically and fluoroscopically. Short-term clinical success was defined as symptom resolution at 30 d after stent placement, inclusive of patients with indwelling stents at day 30 and patients who had elective removal prior to day 30. Long-term clinical success was defined by symptom resolution at 60 d in patients who continued to have indwelling stent, or symptom resolution at 30 d after elective stent removal. Early complications were defined by adverse events pertaining to the stent that occurred either at the time of placement or within 24 h after placement. Late complications were defined by adverse events pertaining to the stent that occurred after 24 h the stent was verified to be placed. Follow-up of stent placement took place via clinic visits, telephone calls, imaging studies and endoscopic surveillance appointments.
A total of 21 patients (mean age 62.6 years, 47.6% males) underwent placement of LAMS for benign GI strictures over the study period at the three centers (Table 2). Anatomic location of strictures included proximal esophagus (5, 23.8%), distal esophagus (4, 19.0%), stomach (6, 28.6%), duodenum (4, 19.0%), and colon (2, 9.5%). Etiology of GI strictures in this study included prior surgical anastomosis (10, 47.6%), prior surgical anastomosis and radiation therapy (4, 19.0%), caustic injury (3, 14.3%), peptic strictures (3, 14.3%) and chronic pancreatitis (1, 4.8%). Sixteen patients (76.2%) had at least one prior endoscopic therapy, which included EBD (n = 14), placement of cSEMS (n = 3), and stricturoplasty (n = 1).
| Proximal esophagus(n = 5) | Distal esophagus(n = 4) | Stomach(n = 6) | Duodenum(n =4) | Colon(n = 2) | Total(n = 21) | |
| Age, mean (yr) | 54 | 68.5 | 59 | 65.8 | 77 | 62.6 |
| Gender | ||||||
| Male | 3 (60.0) | 3 (75.0) | 2 (33.3) | 2 (50.0) | 10 (47.6) | |
| Female | 2 (40.0) | 1 (25.0) | 4 (66.7) | 2 (50.0) | 2 (100.0) | 11 (52.4) |
| Etiology | ||||||
| Post-surgery/radiation | 4 (80.0) | 3 (75.0) | 4 (66.7) | 1 (25.0) | 2 (100.0) | 14 (65.2) |
| Peptic | 1 (16.7) | 2 (50.0) | 3 (13.0) | |||
| Chronic pancreatitis | 1 (25.0) | 1 (4.3) | ||||
| Caustic ingestion | 1 (20.0) | 1 (25.0) | 1 (16.7) | 3 (13.0) | ||
| Types of prior treatments | ||||||
| Balloon dilatation | ||||||
| 1 | 1 | 1 | 1 | 3 | ||
| 2 | 4 | 2 | 2 | 8 | ||
| 3 | 1 | 1 | 2 | |||
| > 3 | 1 | 1 | ||||
| Fully-covered stents | 1 | 1 | 1 | 3 | ||
| Prior migration | 1 | 1 | 1 | 3 | ||
| Stricturoplasty | 1 | 1 |
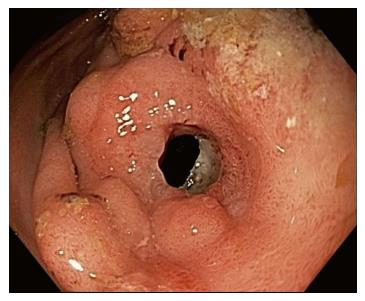
In all cases, procedures were performed using a forward-viewing therapeutic endoscope. A standard guidewire was passed across the stricture under fluoroscopic guidance and contrast may have been utilized via injection to confirm stricture length. Upon the discretion of the endoscopist, the decision was made to use an AXIOS™ stent of 10 or 15 mm in diameter, or a NAGI™ stent measured at 16 mm × 30 mm (Figure 1). The LAMS were deployed under fluoroscopic and endoscopic guidance.
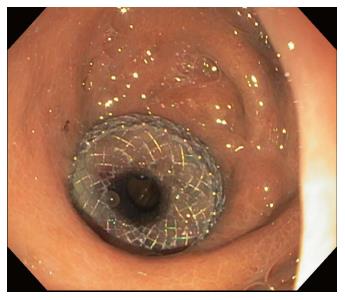
A 15 mm × 10 mm LAMS was placed in 16 patients, a 10 mm × 10 mm LAMS was placed in 2 patients, and a 16 mm × 30 mm NAGI stent was placed in 3 patients (Table 3). Technical success was obtained in 21 out of 21 cases (100.0%). Short-term clinical success was achieved in 19 out of 21 cases (90.5%) (Figure 2). Long-term clinical success was achieved in 12 out of 18 (66.7%). Three cases did not qualify for evaluation for long-term clinical success due to: Currently indwelling at a period of less than 60 d or a period of less than 30 d after already electively removed. Mean (range) dwell time of all cases was 107.2 (28-370) d.
| Proximal esophagus(n = 5) | Distal esophagus(n = 4) | Stomach(n = 6) | Duodenum(n =4) | Colon(n = 2) | Total(n = 21) | |
| LAMS | ||||||
| 15 mm × 10 mm AXIOS | 5 (100.0) | 2 (50.0) | 3 (50.0) | 4 (100.0) | 2 (100) | 16 (76.2) |
| 10 mm × 10 mm AXIOS | 2 (33.3) | 2 (9.5) | ||||
| 16 mm × 30 mm NAGI | 2 (50.0) | 1 (16.7) | 3 (14.3) | |||
| Mean stent dwell time (d) | 67.6 | 56.5 | 151.2 | 167.5 | 55.5 | 107.2 |
| Technical success | 5 (100.0) | 4 (100.0) | 6 (100.0) | 4 (100.0) | 2 (100) | 21 (100.0) |
| Clinical success | 11 | |||||
| Short-term | 5 (100.0) | 3 (75.0) | 5 (83.3) | 4 (100.0) | 2 (100) | 19 (90.5) |
| Long-term | 1 (25.0) | 2 (66.7) | 5 (100.0) | 3 (75.0) | 1 (50.0) | 212 (66.7) |
| Reasons for stent removal | ||||||
| Angulation | 1 | 1 | 2 | |||
| Stent migration | 1 | 1 | 2 | 4 | ||
| Stricture overgrowth | 2 | 1 | 2 | |||
| Ulceration | 1 | 1 | ||||
| Resolution | 1 | 11 | 3 | |||
| Treatments after stent failure | ||||||
| Balloon dilation | 2 | 2 | ||||
| cSEMS | 1 | 1 | ||||
| 15 mm × 10 mm AXIOS | 1 | 1 | 1 | 3 | ||
| 16 mm × 30 mm NAGI | 2 | 1 | 3 |
There were no early adverse events in any of the cases such as bleeding or perforation. There were no serious delayed adverse events in any of the cases. However, after a mean (range) dwell time of 104.3 (28-306) d, 11 LAMS (52.4%) needed to be removed due to the following complications: Ulceration at stent site (n = 1), angulation (n = 2), migration (n = 4), tissue overgrowth (n = 2), and stricture resolution (n = 3). Two patients with LAMS removal did not require further intervention. Overall, there were four cases (19.0%) that involved migration; in one of the cases, it was found that migration occurred likely due to resolution of the stricture. In the 8 cases in which the patients continued to be symptomatic after LAMS removal, the patients underwent repeat dilation, placement cSEMS or repeated placement of LAMS. Median (range) follow-up period was 119 (31-422) d.
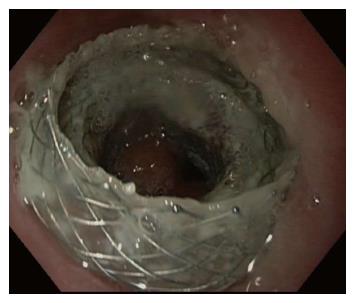
Of the 5 proximal esophageal strictures, 4 (80.0%) were due to prior surgical anastomosis and 1 (20.0%) was due to caustic ingestion. All five underwent prior treatments that included balloon dilatation and placement of cSEMS, which were complicated by recurrence and migration, respectively. All five underwent placement of 15 mm × 10 mm AXIOS™ LAMS. All five cases achieved technical success. All five cases (100.0%) achieved short-term clinical success. One case did not qualify for long-term clinical success evaluation because the patient had an indwelling LAMS less than 60 d. Only one of the remaining four (25%) cases achieved long-term clinical success. The first case that did not meet long-term clinical success involved removal of an AXIOS™ stent after indwell time of 90 d due to perceived resolution of stricture; however, it was found that the stricture had recurred. The second case that did not meet long-term clinical success involved an AXIOS™ stent that had distally migrated after 110 d. The third case that did not meet long-term clinical success involved an AXIOS™ stent that had angulation at stent site after 40 d, in which the lumen of the stent was abutting the oesophageal wall (Figure 3). This led to odynophagia and vomiting that necessitated removal of LAMS.
Of the 4 distal esophageal strictures, 3 (75%) were due to prior surgeries and 1 (25.0%) was due to caustic ingestion. Three (75%) underwent prior therapies including balloon dilatation and placement of cSEMS, which were complicated by recurrence and migration, respectively. Two underwent placement of 15 mm × 10 mm AXIOS™ LAMS, and two underwent placement of 16 mm × 30 mm NAGI™ LAMS. All four cases achieved technical success. Three (75%) achieved short-term clinical success. The one case that did not achieve short-term clinical success was due to a NAGI™ stent that migrated prior to 30 d after stent placement, leading to recurrent symptoms and removal of the stent; thus, this case did not qualify for long-term success evaluation. Two out of the remaining three cases (67%) achieved long-term clinical success. The one case that did not achieve long-term clinical success was due to angulation of a 15 mm × 10 mm AXIOS™ LAMS at the stent site that occurred 45 d after stent placement, which led to vomiting and subsequent removal of the stent.
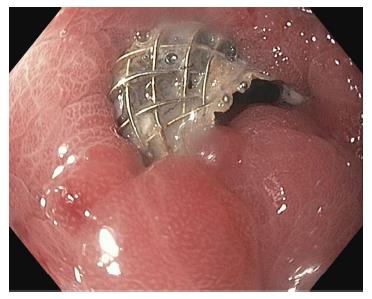
Of the 6 gastric strictures, 4 (67.7%) were due to prior surgery, 1 (16.7%) was due to caustic injury, and 1 (16.7%) was due to peptic ulcer. Three (67.7%) underwent prior therapy, which was balloon dilation that failed due to recurrence. Three underwent placement of 15 mm × 10 mm AXIOS™ LAMS, two underwent placement of 10 mm × 10 mm AXIOS™ LAMS, and one underwent placement of NAGI™ LAMS. All 6 stent placements achieved technical success. Five (83.3%) achieved short-term clinical success. One (16.7%) placement of 15 mm × 10 mm LAMS was complicated by an ulcer at 28 d, requiring removal of LAMS; thus, this case did not qualify for long-term clinical success evaluation. The remaining five cases achieved long-term clinical success. Of note, one case involved a patient that developed tissue overgrowth at the site of a 10 mm × 10 mm AXIOS™ stent placement across a gastrogastric anastomotic stricture after 306 d, which resolved with stricturoplasty (Figure 4). Another case involved a patient that developed tissue overgrowth at the site of a 10 mm × 10 mm AXIOS™ stent placement across a prepyloric anastomotic stricture after 183 d, which led to stent removal (Figure 5).
Of the 4 duodenal strictures, 2 (50%) were peptic ulcer disease, 1 (25%) was due to chronic pancreatitis, and 1 (25%) was an anastomotic stricture from prior Whipple procedure. Two (50%) underwent prior therapies including balloon dilatation and placement of cSEMS, which failed due to recurrence and migration, respectively. All four cases underwent placement of 15 mm × 10 mm AXIOS™ LAMS. All cases achieved technical success and short-term clinical success. Three (75%) cases achieved long-term clinical success. The one case that failed to achieve clinical success was due to proximal migration of AXIOS™ stent that occurred after an indwell time of 150 d, requiring the placement of another AXIOS™ stent. Of note, another case resulted in distal migration of AXIOS™ stent after 60 d. However, upon removal of this stent, the stricture was resolved and no further intervention was needed.
Of the 2 colonic strictures, both were due to prior surgical anastomosis. Both underwent prior treatments with balloon dilatation, which failed due to stricture recurrence. Both underwent placement of 15 mm × 10 mm AXIOS™ LAMS. Technical and clinical successes were obtained in both cases. One case (50%) involved elective removal after indwell time of 48 d due to resolution of the stricture, and patient has been asymptomatic since LAMS removal.
The management of benign GI strictures is often challenging due to the refractory nature of these strictures and failure of conventional therapy, EBD. In our study, the majority of patients who underwent LAMS for benign strictures received prior therapies that failed. Those who had prior EBD or stricturoplasty had developed stricture recurrence, and prior placement of cSEMS had led to migration. These complications are consistent with those that have been previously described[2,3,8]. A recent systematic review and meta-analysis on outcomes following stent placement in refractory benign esophageal strictures reported an overall stent migration rate of 28.6%[14]. In order to prevent the occurrence of migration associated with cSEMS, the utilization of stent suturing as a means of fixation has been described. The use of this external fixation method has been associated with lower migration rates[15]. However, stent suturing with cSEMS is also described to be associated with stricture overgrowth. Furthermore, stent suturing involves a more technically challenging approach for the endoscopist that may affect technical success and feasibility.
In our study, the decision was made to proceed with LAMS prior to considering surgical intervention. Although surgery may provide an opportunity to definitively treat benign strictures, rates of postoperative morbidity and mortality are significant[16-18]. Among the population predisposed to developing benign GI strictures, the risk of surgery is compounded by advanced age, poor nutritional status and other related comorbidities among these patients.
For strictures that are refractory to standard endoscopic therapies, we thus would recommend further consideration of endoscopic therapies, in which LAMS may serve as a feasible and safe alternative. Given the length parameters of LAMS, currently LAMS would be appropriate for benign, short strictures. Specifically, this would be for strictures < 10 mm in length for utilization of AXIOS™ LAMS and < 30 mm in length for utilization of NAGI™ LAMS.
In contrast to the conventional SEMS, LAMS imparts lumen apposition via its wide flanges and provides anchorage, hence potentially reducing the risk of stent migration. In our study, the migration rate for those who underwent LAMS placement was 19.0% and this does appear to be higher than other studies on this topic. In the cases of LAMS migrating, the mean period of time before detected migration was 87.5 d, which appears to be a potentially longer period in comparison to the period associated with cSEMS migrating in our experience. Nonetheless, it is important to highlight that migration may occur with LAMS use, as this has not been a common observation in prior studies.
Overall, technical success was achieved in all cases without incidence of any immediate complications. Of note, all stents were placed by interventional endoscopists that were highly experienced in endoscopic stent placement. There were also no difficulties with evaluation of the stented area or removal of the stent. This supports the feasibility of endoscopic follow-up and surveillance in patients with indwelling LAMSs.
This study supports the high short term clinical success rate found in previous case series[12,13]. We chose 30 d after stent placement to be the measure of short term clinical success as recurrent strictures are defined as those unable to maintain a satisfactory luminal diameter for this length of time[19]. The long term clinical success rate in this series fell quite dramatically however largely due to complications occurring after 30 d. The rate of complications in this study that prevented short or long-term clinical success was high relative to other studies at 38.0%[12,13]. Furthermore, we noted complications not mentioned in the literature previously. In addition to migration, we found angulation to be a potential complication, at a rate of 9.5%. This involved the stent lumen/axis of the LAMS being misaligned within the luminal GI tract. Due to the stent lumen facing the luminal walls, patients developed foreign body sensation and obstructive symptoms. In these cases, this likely occurred due to the short length of LAMS coupled with the angled nature of these particular anastomotic strictures. Therefore, assessment of the stricture angle in relation to adjacent lumen may be an important factor when considering LAMS as a potential therapeutic option. Stricture overgrowth was also encountered in this study as a late complication. In our study, there were two cases of tissue overgrowth leading to stent dysfunction. Of note, one case had the stent placed for 183 d and the other 306 d. In one case, stricturoplasty of the tissue overgrowth with needle knife was successful in recanalizing the stent. In another case, the stent was removed and the stricture has since remained patent. The duration of stent dwell time of these cases was much longer than the mean dwell time of this series which might indicate that a scheduled assessment of the LAMS should occur at a specified duration after placement, possibly 180 d, to ensure tissue overgrowth is not occurring.
Limitations of this study include its retrospective nature with lack of a control arm, lack of symptom severity score, and no standardized method of managing complications. Given the lower volume of benign refractory GI strictures relative to other GI pathological processes, it would be difficult to have a robust control arm for analysis. The utilization of a symptom severity score may have also allowed for ability to better categorize the treatment effect. This data would potentially add clinical significance to our evaluation of LAMS, as we would not only categorize how many patients benefited, but also to the extent of symptom improvement. Lastly, the cases took place in 3 different tertiary care centers with no standardized algorithm of stent management. As a result, decisions of clinical and endoscopic follow-up as well as decisions regarding management of stent related complications were made at the endoscopists’ discretion and best judgment.
In conclusion, we found that the utilization of LAMS is technically feasible and safe as a primary or salvage therapy for benign GI strictures with a high short term clinical success rate. However, late complications related to stricture overgrowth, stent migration, and angulation prevented a sustained symptom-free period in a large proportion of cases. These adverse events highlight the need for further study in this area to better understand which patients and which strictures are most optimal for management with LAMS prior to widespread adoption of this technique for the treatment of benign GI strictures.
Treatment of benign short gastrointestinal (GI) tract strictures has primarily involved endoscopic balloon dilation, intralesional steroid injection and the conventional fully-covered metal stent. The lumen-apposing metal stent (LAMS) exhibit lumen-apposing and dual anchoring capabilities. While it has primarily been used to drain pancreatic fluid collections, LAMS may serve as a more effective alternative to standard endoscopic therapies for benign strictures.
Currently, there are two recent retrospective studies in the literature that describe use of LAMS for benign strictures. Irani and colleagues found that in a group of 25 patients, the placement of AXIOS™ stent led to resolution of symptoms at 6 mo in 60.0% of cases. Yang and colleagues found that in a group of 30 patients, the placement AXIOS™ stent led to resolution of symptoms in 90.0% of cases, and 82.6% continued to have improved symptoms after LAMS removal. The migration rates in the Irani et al and Yang et al are 7.0% and 8.0%, respectively. Currently there have been no prospective studies using LAMS and this may be worthwhile in the future to truly determine the ideal clinical scenarios when LAMS should be used.
In the authors’ group of 21 cases, short-term clinical success was achieved in 90.5% of cases, and long-term clinical success was achieved 66.7% of cases. We also report the outcomes of 16 mm × 30 mm NAGI™ stent that was successfully placed in 3 cases. The migration rate for those who underwent LAMS placement was 19.0%, which appears to be higher than other studies on this topic. Furthermore, in contrast to prior reports, the authors found complications of LAMS placement not described in prior reports. These primarily include angulation and stricture overgrowth, which played significant roles in preventing clinical success in the cases.
The authors found that the utilization of LAMS is technically feasible and safe as a primary or salvage therapy for benign GI strictures with a high short-term clinical success rate. However, the adverse events as described above highlight the need for further study in this area to better understand which patients and which strictures are most optimal for management with LAMS. Uncovering this information will contribute towards the potential widespread adoption of this technique for the treatment of benign GI strictures.
Covered self-expandable metal stent: This stent has been widely used in malignant obstruction. The presence of a covering membrane allows the lumen to remain patent despite structured tissue overgrowth. Furthermore, it prevents the metal wires from burrowing into the wall, which allows for easier retrieval; LAMS: This newer stent has been widely used for drainage of pancreatic fluid collections. It exhibits lumen-apposing and dual anchoring capabilities. The anchoring flanges are thought to lower the risk of stent migration.
An International Multicenter study that is clinically meaningful. It is a retrospective analysis of LAMS placement in three tertiary care hospitals that add value to the knowledge of benign stricture treatment.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lorenzo-Zuniga V, Mitsui K, Seow-Choen F, Souza JLS, Tepes B, Yu B S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc. 2010;2:29-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 2. | Broor SL, Raju GS, Bose PP, Lahoti D, Ramesh GN, Kumar A, Sood GK. Long term results of endoscopic dilatation for corrosive oesophageal strictures. Gut. 1993;34:1498-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Ukleja A, Afonso BB, Pimentel R, Szomstein S, Rosenthal R. Outcome of endoscopic balloon dilation of strictures after laparoscopic gastric bypass. Surg Endosc. 2008;22:1746-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Altintas E, Kacar S, Tunc B, Sezgin O, Parlak E, Altiparmak E, Saritas U, Sahin B. Intralesional steroid injection in benign esophageal strictures resistant to bougie dilation. J Gastroenterol Hepatol. 2004;19:1388-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Vanbiervliet G, Bichard P, Demarquay JF, Ben-Soussan E, Lecleire S, Barange K, Canard JM, Lamouliatte H, Fontas E, Barthet M, Ponchon T, Saurin JC; Research Committee of the French Society of Digestive Endoscopy (SFED). Fully covered self-expanding metal stents for benign colonic strictures. Endoscopy. 2013;45:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Caruso A, Conigliaro R, Manta R, Manno M, Bertani H, Barbera C, Mirante VG, Frazzoni M. Fully covered self-expanding metal stents for refractory anastomotic colorectal strictures. Surg Endosc. 2015;29:1175-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Choi WJ, Park JJ, Park J, Lim EH, Joo MK, Yun JW, Noh H, Kim SH, Choi WS, Lee BJ. Effects of the temporary placement of a self-expandable metallic stent in benign pyloric stenosis. Gut Liver. 2013;7:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Sharaiha RZ, Kumta NA, Doukides TP, Eguia V, Gonda TA, Widmer JL, Turner BG, Poneros JM, Gaidhane M, Kahaleh M. Esophageal Stenting With Sutures: Time to Redefine Our Standards? J Clin Gastroenterol. 2015;49:e57-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Stavropoulos SN, Modayil R, Friedel D. Current applications of endoscopic suturing. World J Gastrointest Endosc. 2015;7:777-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Siddiqui AA, Adler DG, Nieto J, Shah JN, Binmoeller KF, Kane S, Yan L, Laique SN, Kowalski T, Loren DE. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos). Gastrointest Endosc. 2016;83:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 11. | Majumder S, Buttar NS, Gostout C, Levy MJ, Martin J, Petersen B, Topazian M, Wong Kee Song LM, Abu Dayyeh BK. Lumen-apposing covered self-expanding metal stent for management of benign gastrointestinal strictures. Endosc Int Open. 2016;4:E96-E101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Irani S, Jalaj S, Ross A, Larsen M, Grimm IS, Baron TH. Use of a lumen-apposing metal stent to treat GI strictures (with videos). Gastrointest Endosc. 2017;85:1285-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (2)] |
| 13. | Yang D, Nieto JM, Siddiqui A, Riff BP, DiMaio CJ, Nagula S, Ismail AM, Ngamreungphong S, Khashab MA, Wagh MS. Lumen-apposing covered self-expandable metal stents for short benign gastrointestinal strictures: a multicenter study. Endoscopy. 2017;49:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Fuccio L, Hassan C, Frazzoni L, Miglio R, Repici A. Clinical outcomes following stent placement in refractory benign esophageal stricture: a systematic review and meta-analysis. Endoscopy. 2016;48:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Kantsevoy SV, Bitner M. Esophageal stent fixation with endoscopic suturing device (with video). Gastrointest Endosc. 2012;76:1251-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Gerzic ZB, Knezevic JB, Milicevic MN, Jovanovic BK. Esophagocoloplasty in the management of postcorrosive strictures of the esophagus. Ann Surg. 1990;211:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Weiland D, Dunn DH, Humphrey EW, Schwartz ML. Gastric outlet obstruction in peptic ulcer disease: an indication for surgery. Am J Surg. 1982;143:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Little AG, Naunheim KS, Ferguson MK, Skinner DB. Surgical management of esophageal strictures. Ann Thorac Surg. 1988;45:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kochman ML, McClave SA, Boyce HW. The refractory and the recurrent esophageal stricture: a definition. Gastrointest Endosc. 2005;62:474-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |