Published online Feb 25, 2016. doi: 10.4253/wjge.v8.i4.244
Peer-review started: August 6, 2015
First decision: September 23, 2015
Revised: December 20, 2015
Accepted: January 5, 2016
Article in press: January 7, 2016
Published online: February 25, 2016
Processing time: 208 Days and 17.5 Hours
Gastric adenocarcinoma of fundic gland type (GA-FG) with chief cell differentiation was recently proposed as an extremely rare type of gastric adenocarcinoma. Here, we report 4 cases of GA-FG with chief cell differentiation. Endoscopic features included a submucosal tumor shape or a flat shape, whitish discoloration and dilated vessels on the surface. The tumors were located in the upper or middle third of the stomach. All cases were preoperatively diagnosed as GA-FG by biopsy, and endoscopic submucosal dissection was performed. Resected specimens revealed well-differentiated adenocarcinomas resembling chief cells. Tumor cells were diffusely positive for pepsinogen-I, but partially positive for H+/K+-ATPase in scattered locations around the tumor margin. Despite the presence of minimal invasion of the carcinoma into the submucosal layer, which was observed in two cases, neither lymphatic nor venous invasion was detected in any of the cases. Finally, all cases showed less aggressive clinical behavior with low grade malignancy.
Core tip: Gastric adenocarcinoma of fundic gland type (GA-FG) with chief cell differentiation is a new and extremely rare type of gastric adenocarcinoma, and the clinicopathological features of GA-FG have thus not yet been elucidated. In the present study, we discuss 4 cases of GA-FG that displayed low grade malignancy, slow-growth and less aggressive clinical behavior. Endoscopic submucosal dissection was performed and complete tumor resection was confirmed pathologically. None of the patients showed any signs of recurrence during the follow-up periods. We decided to report these rare cases because of their distinct endoscopic and clinicopathological features and unique biological behaviors.
- Citation: Tohda G, Osawa T, Asada Y, Dochin M, Terahata S. Gastric adenocarcinoma of fundic gland type: Endoscopic and clinicopathological features. World J Gastrointest Endosc 2016; 8(4): 244-251
- URL: https://www.wjgnet.com/1948-5190/full/v8/i4/244.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i4.244
Gastric carcinoma has been histologically classified into differentiated and undifferentiated types[1]. Following the advent of recent advanced techniques such as mucin histochemistry and immunohistochemistry, gastric adenocarcinomas can be classified as having either a gastric or an intestinal phenotype[2,3], irrespective of their histological features. Among the types of differentiated adenocarcinomas with gastric phenotypes, tumors of both the foveolar type and the pyloric gland type have been reported. However, gastric adenocarcinomas of fundic gland type (GA-FG) have been considered extremely rare. After several cases of GA-FG with a parietal cell phenotype were reported[4-9], a GA-FG with a chief cell differentiation was reported[10] and was proposed as a new entity of gastric adenocarcinoma[11]. Since this proposal, several cases of GA-FG have been reported[12-20], but it is still considered an extremely rare type of gastric cancer. In the present study, we describe 4 cases of GA-FG with a chief cell differentiation, and evaluate their endoscopic findings, clinicopathological features and biological behaviors.
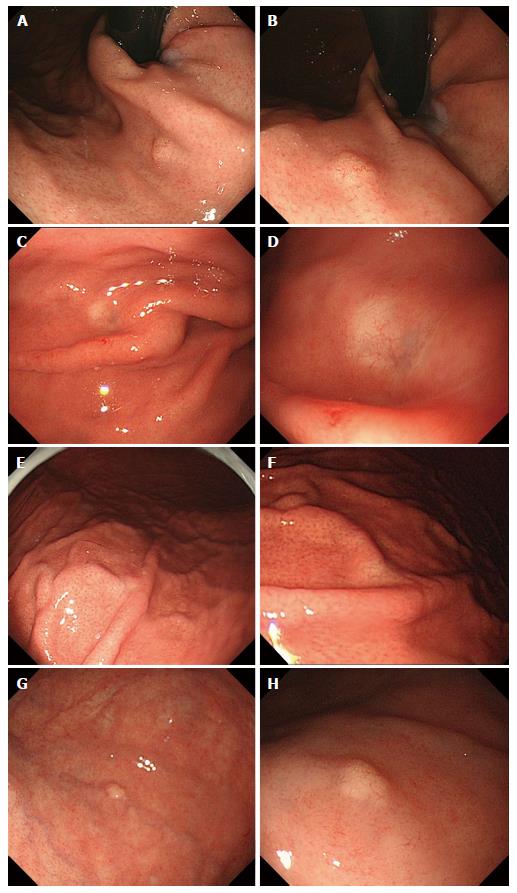
Between October 2010 and September 2014, among the 30182 patients who underwent upper gastrointestinal endoscopy as part of a yearly check-up, 4 patients were diagnosed with GA-FG. The clinicopathological findings are summarized in Table 1. The two male and two female patients were between 42 and 62 years of age. Concerning the gastric mucosa, atrophic changes with Helicobacter pylori (H. pylori) infection were observed in two of the 4 cases. The tumors were located in the upper or middle third of the stomach, and some appeared as either submucosal tumor (SMT)-like elevated lesions, whereas others had a flat shape. Faded or whitish discoloration and dilated vessels on the surface were also observed by conventional endoscopy (Figure 1). Neither irregular microvascular architecture nor an irregular microsurface pattern was detected by magnifying endoscopy with narrow band imaging. All of the tumors were smaller than 5 mm in diameter. Histological examination of the biopsy specimens showed well-differentiated adenocarcinomas composed of chief cell-like cells. No evidence of metastasis was observed on computed tomography. After a preoperative diagnosis of GA-FG, endoscopic submucosal dissection (ESD) was performed.
| Case 1 | Case 2 | Case 3 | Case 4 | |
| Age (yr) | 42 | 60 | 62 | 50 |
| Sex | Male | Female | Male | Female |
| Location | Upper third | Upper third | Middle third | Upper third |
| H. pylori | Negative | Positive | Negative | Positive |
| Morphological type | SMT-like | 0IIb | 0IIb | SMT-like |
| Size (mm) | 4 × 3 | 5 × 4 | 5 × 5 | 2 × 2 |
| Depth of invasion | M | M | SM | SM |
| Lymphatic invasion | - | - | - | - |
| Venous invasion | - | - | - | - |
| Observation time | 10 mo | 16 mo | 28 mo | 57 mo |
| Recurrence | - | - | - | - |
| Outcome | Alive | Alive | Alive | Alive |
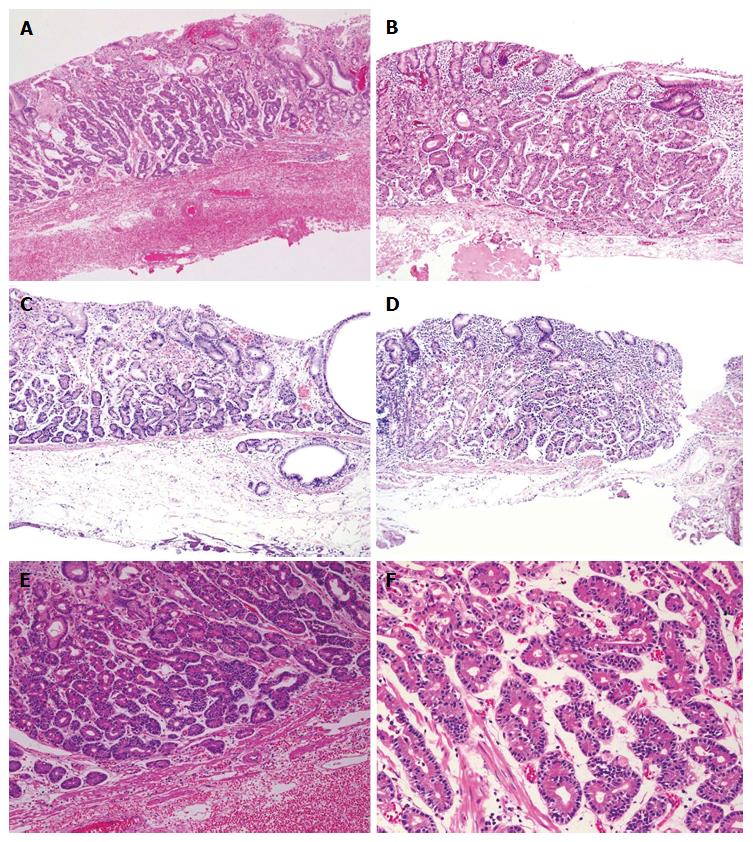
According to the hematoxylin and eosin staining, the tumors were primarily composed of well-differentiated adenocarcinoma with columnar cells that mimicked fundic gland cells (Figure 2). Irregularly anastomosing glands with mildly enlarged and hyperchromatic nuclei were also observed. Histologically, in all cases, the tumors arose within the deeper zone of the gastric mucosa and were covered with a non-neoplastic epithelium. Despite the small sizes of the tumors, minimal invasion of the submucosal layer by tumor cells was observed in 2 cases. However, none of the cases demonstrated lymphatic or venous invasion. Moreover, none of the endoscopically resected specimens showed atrophic changes or intestinal metaplasia in the surrounding mucosa.
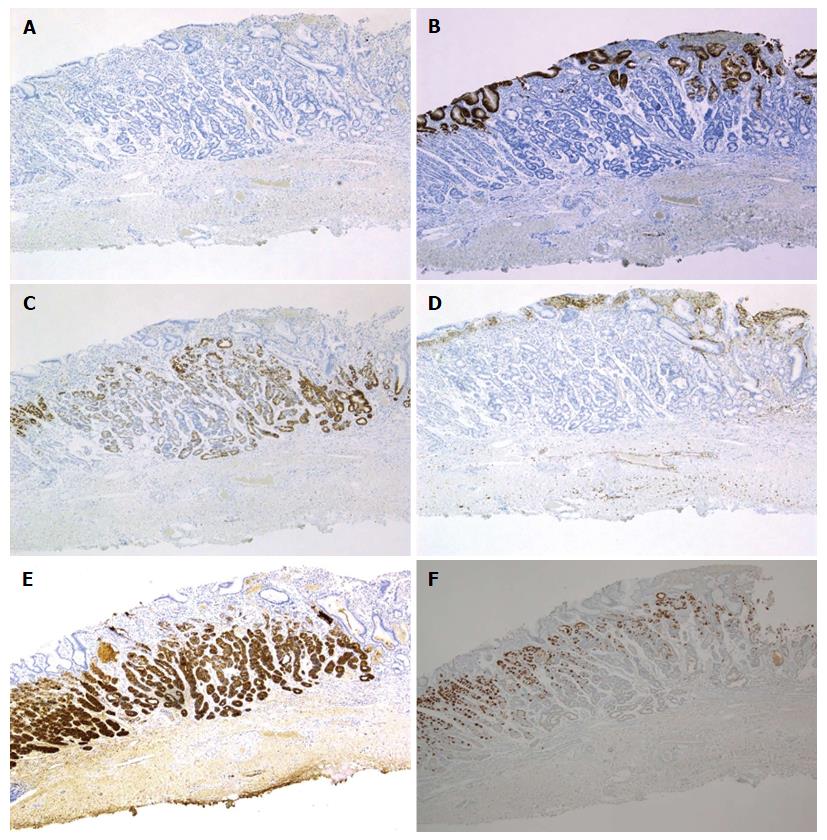
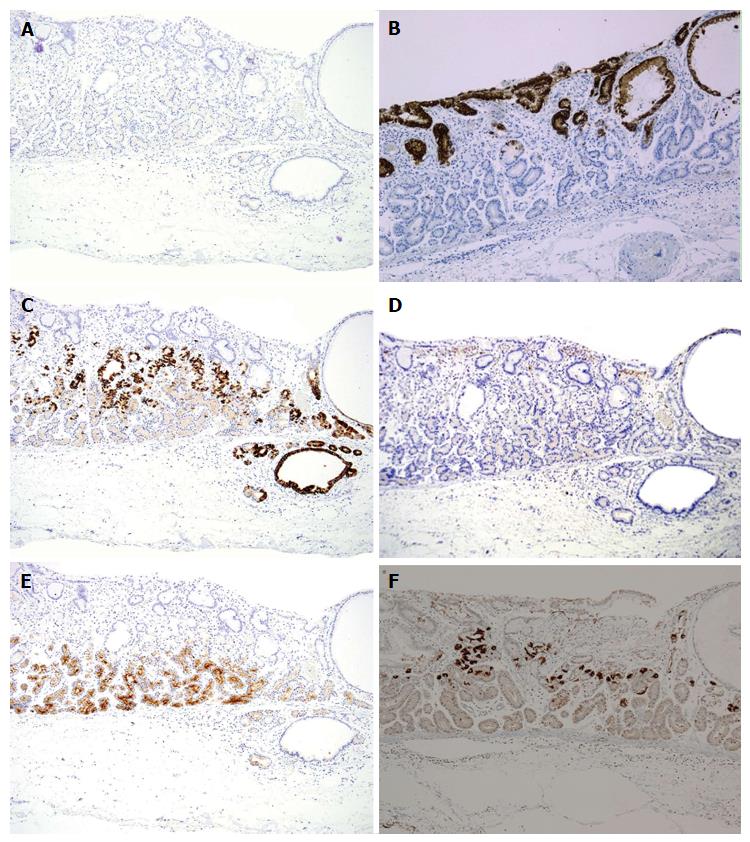
Cell differentiation according to the expression of Mucin 2 (MUC2) (a marker of goblet cells), MUC5AC (a marker of gastric foveolar epithelium), MUC6 (a marker of mucous neck cells and pyloric glands), CD10 (a marker of the brush border), pepsinogen-I (a marker of chief cells), H +/K +-ATPase (a marker of parietal cells) was evaluated immunohistochemically (Table 2). The tumor cells were classified as the gastric mucin phenotype based on diffuse positive staining for MUC6 and negativity for MUC2 and CD10. Immunohistochemical staining showed diffuse positive staining for pepsinogen-I and limited positivity for H +/K +-ATPase in scattered locations around the tumor margin (Figures 3 and 4). Finally, all 4 cases were diagnosed as GA-FG with chief cell differentiation. Cell proliferation, which was based on the Ki-67 (MIB-1) labeling index, was lower than 5% in all cases. Complete tumor resection was confirmed pathologically without further treatment. No complications associated with ESD were observed. None of the patients showed any signs of recurrence during the follow-up periods.
| Case 1 | Case 2 | Case 3 | Case 4 | |
| MUC 2 | Negative | Negative | Negative | Negative |
| MUC 5AC | Negative | Negative | Negative | Negative |
| MUC 6 | Positive | Positive | Positive | Positive |
| CD 10 | Negative | Negative | Negative | Negative |
| Pepsinogen-I | Positive | Positive | Positive | Positive |
| H+/K+-ATPase | NS | NS | NS | NS |
Histologically, GA-FG is typically classified into a chief cell-predominant type, a parietal cell-predominant type, or a mixed type. GA-FG with chief cell differentiation has been considered extremely rare and has been proposed as a new entity and pathologic subtype of gastric adenocarcinoma[11]. GA-FG is associated with distinct clinicopathological characteristics, tumor location (they arise within a deeper zone of the gastric mucosa), and histological features (high-frequency of submucosal invasion independent of tumor size) as well as mucin expression (stomach type), and low-grade malignancy (mild atypism, lack of lymphovascular invasion and low proliferative activity). Moreover, GA-FG is not associated with H. pylori infection[11]. In the present study, we describe 4 cases of GA-FG with chief cell differentiation.
During the diagnostic process of GA-FG, endoscopic features should be taken into consideration. According to previous reports[11,18], GA-FG has distinct endoscopic features, such as an SMT shape or a flat/depressed shape, a faded/whitish color and dilated vessels on the surface, which can provide valuable information in the diagnostic process. In the present study, 2 cases revealed an SMT-like morphology on endoscopy. For these cases, we would have to consider the possibility of sporadic carcinoid tumors that may also have been centered in the deeper zone of the gastric mucosa. The other 2 cases featured a flat mucosa with faded/whitish discoloration. For these cases, undifferentiated adenocarcinoma, mucosa-associated lymphoid tissue lymphoma, and normal gastric mucosa with focal atrophy would have to be considered as the differential diagnoses. Although distinct endoscopic features might be helpful in the diagnosis of GA-FG, it may still be difficult to discriminate among the differential diagnoses by endoscopic findings.
In the process of a histological diagnosis of GA-FG, dysplasia and adenocarcinoma in the fundic gland polyps (FGPs) have to be considered as the differential diagnoses. According to previous reports[21,22], dysplasia and adenocarcinoma in the FGPs usually arise from the foveolar epithelium, which is different from the epithelium of the fundic glands. Therefore, these diseases can be distinguished from GA-FG. Cases of FGPs with chief cell hyperplasia and morphologic atypia have also been reported[23,24]. In these reports, structural and nuclear atypia in chief cell hyperplastic lesions were detected, but they were not diagnosed as adenocarcinoma due to a low Ki-67 labeling index. However, it is possible that such cases may become carcinomas because they are similar to the tumors of GA-FG chief cell-predominant type.
In the present study, all cases of GA-FG were discovered in the early stages. Neither lymphatic nor venous invasion was observed despite the presence of minimal tumor invasion into the submucosal layer. A high Ki-67 labeling index is considered to be related to a poor prognosis of gastric adenocarcinoma[25,26]. In our study, all cases show a low proliferative index in regards to Ki-67 expression. No signs of recurrence or metastasis were observed during the follow-up periods. Patients with GA-FG are considered to have a favorable prognosis[11,16-18]. In the current report, long-term follow-up of patients with GA-FG using upper gastrointestinal endoscopy showed changes that were barely detectable[17]. In our study, a 50-year-old woman who was diagnosed with GA-FG underwent follow-up endoscopy every 8 mo. However, no detectable changes in the tumor were observed, and ESD was performed after three years of observation. Minimal invasion by the cancer cells into the submucosa was observed, but no lymphovascular invasion was noted. Our cases also suggest that GA-FG displays slow-growth and less aggressive clinical behavior compared with typical gastric adenocarcinomas.
Base on the less aggressive biological behavior of GA-FG, a reclassification of GA-FG as a benign, oxyntic gland polyp/adenoma was proposed[16]. However, advanced stage GA-FG with unusual clinicopathological features has since been reported[19]. It has also been reported that GA-FG shows a favorable prognosis with less aggressive clinical behavior but that some cases of GA-FG might transform into high-grade malignancies during tumor progression[11,19]. A recent study clarified that the molecular pathway of GA-FG is different from that of conventional type gastric adenocarcinomas[27-29]. Because only a few case reports of early stage GA-FG have been published, the pathogenesis of GA-FG has not yet been elucidated. Further research is needed to analyze the molecular mechanism of GA-FG and to evaluate the clinicopathological features including those of tumor development.
In conclusion, we reported 4 cases of well-differentiated adenocarcinoma of GA-FG with chief cell differentiation. GA-FG is extremely rare and has distinct characteristics that distinguish it from the more common types of gastric adenocarcinoma. Future studies will be expected to analyze the clinical behavior of GA-FG.
Four middle-aged patients without symptom underwent upper gastrointestinal endoscopy as a yearly check-up, and diagnosed as gastric adenocarcinoma of fundic gland type (GA-FG) with chief cell differentiation.
Upon physical examination, no significant physical findings were observed.
Carcinoid tumors, undifferentiated adenocarcinoma, mucosa-associated lymphoid tissue lymphoma, normal gastric mucosa with focal atrophy, and adenocarcinoma in the fundic gland polyps were considered as differential diagnoses.
All laboratory data were within normal limits.
In the upper or middle third of the stomach, a submucosal tumor-like elevated lesions or a flat shape with faded/whitish discoloration and dilated vessels on the surface were observed by upper gastrointestinal endoscopy.
The tumors were primarily composed of well-differentiated adenocarcinoma resembling fundic gland cells, and diagnosed as GA-FG with chief cell differentiation by immunohistochemical staining.
In all cases, endoscopic submucosal dissection was performed and complete tumor resection was confirmed pathologically.
GA-FG is a novel diagnostic entity with getting more reported cases worldwide. GA-FG usually shows low grade malignancy and less aggressive clinical behavior. Future studies will be expected to analyze the long-term prognosis of GA-FG.
GA-FG is a new diagnostic entity of gastric adenocarcinoma. Endoscopic treatment is often performed because of its low grade malignancy and less aggressive clinical behavior.
GA-FG has been reported as a new entity and pathologic subtype of gastric adenocarcinoma. Recognizing the endoscopic and clinicopathological features of GA-FG can prevent misdiagnosis and provide adequate treatment.
GA-FG has been recently reported as a novel diagnostic entity of gastric adenocarcinoma. There is little information about GA-FG. In this study, the clinicopathologic findings of GA-FG are summarized. It is worthwhile for the readers because of its rarity or being less noticed.
P- Reviewer: Lee CL, Ozkan OV S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968;59:251-258. [PubMed] |
| 2. | Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M. Gastric or intestinal phenotypic expression in the carcinomas and background mucosa of multiple early gastric carcinomas. Histopathology. 2000;37:513-522. [PubMed] |
| 3. | Matsui N, Yao T, Akazawa K, Nawata H, Tsuneyoshi M. Different characteristics of carcinoma in the gastric remnant: histochemical and immunohistochemical studies. Oncol Rep. 2001;8:17-26. [PubMed] |
| 4. | Capella C, Frigerio B, Cornaggia M, Solcia E, Pinzon-Trujillo Y, Chejfec G. Gastric parietal cell carcinoma--a newly recognized entity: light microscopic and ultrastructural features. Histopathology. 1984;8:813-824. [PubMed] |
| 5. | Byrne D, Holley MP, Cuschieri A. Parietal cell carcinoma of the stomach: association with long-term survival after curative resection. Br J Cancer. 1988;58:85-87. [PubMed] |
| 6. | Hedenbro JL, Hägerstand I, Rychterova V. Parietal cell carcinoma. A new differential diagnosis for submucosal gastric tumors. Endoscopy. 1990;22:47-48. [PubMed] |
| 7. | Rychterova V, Hägerstrand I. Parietal cell carcinoma of the stomach. APMIS. 1991;99:1008-1012. [PubMed] |
| 8. | Takubo K, Honma N, Sawabe M, Arai T, Izumiyama-Shimomura N, Kammori M, Sasajima K, Esaki Y. Oncocytic adenocarcinoma of the stomach: parietal cell carcinoma. Am J Surg Pathol. 2002;26:458-465. [PubMed] |
| 9. | Yang GY, Liao J, Cassai ND, Smolka AJ, Sidhu GS. Parietal cell carcinoma of gastric cardia: immunophenotype and ultrastructure. Ultrastruct Pathol. 2003;27:87-94. [PubMed] |
| 10. | Tsukamoto T, Yokoi T, Maruta S, Kitamura M, Yamamoto T, Ban H, Tatematsu M. Gastric adenocarcinoma with chief cell differentiation. Pathol Int. 2007;57:517-522. [PubMed] |
| 11. | Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Fukatsu H, Miyoshi H, Ishiki K, Tamura M, Yao T. Gastric adenocarcinoma of fundic gland type (chief cell predominant type) treated with endoscopic aspiration mucosectomy. Dig Endosc. 2011;23:244-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Terada T. Well differentiated adenocarcinoma of the stomach composed of chief cell-like cells and parietal cells (Gastric adenocarcinoma of fundic gland type). Int J Clin Exp Pathol. 2011;4:797-798. [PubMed] |
| 14. | Park ES, Kim YE, Park CK, Yao T, Kushima R, Kim KM. Gastric adenocarcinoma of fundic gland type: report of three cases. Korean J Pathol. 2012;46:287-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Chen WC, Rodriguez-Waitkus PM, Barroso A, Balsaver A, McKechnie JC. A Rare Case of Gastric Fundic Gland Adenocarcinoma (Chief Cell Predominant Type). J Gastrointest Cancer. 2012;43 Suppl 1:S262-S265. [PubMed] |
| 16. | Singhi AD, Lazenby AJ, Montgomery EA. Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma. Am J Surg Pathol. 2012;36:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Abe T, Nagai T, Fukunaga J, Okawara H, Nakashima H, Syutou M, Kajimoto N, Wake R, Oyama T, Yao T. Long-term follow-up of gastric adenocarcinoma with chief cell differentiation using upper gastrointestinal tract endoscopy. Intern Med. 2013;52:1585-1588. [PubMed] |
| 18. | Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Ueo T, Yonemasu H, Ishida T. Gastric adenocarcinoma of fundic gland type with unusual behavior. Dig Endosc. 2014;26:293-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Hori K, Ide YH, Hirota S, Toyoshima F, Takagawa T, Nakamura S. Early gastric adenocarcinoma of the fundic gland type. Endoscopy. 2015;47 Suppl 1 UCTN:E177-E178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Jalving M, Koornstra JJ, Boersma-van Ek W, de Jong S, Karrenbeld A, Hollema H, de Vries EG, Kleibeuker JH. Dysplasia in fundic gland polyps is associated with nuclear beta-catenin expression and relatively high cell turnover rates. Scand J Gastroenterol. 2003;38:916-922. [PubMed] |
| 22. | Stolte M, Vieth M, Ebert MP. High-grade dysplasia in sporadic fundic gland polyps: clinically relevant or not? Eur J Gastroenterol Hepatol. 2003;15:1153-1156. [PubMed] |
| 23. | Müller-Höcker J, Rellecke P. Chief cell proliferation of the gastric mucosa mimicking early gastric cancer: an unusual variant of fundic gland polyp. Virchows Arch. 2003;442:496-500. [PubMed] |
| 24. | Matsukawa A, Kurano R, Takemoto T, Kagayama M, Ito T. Chief cell hyperplasia with structural and nuclear atypia: a variant of fundic gland polyp. Pathol Res Pract. 2005;200:817-821. [PubMed] |
| 25. | Kikuyama S, Kubota T, Shimizu K, Miyakita M. Ki-67 antigen expression in relation to clinicopathological variables and prognosis in gastric cancer. Oncol Rep. 1998;5:867-870. [PubMed] |
| 26. | Goishi H, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Predictive value of cathepsin D and Ki-67 expression at the deepest penetration site for lymph node metastases in gastric cancer. Oncol Rep. 2000;7:713-718. [PubMed] |
| 27. | Nomura R, Saito T, Mitomi H, Hidaka Y, Lee SY, Watanabe S, Yao T. GNAS mutation as an alternative mechanism of activation of the Wnt/β-catenin signaling pathway in gastric adenocarcinoma of the fundic gland type. Hum Pathol. 2014;45:2488-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Kushima R, Sekine S, Matsubara A, Taniguchi H, Ikegami M, Tsuda H. Gastric adenocarcinoma of the fundic gland type shares common genetic and phenotypic features with pyloric gland adenoma. Pathol Int. 2013;63:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Hidaka Y, Mitomi H, Saito T, Takahashi M, Lee SY, Matsumoto K, Yao T, Watanabe S. Alteration in the Wnt/β-catenin signaling pathway in gastric neoplasias of fundic gland (chief cell predominant) type. Hum Pathol. 2013;44:2438-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |