Published online Feb 10, 2016. doi: 10.4253/wjge.v8.i3.186
Peer-review started: September 16, 2015
First decision: October 21, 2015
Revised: November 8, 2015
Accepted: December 8, 2015
Article in press: December 11, 2015
Published online: February 10, 2016
Processing time: 142 Days and 11.5 Hours
AIM: To elucidate the safety of percutaneous endoscopic gastrostomy (PEG) under steady pressure automatically controlled endoscopy (SPACE) using carbon dioxide (CO2).
METHODS: Nine patients underwent PEG with a modified introducer method under conscious sedation. A T-tube was attached to the channel of an endoscope connected to an automatic surgical insufflator. The stomach was inflated under the SPACE system. The intragastric pressure was kept between 4-8 mmHg with a flow of CO2 at 35 L/min. Median procedure time, intragastric pressure, median systolic blood pressure, partial pressure of CO2, abdominal girth before and immediately after PEG, and free gas and small intestinal gas on abdominal X-ray before and after PEG were recorded.
RESULTS: PEG was completed under stable pneumostomach in all patients, with a median procedural time of 22 min. Median intragastric pressure was 6.9 mmHg and median arterial CO2 pressure before and after PEG was 42.1 and 45.5 Torr (NS). The median abdominal girth before and after PEG was 68.1 and 69.6 cm (NS). A mild free gas image after PEG was observed in two patients, and faint abdominal gas in the downstream bowel was documented in two patients.
CONCLUSION: SPACE might enable standardized pneumostomach and modified introducer procedure of PEG.
Core tip: We report the safety of percutaneous endoscopic gastrostomy (PEG) under steady pressure automatically controlled endoscopy (SPACE) using carbon dioxide (CO2). Nine patients underwent PEG with a modified introducer method under conscious sedation. The stomach was inflated under the SPACE system. PEG was completed under stable pneumostomach in all patients. Median arterial CO2 pressure before and after PEG was 42.1 and 45.5 Torr (NS). The median abdominal girth before and after PEG was 68.1 and 69.6 cm (NS). A mild free gas image after PEG was observed in two patients. SPACE might enabled standardized pneumostomach which leads to easier and safer PEG procedures.
- Citation: Imaeda H, Nakajima K, Hosoe N, Nakahara M, Zushi S, Kato M, Kashiwagi K, Matsumoto Y, Kimura K, Nakamura R, Wada N, Tsujii M, Yahagi N, Hibi T, Kanai T, Takehara T, Ogata H. Percutaneous endoscopic gastrostomy under steady pressure automatically controlled endoscopy: First clinical series. World J Gastrointest Endosc 2016; 8(3): 186-191
- URL: https://www.wjgnet.com/1948-5190/full/v8/i3/186.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i3.186
Percutaneous endoscopic gastrostomy (PEG) has been widely accepted for external feeding since Gauderer et al[1] first reported it in 1980. A conventional on-demand insufflation using atmospheric air through the endoscope has been a gold standard in performing PEG, not only for optimal visualization but also for maintaining pneumostomach to keep puncture sites on the gastric/abdominal walls stabilized. Abdominal distension and pneumoperitoneum often occur after PEG[2-7]. Carbon dioxide (CO2) insufflation has been initially reported for colonoscopic electrosurgical polypectomy in the field of gastrointestinal (GI) endoscopy[8]. CO2 is now increasingly being used instead of atmospheric air in GI endoscopic procedures since CO2 is rapidly absorbed via the gut lining. Total colonoscopy[9-13], endoscopic retrograde cholangiopanreatography[14-17], peroral cholangioscopy[18], double-balloon enteroscopy[19], PEG[20], gastric and colonic endoscopic submucosal dissection (ESD)[21-25], and upper GI intragastric endoscopy during laparoscopic surgery under CO2 insufflation[26] have been reported to be safe and more comfortable compared with air insufflation.
GI endoscopy has been performed under on-demand insufflation by endoscopists through the endoscope itself in a manual manner without pressure monitoring. This practice has been justified because the gastrointestinal tract allows migration of excessive gas into the upstream/downstream bowel. Excessive air supply may result in gaseous regurgitation, vomiting, and abdominal bloating. Steady pressure automatically controlled endoscopy (SPACE) using CO2, developed by Nakajima et al[27,28], Kato et al[29] and Yamada et al[30] is expected to improve and standardize endoscopic visualization and working space in the GI lumen. Although SPACE has been reported to shorten procedural time and improve the safety of endoscopic intervention[28-30], CO2 narcosis is of concern during PEG under sedation, since patients usually suffer from respiratory disease and/or consciousness disturbance. The SPACE system consists of a standard commercially available endoscope overtube (Top Co., Ltd., Tokyo, Japan) and a newly developed detachable leak-proof device with an anti-reflux valve and a Luer lock connection (Leak Cutter, Top)[28,29]. A commercially available automatic surgical insufflator is then connected to the system. Esophageal ESD under SPACE has been reported to be feasible and safe[28,29]. Recently, gastric ESD under SPACE has been also reported to be feasible and safe in an preclinical study[30].
The aim of this study is to elucidate the safety of PEG under the SPACE system. To our knowledge, this is the first clinical study regarding application of SPACE technology in PEG.
Ten patients undergoing treatment at our institutions were enrolled in the study. Patients who had CO2 retention due to chronic obstructive pulmonary dysfunction were excluded. One of the ten enrolled patients was excluded because he withdrew his consent after informed consent was obtained. Therefore, a total of nine patients, six males and three females, underwent PEG under SPACE. The mean age of patients was 78 years (ranging from 61 to 89). Four patients had Parkinson’s disease, one had cerebrovascular disease, one had amyotrophic lateral sclerosis, one had necrotizing fasciitis, one had disuse syndrome, and one had laryngeal cancer (Table 1).
| Clinical characteristics | Data |
| Male/female | 6/3 |
| Mean age | 78 (61–89) |
| Comorbid disease | |
| Parkinson's disease | 4 |
| Cerebrovascular disease | 1 |
| Amyotrophic lateral sclerosis | 1 |
| Necrotizing fasciitis | 1 |
| Disuse syndrome | 1 |
| Laryngeal cancer | 1 |
PEG was performed under conscious sedation using intravenous injection of 35 mg pethidine chloride and 0.1-0.2 mg of flunitrazepam or 1-2 mg of midazolam and oxygen inhalation. A T-tube with two junctions (MD-807, Olympus Medical Systems Co. Ltd., Tokyo, Japan) was connected directly to the channel of the flexible gastroscope (GIF-H260, Olympus Medical Systems Co. Ltd., Tokyo, Japan) (Figure 1). One of the junctions was connected to a commercially available automatic surgical insufflator (UHI-3, Olympus Medical Systems Co. Ltd., Tokyo, Japan) that feeds 35 L of CO2 per minute into the stomach through the channel (Figure 2). The intragastric pressure was kept between 4-8 mmHg. PEG was performed using a modified introducer procedure and a dedicated kit (Direct Ideal PEG kit, Olympus Medical Systems Co. Ltd., Tokyo, Japan). The gastroscope was inserted from the mouth to the esophagus under conventional manual air insufflation. After insertion into the stomach, conventional manual air insufflation was switched to the SPACE system. First, percutaneous gastropexy was conducted at two sites while the stomach was inflated under the SPACE system through the endoscope channel. Second, after puncture using an indwelling needle was performed between the two gastropexy sites, a guide-wire was replaced with the needle. Third, the PEG site was dilated by the dilator through the guide-wire. When the dilator was withdrawn, the CO2 supply was temporarily stopped, the PEG tube was inserted through the guide-wire, and the CO2 supply was restarted and checked to ensure it had been located correctly.
Data such as mean procedure time, intragastric pressure, mean systolic blood pressure, partial pressure of CO2 (PaCO2), abdominal circumference before and soon after PEG, and change of free gas and small intestinal gas on abdominal X-ray before and immediately after PEG were obtained and prospectively recorded in the database.
The study protocol was in accordance with the tenets of the revised Declaration of Helsinki (1989) and was approved by the institutional review board at our institutions. Written informed consent was obtained from all the patients.
Statistical analysis was performed by Fischer’s test using SPSS software, version 17 (SPSS Inc., Chicago, IL). For therapeutic performance, sensitivity, specificity, and accuracy are presented as percentages with 95%CIs. All probability values calculated in this analysis were sided, and P < 0.05 was considered significant.
The median procedural time was 22 min (14-38 min) (Table 2). It was possible to maintain a good endoscopic visualization and a sufficient pneumostomach to keep puncture sites stabilized during PEG, which was completed easily in all 9 patients. Visualization after intentional suction was regained more quickly than with conventional endoscopy (Video 1). PEG was established exactly in the scheduled puncture sites. Median intragastric pressure was kept at 6.9 mmHg as preset (5-8 mmHg). Median O2 inhalation was 1.7 L/min (0-3). Median systolic blood pressure before and immediately after PEG was 129.3 mmHg (101-158 mmHg) and 120.6 mmHg (90-145 mmHg). There was no significant difference in these data (P = 0.33). Median PaCO2 before and after PEG was 42.1 Torr (35.2-45.7 Torr) and 45.5 Torr (41.0-54.6 Torr). There was a tendency to an elevated median PaCO2 after PEG compared with prior values (P = 0.10); however no CO2 narcosis was encountered in the series.
| Clinical outcomes | P value | |
| Median procedural time (min) | 22 (14-38) | |
| Median intragastric pressure (mmHg) | 6.9 (5-8) | |
| Median systolic pressure | ||
| Before PEG (mmHg) | 129.3 (101-158) | |
| Soon after PEG (mmHg) | 120.6 (90-145) | 0.33 |
| Median PaCO2 | ||
| Before PEG (Torr) | 42.1 (35.2-45.7) | |
| Soon after PEG (Torr) | 45.5 (41.0-54.6) | 0.10 |
| Median abdominal girth | ||
| Before PEG (cm) | 68.1 (58-85) | |
| Soon after PEG (cm) | 69.6 (60-86) | 0.38 |
| Mild free gas after PEG (n) | 2 | |
| Mild increase of small intestinal gas after PEG (n) | 2 |
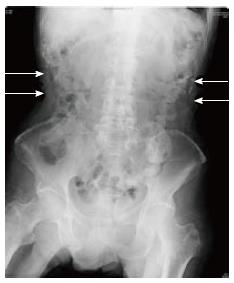
The median abdominal girth before and immediately after PEG was 68.1 cm (58-85 cm) and 69.6 cm (60-86 cm), and there was no significant difference (P = 0.38). Mild free gas was observed postoperatively in two patients, and small intestinal gas was slightly increased in two patients (Figure 3). All these were subclinical, and no other serious adverse events were encountered in any patients.
Several endoscopic procedures under CO2 insufflation have been reported to be safe and more comfortable compared with air insufflation because CO2 is absorbed rapidly via the gut lining. CO2 insufflation during PEG reduces risk of pneumoperitonium and bloating[8-25]. Technically, it is a key point to maintain pneumostomach stabilized during PEG so that PEG can be fashioned in the scheduled puncture sites.
In our study, although PaCO2 was subclinically elevated during and after the procedure, there were no adverse events associated with CO2 insufflation. The insufflation is mandatory in PEG for maintaining a pneumostomach to keep puncture sites stabilized. Nishiwaki et al[20] reported that PEG under CO2 insufflation compared with air insufflation was safer and more comfortable because of the lower incidence of pneumoperitoneum, less distension of the small bowel, and no adverse events. Our present data first showed that PEG is safely fashioned under SPACE.
Nakajima et al[27] reported that a steady-pressure pneumostomach was successfully created and maintained for 100 min on average without clamping the downstream bowel in laparoscopic intragastric surgery (LIGS). The stomach was insufflated with a UHI-3 surgical insufflation unit connected to a transgastric port at an intragastric pressure of 6-8 mmHg. No adverse events were noted during LIGS, and no postoperative abdominal distention was observed. Nakajima et al[28] have also reported esophageal ESD under SPACE using a standard endoscopic overtube and a detachable leak-proof valve with a luer-lock connection in an animal model. Moreover, Kato et al[29] reported on the feasibility and safety of esophageal ESD under SPACE in a clinical study, and Yamada et al[30] reported on the feasibility and safety of gastric ESD under SPACE in an animal model. In SPACE, endoscopic visualization is automatically obtained once the insufflation pressure and flow rate are set. Visualization after suction is automatically regained more quickly than with conventional endoscopy. The flow capacity of current surgical insufflators is higher than that of manual endoscopic insufflators and is considered responsible for the rapid regaining. UHI-3 can supply 35 L of CO2 per minute and these flow rates are significantly higher than those of actual endoscopic flow with manual CO2 insufflation (1.4 L/min). The insufflation process is automatic in SPACE. Air/water button manipulation is no longer necessary, leaving the endoscopist free to focus on the intervention itself. SPACE can prevent excessive CO2 supply, which may result in gaseous regurgitation, vomiting, and abdominal bloating[30].
In this study, CO2 was successfully supplied through the endoscopic channel using a T-tube without an overtube. The intragastric pressure was kept from 5 to 8 mmHg throughout the procedure. PEG under SPACE had no negative effects such as vomiting or abdominal bloating and no impact on vital signs. Mild postprocedural free gas was observed in two patients and abdominal gas was slightly increased in another two patients. There were, however, no adverse events in any patients. Even if CO2 is leaked into the abdominal cavity through the PEG site, CO2 can be absorbed quickly via the peritoneal lining and abdominal distention will be resolved immediately. Nishiwaki et al[20] reported that pneumoperitoneum was not observed in the CO2 insufflation group. In our study, pneumoperitoneum might have occurred because of the leakage of remnant air in the stomach. Nishiwaki et al[20] performed a pull method of the PEG procedure, while in our study, a modified introducer method was performed. After the dilator was withdrawn, the PEG tube was inserted during the modified introducer method, and it was possible that intragastric gas (air) might have leaked into the abdominal cavity at this time. Thus we hypothesized that postprocedural pneumoperitoneum might be caused by the difference of the PEG procedure. Yamada et al[30] reported the potential safety of pneumoperitoneum under SPACE, because intra-gastric pressure was regulated within the preset pressure range to prevent excessive transmural insufflation. Nakajima et al[28] have reported that the migration of CO2 over the proximal jejunum does not occur because of a pinch-cock phenomenon and intestinal surface tension. In this pinch-cock phenomenon, the distended upstream bowel (stomach and duodenum) acts as a cock that compresses the downstream bowel, resulting in the prevention of massive gas migration. The surface tension in the collapsed gut lumen may work as another pressure barrier. The insufflated gas volume was sufficiently low in each SPACE, suggesting no major gas migration into the downstream bowel during SPACE. In fact, CO2 outflow stopped automatically whenever the stomach was insufflated.
Although conscious sedation is necessary during PEG procedure, most patients who undergo PEG have cerebrovascular diseases and aspiration pneumonia, which means they are at high risk for developing respiratory dysfunction. CO2 narcosis might develop in patients with chronic pulmonary diseases, so they were excluded from this study. There was a tendency to an elevated PaCO2 median after PEG compared with before PEG, but CO2 narcosis did not occur in any cases. This elevation might be caused by PEG under SPACE, but it could also be caused by the administration of sedative drugs that suppress the respiratory function.
There were several limitations in this study. First, as this was a pilot study, the sample size was very small. We need to accumulate more clinical data such as a randomized controlled trial between PEG under conventional manual air or CO2 insufflation and that under SPACE system in near future. There was a tendency to an elevated median PaCO2 after PEG compared with previous values, indicating that a randomized controlled trial to compare PEG under SPACE and under manual air insufflation is necessary. We examined PaCO2 only twice: once before and once after PEG. Ideally we should examine the course of PCO2 during PEG using the monitor of transcutaneous measurement of PCO2. Most patients cannot complain of abdominal pain or distention because of comorbid diseases such as cerebrovascular disease, so the complaints of all patients cannot be detected. We have to examine the gas volume in the small intestine and the pneumoperitoneum in the abdominal X-ray and/or CT scan. The channel is free during a modified introducer procedure of PEG, therefore, the SPACE system is available during PEG procedure. The introduction of snares or forceps through the channel affects the SPACE system.
In conclusion, PEG under SPACE might be feasible and safe. SPACE might enable standardized pneumostomach which leads to easier and safer PEG procedures.
“On-demand” insufflation using atmospheric air has been a gold standard in performing percutaneous endoscopic gastrostomy (PEG), not only for optimal visualization but also for maintaining pneumostomach to keep puncture sites stabilized. However, excessive air insufflation may result in gaseous regurgitation, vomiting, and abdominal bloating.
PEG under steady pressure automatically controlled endoscopy (SPACE) using carbon dioxide (CO2) has not been reported.
PEG under SPACE was feasible and safe.
SPACE enables standardized pneumostomach which leads to easier and safer PEG procedures.
The authors evaluated the safety of PEG under SPACE using CO2. PEG was completed under stable pneumostomach in all nine patients. Further clinical trials in a randomized controlled study between PEG under conventional manual air or CO2 insufflation and that under SPACE system will be necessary.
P- Reviewer: Casadesus D, Kapetanos D, Trevisani L S- Editor: Wang JL L- Editor: A E- Editor: Lu YJ
| 1. | Gauderer MW, Ponsky JL, Izant RJ. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872-875. [PubMed] |
| 2. | Gottfried EB, Plumser AB, Clair MR. Pneumoperitoneum following percutaneous endoscopic gastrostomy. A prospective study. Gastrointest Endosc. 1986;32:397-399. [PubMed] |
| 3. | Wojtowycz MM, Arata JA. Subcutaneous emphysema after percutaneous gastrostomy. Am J Roentgenol. 1988;151:311-312. [PubMed] |
| 4. | Dulabon GR, Abrams JE, Rutherford EJ. The incidence and significance of free air after percutaneous endoscopic gastrostomy. Am Surg. 2002;68:590-593. [PubMed] |
| 5. | Wiesen AJ, Sideridis K, Fernandes A, Hines J, Indaram A, Weinstein L, Davidoff S, Bank S. True incidence and clinical significance of pneumoperitoneum after PEG placement: a prospective study. Gastrointest Endosc. 2006;64:886-889. [PubMed] |
| 6. | Schrag SP, Sharma R, Jaik NP, Seamon MJ, Lukaszczyk JJ, Martin ND, Hoey BA, Stawicki SP. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J Gastrointestin Liver Dis. 2007;16:407-418. [PubMed] |
| 7. | Blum CA, Selander C, Ruddy JM, Leon S. The incidence and clinical significance of pneumoperitoneum after percutaneous endoscopic gastrostomy: a review of 722 cases. Am Surg. 2009;75:39-43. [PubMed] |
| 8. | Rogers BH. The safety of carbon dioxide insufflation during colonoscopic electrosurgical polypectomy. Gastrointest Endosc. 1974;20:115-117. [PubMed] |
| 9. | Hussein AM, Bartram CI, Williams CB. Carbon dioxide insufflation for more comfortable colonoscopy. Gastrointest Endosc. 1984;30:68-70. [PubMed] |
| 10. | Stevenson GW, Wilson JA, Wilkinson J, Norman G, Goodacre RL. Pain following colonoscopy: elimination with carbon dioxide. Gastrointest Endosc. 1992;38:564-567. [PubMed] |
| 11. | Yamano HO, Yoshikawa K, Kimura T, Yamamoto E, Harada E, Kudou T, Katou R, Hayashi Y, Satou K. Carbon dioxide insufflation for colonoscopy: evaluation of gas volume, abdominal pain, examination time and transcutaneous partial CO2 pressure. J Gastroenterol. 2010;45:1235-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Uraoka T, Kato J, Kuriyama M, Hori K, Ishikawa S, Harada K, Takemoto K, Hiraoka S, Fujita H, Horii J. CO(2) insufflation for potentially difficult colonoscopies: efficacy when used by less experienced colonoscopists. World J Gastroenterol. 2009;15:5186-5192. [PubMed] |
| 13. | Yasumasa K, Nakajima K, Endo S, Ito T, Matsuda H, Nishida T. Carbon dioxide insufflation attenuates parietal blood flow obstruction in distended colon: potential advantages of carbon dioxide insufflated colonoscopy. Surg Endosc. 2006;20:587-594. [PubMed] |
| 14. | Bretthauer M, Seip B, Aasen S, Kordal M, Hoff G, Aabakken L. Carbon dioxide insufflation for more comfortable endoscopic retrograde cholangiopancreatography: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:58-64. [PubMed] |
| 15. | Maple JT, Keswani RN, Hovis RM, Saddedin EZ, Jonnalagadda S, Azar RR, Hagen C, Thompson DM, Waldbaum L, Edmundowicz SA. Carbon dioxide insufflation during ERCP for reduction of postprocedure pain: a randomized, double-blind, controlled trial. Gastrointest Endosc. 2009;70:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Dellon ES, Velayudham A, Clarke BW, Isaacs KL, Gangarosa LM, Galanko JA, Grimm IS. A randomized, controlled, double-blind trial of air insufflation versus carbon dioxide insufflation during ERCP. Gastrointest Endosc. 2010;72:68-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Nelson DB, Freeman ML, Silvis SE, Cass OW, Yakshe PN, Vennes J, Stahnke LL, Herman M, Hodges J. A randomized, controlled trial of transcutaneous carbon dioxide monitoring during ERCP. Gastrointest Endosc. 2000;51:288-295. [PubMed] |
| 18. | Ueki T, Mizuno M, Ota S, Ogawa T, Matsushita H, Uchida D, Numata N, Ueda A, Morimoto Y, Kominami Y. Carbon dioxide insufflation is useful for obtaining clear images of the bile duct during peroral cholangioscopy (with video). Gastrointest Endosc. 2010;71:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Domagk D, Bretthauer M, Lenz P, Aabakken L, Ullerich H, Maaser C, Domschke W, Kucharzik T. Carbon dioxide insufflation improves intubation depth in double-balloon enteroscopy: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:1064-1067. [PubMed] |
| 20. | Nishiwaki S, Araki H, Hayashi M, Takada J, Iwashita M, Tagami A, Hatakeyama H, Hayashi T, Maeda T, Saito K. Inhibitory effects of carbon dioxide insufflation on pneumoperitoneum and bowel distension after percutaneous endoscopic gastrostomy. World J Gastroenterol. 2012;18:3565-3570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Maeda Y, Hirasawa D, Fujita N, Obana T, Sugawara T, Ohira T, Harada Y, Yamagata T, Suzuki K, Koike Y. A prospective, randomized, double-blind, controlled trial on the efficacy of carbon dioxide insufflation in gastric endoscopic submucosal dissection. Endoscopy. 2013;45:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Nonaka S, Saito Y, Takisawa H, Kim Y, Kikuchi T, Oda I. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. [PubMed] |
| 24. | Kikuchi T, Fu KI, Saito Y, Uraoka T, Fukuzawa M, Fukunaga S, Sakamoto T, Nakajima T, Matsuda T. Transcutaneous monitoring of partial pressure of carbon dioxide during endoscopic submucosal dissection of early colorectal neoplasia with carbon dioxide insufflation: a prospective study. Surg Endosc. 2010;24:2231-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Suzuki T, Minami H, Komatsu T, Masusda R, Kobayashi Y, Sakamoto A, Sato Y, Inoue H, Serada K. Prolonged carbon dioxide insufflation under general anesthesia for endoscopic submucosal dissection. Endoscopy. 2010;42:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Souma Y, Nakajima K, Takahashi T, Nishimura J, Fujiwara Y, Takiguchi S, Miyata H, Yamasaki M, Doki Y, Nishida T. The role of intraoperative carbon dioxide insufflating upper gastrointestinal endoscopy during laparoscopic surgery. Surg Endosc. 2009;23:2279-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Nakajima K, Nishida T, Milsom JW, Takahashi T, Souma Y, Miyazaki Y, Iijima H, Mori M, Doki Y. Current limitations in endoscopic CO2 insufflation for NOTES: flow and pressure study. Gastrointest Endosc. 2010;72:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Nakajima K, Moon JH, Tsutsui S, Miyazaki Y, Yamasaki M, Yamada T, Kato M, Yasuda K, Sumiyama K, Yahagi N. Esophageal submucosal dissection under steady pressure automatically controlled endoscopy (SPACE): a randomized preclinical trial. Endoscopy. 2012;44:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Kato M, Nakajima K, Yamada T, Hirota M, Miyazaki Y, Yamasaki M, Nishida T, Mori M, Doki Y, Tsujii M. Esophageal submucosal dissection under steady pressure automatically controlled endoscopy (SPACE): a clinical feasibility study. Endoscopy. 2014;46:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Yamada T, Hirota M, Tsutsui S, Kato M, Takahashi T, Yasuda K, Sumiyama K, Tsujii M, Takehara T, Mori M. Gastric endoscopic submucosal dissection under steady pressure automatically controlled endoscopy (SPACE); a multicenter randomized preclinical trial. Surg Endosc. 2015;29:2748-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |