Published online Aug 25, 2016. doi: 10.4253/wjge.v8.i16.558
Peer-review started: April 6, 2016
First decision: May 17, 2016
Revised: June 7, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: August 25, 2016
Processing time: 142 Days and 17.1 Hours
To clarify the diagnostic efficacy and limitations of endoscopic ultrasonography (EUS) and the characteristics of early gastric cancers (EGCs) that are indications for EUS-based assessment of cancer invasion depth.
We retrospectively investigated the cases of 153 EGC patients who underwent conventional endoscopy (CE) and EUS (20 MHz) before treatment.
We found that 13.7% were “inconclusive” cases with low-quality EUS images, including all nine of the cases with protruded (0-I)-type EGCs. There was no significant difference in the diagnostic accuracy between CE and EUS. Two significant independent risk factors for misdiagnosis by EUS were identified-ulcer scarring [UL(+); odds ratio (OR) = 4.49, P = 0.003] and non-indication criteria for endoscopic resection (ER) (OR = 3.02, P = 0.03). In the subgroup analysis, 23.1% of the differentiated-type cancers exhibiting SM massive invasion (SM2) invasion (submucosal invasion ≥ 500 μm) by CE were correctly diagnosed by EUS, and 23.1% of the undifferentiated-type EGCs meeting the expanded-indication criteria for ER were correctly diagnosed by EUS.
There is no need to perform EUS for UL(+) EGCs or 0-I-type EGCs, but EUS may enhance the pretreatment staging of differentiated-type EGCs with SM2 invasion without UL or undifferentiated-type EGCs revealed by CE as meeting the expanded-indication criteria for ER.
Core tip: With the increasingly expanded indications of endoscopic resection for early gastric cancer (EGC), the accurate diagnosis of the invasion depth has become more important in the pretreatment strategy. Although there have been many investigations comparing the efficacy of endoscopic ultrasonography (EUS) and conventional endoscopy (CE) for invasion depth diagnosis of EGCs, much controversy remains. Our results revealed that there is no need to perform EUS for EGCs that are protruded type or those that have an ulcer scar, but EUS may have an add-on effect in the pretreatment staging of differentiated-type EGCs diagnosed as SM2 (submucosal invasion ≥ 500 μm) and undifferentiated-type EGCs diagnosed by CE as meeting the expanded-indication criteria for endoscopic resection.
- Citation: Watari J, Ueyama S, Tomita T, Ikehara H, Hori K, Hara K, Yamasaki T, Okugawa T, Kondo T, Kono T, Tozawa K, Oshima T, Fukui H, Miwa H. What types of early gastric cancer are indicated for endoscopic ultrasonography staging of invasion depth? World J Gastrointest Endosc 2016; 8(16): 558-567
- URL: https://www.wjgnet.com/1948-5190/full/v8/i16/558.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i16.558
Until recently, the Japanese Gastric Cancer Treatment Guidelines[1] stipulated that mucosal lesions < 2 cm in size and without ulceration are indicated for endoscopic resection (ER). However, in response to a report by Gotoda et al[2] on the low incidence of lymph node metastasis from early gastric cancers (EGCs), the indications for ER described in those Guidelines have been expanded to include EGCs with a very low risk of lymph node metastasis. Another part of the rationale behind this decision was that endoscopic submucosal dissection (ESD), which was developed in Japan[3-7], has made en bloc resection possible for lesions of all sizes. Along with the expanded indications for the ER of EGCs, therefore, the accurate diagnosis of invasion depth has become a very important component of pretreatment strategies.
Conventional endoscopy (CE) remains a useful modality for detecting EGCs and gauging their invasion depth. Although there have been many investigations, mostly in Japan, of the ability of CE to gauge the invasion depth of mucosal (M) and submucosal (SM) invasive cancers, collectively the rate of successful depth measurement has ranged from 62% to 80%[8-10]. Thus it is sometimes difficult to establish diagnostic criteria for differentiating M from SM cancers by CE alone. Endoscopic ultrasonography (EUS) permits a more objective assessment by providing a tomographic image, and is thus sometimes used as an adjunct diagnostic tool for determining the depth of gastric cancer invasion.
Several studies have compared the accuracy of invasion depth measurement between CE and EUS, and some of these reports clearly demonstrated the superiority of EUS for diagnosing EGC invasion depth[11-14] whereas others did not[9,15]. Two recent meta-analyses showed that EUS has relatively low accuracy for staging the depth of EGC invasion, and thus EUS may not be indispensable in the staging of EGCs[16,17]. It has also been reported that the accurate determination of invasion depth is difficult in cases with a large tumor size[11,15,18-21], upper location[15,18,20], depressed-type lesion[11,20], undifferentiated histology[15,21] or ulcerous finding (UL)[15,19,21,22].
There are also a number of practical technical difficulties that impede the production of suitable EUS images, and the use of poor-quality EUS images to determine the depth of EGCs may lead to incorrect results[23]. Unfortunately, most of the previous comparative studies (with the exception of the study by Tsujii et al[24]) analyzed only cases in which good-quality EUS images were obtained, and thus their findings may not show the true diagnostic capability of EUS in actual practice.
Along with the expanded indications for EGC dissection, it is expected that the number of ESDs of EGCs will increase, and the precise invasion depth staging of EGCs will therefore be important. Accordingly, the aims of the present study were to clarify: (1) the comparative diagnostic efficacies and limitations of EUS and CE for the pre-operative staging of EGC; and (2) the characteristic(s) of EGCs that are indications for the use of EUS as an adjunct diagnostic tool for measuring invasion depth.
Between April 2012 and March 2015, 452 consecutive patients with a total of 510 neoplasias comprised of gastric adenomas and EGCs were treated with ESD (360 neoplasias) and surgery (150 neoplasias) at Hyogo College of Medicine Hospital in Nishinomiya, Japan. Among them, 153 EGCs in 140 patients were examined using both CE and EUS. Both the absolute-indication and the expanded-indication criteria for the ER of EGCs followed the Japanese Gastric Cancer Treatment Guidelines[1]. The absolute-indication criteria for ER are: M cancer, differentiated-type adenocarcinoma, UL(-), and < 2 cm in dia. The proposed extended-indication criteria for ER are as follows: (1) M cancer, differentiated-type adenocarcinoma, UL(-) and any tumor size; (2) M cancer, differentiated-type adenocarcinoma, UL(+) and < 3 cm in size; (3) minute submucosal cancer (< 500 μm invasion into the submucosa, SM1), differentiated-type adenocarcinoma and < 3 cm in size; and (4) M cancer, undifferentiated-type carcinoma, UL(-) and < 2 cm in size.
Written informed consent was obtained from all patients prior to the procedures and treatment, and the study design was approved by the Ethics Committee of Hyogo College of Medicine (No. 2109).
When the invasion depth of an EGCs is being diagnosed, close endoscopic observation is necessary to adjust the air volume in the patient’s stomach. The endoscopic criteria for cancer invasion in the present patient series were judged based on previous reports[8-10,15,24-26]. Briefly, in the CE diagnosis, the presence or absence of the following CE findings of SM massive invasion was determined: (1) irregular surface including nodules in the depressed area; (2) submucosal tumor-like elevation without flexibility; (3) abnormal converging folds such as clubbing and fusion; and (4) deep ulceration with marked marginal elevation. All endoscopic observations were performed by chromoendoscopy using an endoscope (GIF-Q260, H260, H260Z, H290Z, H290 or HQ290; Olympus Medical Systems, Tokyo) followed by EUS.
EUS was performed with a 20-MHz miniature probe UM-3R (Olympus Medical Systems), which was connected to an endoscopic ultrasonic observation unit (EU-M2000; Olympus Medical Systems). Approximately 200-500 mL of deaerated water was instilled in the stomach to improve the transmission of the ultrasound beam. In the EUS diagnoses, lesions confined to the 1st and 2nd sonographic layers were considered mucosal cancer. Massive submucosal invasion was defined as obvious irregular narrowing or budding into the 3rd sonographic layer as shown in previous reports[9-11,14,15,20,21,23-26].
In the UL(+) lesions, the previous criteria for EUS diagnosis were used[13,27]; namely, if a fan-shaped hypoechoic area was demonstrated in the 3rd layer, the lesion was defined as M/SM1, and when an arch-shaped hypoechoic area was observed in the 3rd layer, the lesions were regard as SM massive invasion (SM2). In the cases in which at least five layers of the gastric wall, including the lesion, were unclear and an assessment by EUS was difficult due to the low-quality image, the lesions were judged to be “inconclusive”[24].
It is very difficult to discriminate SM1 from M cancer even by CE or EUS, and the therapeutic strategies for these lesions are also similar. We therefore clinically divided these lesions into two groups: The M/SM1 group, for which ER may be suitable, and the SM2 group, for which surgery was indicated.
In this retrospective study, two endoscopists (Jiro Watari and Shigemitsu Ueyama) with 29 and 17 years of endoscopic practice experience, respectively and board certification from the Japan Gastroenterological Endoscopy Society independently reviewed the CE and EUS images without any pathologic information. The results were used for the calculation of interobserver agreement (κ value).
Resected specimens were sectioned at 2-mm intervals for ESD and 5-mm intervals for surgical resection. The histology, tumor location, gross morphologic type, and depth of invasion fulfilled the criteria of the Japanese Research Society for Gastric Cancer[28]. We histologically classified the specimens into two groups based on their depth of submucosal invasion: Invasion into the SM1 (invasion < 500 μm) or SM2 (invasion ≥ 500 μm) layer. The largest measured tumor size of the resected specimen was recorded histologically as the tumor dia.
We assessed the data by performing the Mann-Whitney U test for comparisons between two independent groups, and the χ2 test or Fisher’s exact test was used to examine differences between two proportions. Statistical significance was defined as a P value < 0.05. Risk factors for the misdiagnosis of the depth of cancer invasion by EUS that were found to be significant with a P value of < 0.05 in a univariate analysis were entered into a multiple logistic regression model and analyzed using a backward approach. Odds ratios (ORs) and 95%CIs were calculated for each risk factor.
The interobserver agreement for the CE imaging and the EUS imaging evaluations was calculated by κσstatistics, which were interpreted as follows: Poor (≤ 0.2), mild (0.2-0.4), moderate (0.4-0.6), good (0.6-0.8), and excellent (0.8-1.0). Differences at P < 0.05 were considered significant. All statistical analyses were performed using the StatView software program, ver. 5.0 (SAS Institute, Cary, NC).
Table 1 shows the characteristics of the 140 patients and a summary of the 153 studied EGCs. The mean age of the patients was 68.7 ± 10.4 years (range 23-87 years), and women accounted for 27.1% of the patients. The mean tumor size was 20.5 ± 14.4 mm in dia. The numbers of lesions that met the absolute- and expanded-indication criteria for ER were 51 and 38 lesions, respectively. The lesions were located mainly in the middle portion of the stomach.
| Total no. of lesions (patients) | 153 (140) |
| Mean (± SD) age, years | 68.7 ± 10.4 |
| Sex, male/female | 102/38 |
| Macroscopic type | |
| 0-I /0-IIa/0-IIb /0-IIc | 9/51/1/92 |
| Location | |
| Upper/middle/lower | 45/69/39 |
| Mean (± SD) tumor size, mm | 20.5 ± 14.4 |
| Depth of invasion | |
| M/SM1/SM2 | 93/17/43 |
| Histology | |
| Differentiated/undifferentiated | 118/35 |
| Ulcer scar | |
| Positive/negative | 29/124 |
| Criteria for endoscopic resection | |
| Absolute/expanded/non-indication | 51/38/64 |
Twenty-one (13.7%) of the 153 EGCs were judged as “inconclusive”. As shown in Table 2, all nine of the protruded-type (0-I) cancers yielded low-quality images. The inconclusive rate was significantly higher in the lower portion of the stomach than in other portions (P = 0.03). There was no significant difference in the inconclusive rate between the lesions with and without UL.
| Tumor-related factors | No. of inconclusive cases (%) | P value | |
| Macroscopic type | < 0.0001 | ||
| I | (n = 9) | 9 (100) | |
| IIa | (n = 51) | 7 (13.7) | |
| IIc | (n = 92) | 5 (5.4) | |
| Location | 0.03 | ||
| Upper | (n = 45) | 3 (6.7) | |
| Middle | (n = 69) | 8 (11.6) | |
| Lower | (n = 39) | 10 (25.6) | |
| Histology | 0.16 | ||
| Differentiated | (n = 118) | 19 (16.1) | |
| Undifferentiated | (n = 35) | 2 (5.7) | |
| Ulcer scar | 0.37 | ||
| Positive | (n = 29) | 2 (6.9) | |
| Negative | (n = 124) | 19 (15.3) | |
| Criteria for ER | 0.58 | ||
| Absolute | (n = 51) | 9 (17.6) | |
| Expanded | (n = 38) | 5 (13.2) | |
| Non-indication | (n = 64) | 7 (10.3) | |
The ĸ-values for the interobserver agreement for the invasion depth diagnosis between the two endoscopists were 0.78 (95%CI: 0.68-0.89) for EUS and 0.82 (95%CI: 0.72-0.92) for CE. Thus the interobserver agreement for invasion depth diagnosis by EUS and CE was good to excellent. When the results of the diagnostic accuracy by one endoscopist whose accuracy rate was higher than that of the other endoscopist were used in both modalities, the accuracy rate of EUS was 71.2% (109 of 153 lesions) (Table 3), and when the accuracy was calculated in 132 lesions (omitting 21 inconclusive cases), the rate increased to 82.6% (109 of 132).
| Clinical diagnosis | Histologic diagnosis | EUSdiagnosis | P2 | Histologicdiagnosis | Accuracy | P (vs EUS) | ||||
| M/SM1 | SM2 | Overall accuracy | Accuracy1 | M/SM1 | SM2 | |||||
| Diagnosis | M/SM1 | 81 | 9 | 71.2 | 82.6 | 97 | 18 | 79.7 | 0.54 | |
| SM2 | 14 | 28 | 13 | 25 | ||||||
| Macroscopic type | 0.30 | |||||||||
| I | M/SM1 | - | - | - | - | 5 | 1 | 88.9 | - | |
| SM2 | - | - | 0 | 3 | ||||||
| IIa/IIb | M/SM1 | 26 | 4 | 67.3 | 77.8 | 32 | 5 | 78.8 | 0.90 | |
| SM2 | 6 | 9 | 6 | 9 | ||||||
| IIc | M/SM1 | 55 | 5 | 80.4 | 85.1 | 60 | 12 | 79.3 | 0.32 | |
| SM2 | 8 | 19 | 7 | 13 | ||||||
| Location | 0.55 | |||||||||
| Upper | M/SM1 | 21 | 2 | 74.4 | 80 | 24 | 4 | 80.0 | > 0.99 | |
| SM2 | 6 | 11 | 5 | 12 | ||||||
| Middle | M/SM1 | 40 | 7 | 69.9 | 78.5 | 44 | 11 | 78.3 | 0.98 | |
| SM2 | 7 | 11 | 4 | 10 | ||||||
| Lower | M/SM1 | 19 | 2 | 62.2 | 85.2 | 29 | 3 | 82.1 | > 0.99 | |
| SM2 | 2 | 4 | 4 | 3 | ||||||
| Histology | 0.79 | |||||||||
| Diff. | M/SM1 | 71 | 10 | 70.4 | 83 | 77 | 12 | 80.5 | 0.63 | |
| SM2 | 8 | 17 | 11 | 18 | ||||||
| Undiff. | M/SM1 | 9 | 1 | 75.0 | 84 | 20 | 6 | 77.1 | > 0.99 | |
| SM2 | 4 | 12 | 2 | 7 | ||||||
| Ulcer scar | < 0.0001 | |||||||||
| Positive | M/SM1 | 3 | 2 | 46.7 | 50 | 7 | 4 | 58.6 | 0.51 | |
| SM2 | 12 | 11 | 8 | 10 | ||||||
| Negative | M/SM1 | 77 | 7 | 75.6 | 89.4 | 90 | 14 | 84.7 | 0.29 | |
| SM2 | 4 | 16 | 5 | 15 | ||||||
| Indication for ER | < 0.0001 | |||||||||
| Absolute | M/SM1 | 37 | - | 80.4 | 97.4b | 43 | - | 84.3f | 0.07 | |
| SM2 | 1 | - | 8 | - | ||||||
| Expanded | M/SM1 | 28 | - | 75.7 | 87.5d | 33 | - | 86.8h | > 0.99 | |
| SM2 | 4 | - | 5 | - | ||||||
| Non-indication | M/SM1 | 12 | 13 | 56.1 | 62.7bd | 16 | 18 | 64.1fh | > 0.99 | |
| SM2 | 9 | 25 | 5 | 25 | ||||||
The sensitivity of EUS for diagnosing M/SM1 lesions was 85.3% (81 of 95 cases), the specificity was 75.7% (28 of 37), the positive predictive value (PPV) was 90.0% (81 of 90), and the negative predictive value (NPV) was 66.7% (28 of 42). The diagnostic accuracy of EUS was not significantly different among the three macroscopic types or the three tumor locations, or between the histological types, i.e., the differentiated type and the undifferentiated type.
However, UL(+) and the non-indication criteria for ER were significantly associated with the incorrect diagnosis of tumor invasion depth by EUS (P < 0.0001 and P = 0.0004, respectively). In addition, UL(+) (OR = 4.49; 95%CI: 1.68-11.97; P = 0.003) and the non-indication criteria for ER (OR = 3.02; 95%CI: 1.14-8.00; P = 0.03) were significant and independent risk factors affecting misdiagnosis by EUS in our multivariate logistic regression analysis.
There were no significant differences in the accuracy or other parameters between EUS and CE; the sensitivity of CE diagnosis for M/SM1 was 88.2% (97 of 110 cases), the specificity was 58.1% (25 of 43), the PPV was 84.3% (97 of 115), and the NPV was 65.8% (25 of 38). As shown in Table 3, the accuracy rate obtained for the absolute-indication criteria lesions was very high for both modalities, and was significantly higher than that of the non-indication criteria lesions (P < 0.0001 in EUS and P = 0.01 in CE). There were also significant differences in the accuracy between the lesions with the expanded-indication criteria and those with the non-indication criteria for ER (P = 0.02 in both EUS and CE). However, no significant differences in diagnostic accuracy between the two modalities were observed within the expanded-indication criteria group or the non-indication criteria group (Table 3).
As shown in Table 4, the number of lesions that showed a correct diagnosis by CE and an incorrect diagnosis by EUS was almost the same as the number of lesions that showed an incorrect diagnosis by CE and a correct diagnosis by EUS in both the expanded-indication criteria group and the non-indication criteria group, irrespective of histology. This result may indicate that there is no additive effect of EUS in the diagnosis of invasion depth.
| Indication for endoscopic resection | ||||
| Diagnosis | Absolute criteria | Expanded criteria | Non-indication | |
| Differentiated-type cancer (n = 99) | ||||
| EUS | CE | (n = 42) (%) | (n = 25) (%) | (n = 32) (%) |
| Correct | Correct | 39 (92.9) | 19 (76) | 20 (62.5) |
| Incorrect | Incorrect | 0 (0) | 3 (12) | 11 (34.4) |
| Correct | Incorrect | 1 (4.8) | 1 (4) | 1 (3.1) |
| Incorrect | Correct | 1 (2.4) | 2 (8) | 0 (0) |
| Undifferentiated-type cancer (n = 33) | ||||
| EUS | CE | (n = 8) (%) | (n = 25) (%) | |
| Correct | Correct | - | 8 (100) | 15 (60) |
| Incorrect | Incorrect | - | 0 (0) | 1 (4) |
| Correct | Incorrect | - | 0 (0) | 5 (20) |
| Incorrect | Correct | - | 0 (0) | 4 (16) |
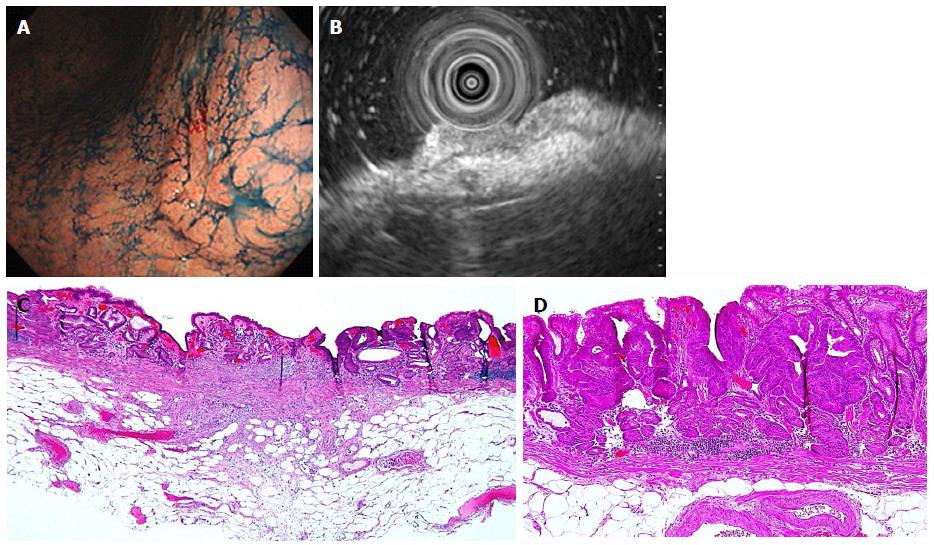
In the subgroup analysis of a total of 13 differentiated-type cancers without UL and with SM2 invasion diagnosed by CE, three (23.1%) cases that were misdiagnosed by CE were correctly diagnosed as M/SM1 lesions by EUS (Table 5 and Figure 1). We identified two cases (20.0%, 2 of 10) lesions that were ≤ 2 cm and three cases (25.0%, 3 of 12) that were 3 cm in size. These cases were subsequently treated with ESD, avoiding surgery. The reverse phenomenon, i.e., cases misdiagnosed by EUS but correctly diagnosed by CE - was not seen.
| EUS | CE | n (%) |
| Correct | Correct | 10 (76.9) |
| Correct | Incorrect | 3 (23.1) |
| Incorrect | Correct | 0 (0) |
| Incorrect | Incorrect | 0 (0) |
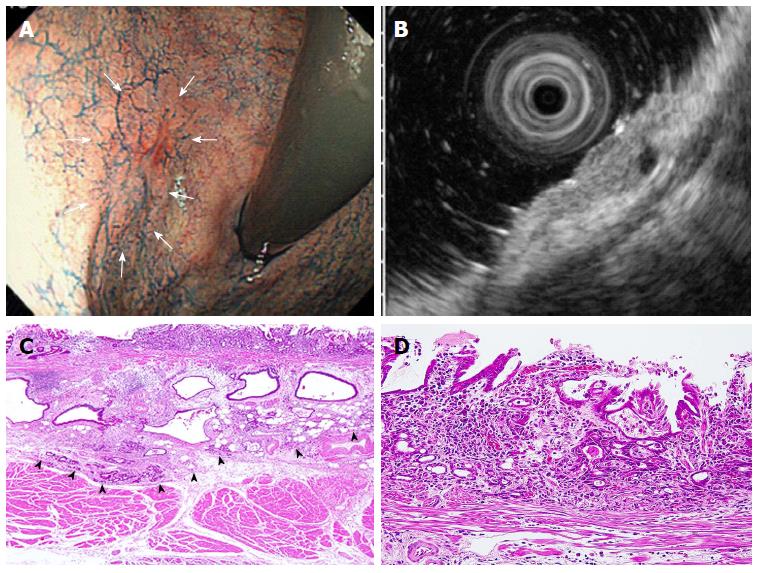
Similarly, in our subgroup analysis of 13 undifferentiated-type cases that met the expanded-indication criteria for ER, which were judged endoscopically as M/SM1 lesions, UL(-) and ≤ 2 cm in size, three cases (23.1%) were correctly diagnosed by EUS as having SM2 invasion (Table 6 and Figure 2). These three cases were thus adequately treated with surgery.
| EUS | CE | n (%) |
| Correct | Correct | 10 (76.9) |
| Correct | Incorrect | 3 (23.1) |
| Incorrect | Correct | 0 (0) |
| Incorrect | Incorrect | 0 (0) |
Although there have been many investigations comparing the efficacy of EUS and CE for the pretreatment staging of EGCs, much controversy remains. In our present study, the overall accuracy of EUS for diagnosing invasion depth was lower than that of CE, but not significantly so. The accuracy of EUS was 82.6% (71.1% in overall accuracy), which was similar to the values reported in previous studies[13,14,19,22-25,27] but higher than the values obtained in other studies[9,11,12,15,20,21,26]. In recent meta-analyses, most of the cited studies showed that EUS has only a limited effect on determining the optimal therapeutic strategy[15,18-20,25]. Our present findings clearly demonstrated the limitations of EUS and the characteristics of EGCs that make them suitable for analysis by EUS.
In the present study, all nine of the 0-I-type cancers (protruded-type) yielded low-quality EUS images and were thus judged as inconclusive cases, as mentioned above[11,22]. The main cause of inconclusiveness was ultrasound attenuation due to the use of a high-frequency ultrasound probe (20 MHz); the submucosal layer could not be clearly visualized. If a low-frequency EUS or probe had been used, the number of inconclusive cases among those types of cancers might have been lower. However, in 0-I-type cancer the mucosa is thick and the muscularis mucosae elevates toward the mucosa from the submucosa, and it may thus be difficult to make an accurate diagnosis even if low-frequency EUS is performed.
In addition, the accuracy rate of EUS in the UL(+) lesions was extremely low (≤ 50%), and significantly lower than that in the UL(-) lesions (P < 0.0001). Regarding the reason for this finding, most of the lesions (80%, 12 of 15) of M/SM1 cancers with UL were over-diagnosed due to submucosal fibrosis, which is in agreement with previous reports[12,15,19,21,23]. In the report by Mandai et al[21], the accuracy rate of EUS decreased from 86.5% to 28.9% in the UL(-) lesions. Although a few studies have introduced a method that distinguishes cancer invasion from ulcer fibrosis[13,27], it may be difficult in practice to differentiate between those two conditions. In our multivariate logistic regression analysis, UL was a significant and independent risk factor affecting misdiagnosis by EUS, and thus it may be futile to perform EUS for UL(+) lesions.
There was no significant difference in the accuracy rate of EUS among the three tumor locations of the stomach, but inconclusive cases were observed significantly more frequently in the lower third of the stomach than in the other portions (P = 0.03). Several studies showed that the diagnostic accuracy of the invasion depth was diminished for lesions in the upper portion of the stomach[8,12,14,15,19,23]. Tsuzuki et al[25] reported that the submucosal layer in the upper third of the stomach is relatively thin and tends to have fibrosis and many vessels, making signs of submucosal invasion difficult to diagnosis and leading to incorrect staging. For other reasons, it is considered that it is difficult to fill this region with deaerated water[8,19,25]. However, this problem can be overcome by adjusting the volumes of air and deaerated water. In our patient population, it was often difficult to achieve the necessary pool of deaerated water in the lower third of the stomach, and there were technical problems with scanning this portion.
The diagnostic accuracy of EUS has been reported to be low for undifferentiated-type lesions[10-12,14,18,22] and larger-size lesions[11,12,18,19,21], which were categorized mainly as meeting the expanded-indication criteria or non-indication criteria for ER. In the present study, the diagnostic accuracy for the lesions meeting the absolute-indication criteria for ER was very high for both EUS (97.4%) and CE (84.3%) as expected, whereas the accuracy rates of EUS and CE were significantly lower for the lesions that met the non-indication criteria for ER compared to those that met other criteria for ER.
If EUS is going to be performed for many lesions meeting the absolute-indication criteria for ER, the overall accuracy of EUS may naturally increase, but not to a clinically significant degree. It has been reported that magnifying endoscopy with narrow-band imaging (ME-NBI) is useful for determining the invasion depth diagnosis of EGC[29,30]; however the diagnostic criteria for SM2 are complex[29] and the diagnostic specificity of ME-NBI may be relatively low[30]. Actually, when the staging of an EGC is doubtful by CE, EUS is likely to provide helpful information to stage the EGC, i.e., to determine the M/SM1 or SM2 status[16]. In such cases EUS may correct a misdiagnosis by CE, especially with respect to the expanded-indication and non-indication criteria for ER.
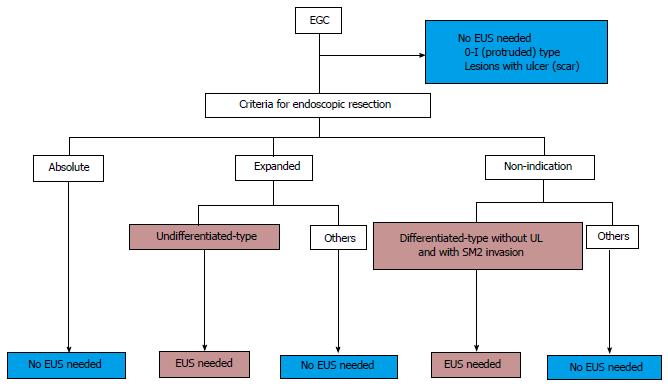
Taking past findings into consideration along with our present results, we propose that EUS may be considered for the following lesions: (1) differentiated-type cancers without UL diagnosed as invading to SM2; and (2) undifferentiated-type cancers diagnosed by CE as meeting the expanded-indication criteria for ER. When EUS is performed for these lesions, the additive effect of EUS will increase the accuracy by 23.1%. It should be noted, however, that we studied only a small number of either type of lesions, i.e., three lesions of type (1) and three lesions of type (2). In contrast, it should also be emphasized that there were no lesions of either type which were correctly diagnosed by CE and incorrectly diagnosed by EUS. Based on our conclusion, we have summarized the indications of EUS for the pretreatment diagnosis of EGCs in Figure 3.
Our study has several potential limitations. First, it was a retrospective study at a single institution. Second, the sample size was relatively small. However, we did not perform EUS for most of the lesions that met the absolute-indication criteria, which could be definitely diagnosed as mucosal cancer by CE as mentioned above. Indeed, of the 186 EGCs that met the absolute-indication criteria for ER and that were treated with ER during this study period, only 50 lesions (26.9%) underwent EUS. This result may thus have resulted in a selection bias because there were no eligibility criteria for performing EUS in this study. Third, only the patients with histologically confirmed EGC who underwent EUS and ESD or surgery were evaluated, which might also have introduced a potential selection bias. Fourth, since EUS was performed under CE by an endosonographer, the construction of EUS images may have been affected by the endoscopic appearance of the lesions and the experience of the endosonographer[31]. In addition, one observer might have been involved in both of the examinations, i.e., CE and EUS, in some cases. In general, the observer who validates the criteria should not have been involved in the evaluation of the EUS and CE images[24].
In conclusion, our analyses revealed that: (1) EUS may not be necessary to determine the pretreatment staging of 0-I type and UL(+) or absolute-indication criteria lesions; and (2) EUS may be considered for the following lesions: (1) differentiated-type cancers diagnosed without UL and with invasion to SM2; and (2) undifferentiated-type cancers diagnosed as meeting the expanded-indication criteria for ER by CE.
It is sometimes difficult to establish diagnostic criteria for differentiating mucosal cancer from submucosal invasive cancer by conventional endoscopy (CE) alone. Although endoscopic ultrasonography (EUS) permits a more objective assessment by providing a tomographic image, recent meta-analyses showed that EUS has relatively low accuracy for staging the depth of early gastric cancer (EGC) invasion.
According to the previous studies, some of these reports clearly demonstrated the superiority of EUS for diagnosing EGC invasion depth whereas others did not. The authors retrospectively investigated the application of EUS in the pre-treatment staging of EGD.
All protruded-type EGCs were “inconclusive” cases with low-quality EUS images. There was no significant difference in the diagnostic accuracy between CE and EUS. The lesions with ulcer scar (UL) and non-indication criteria for endoscopic resection (ER) were significant independent risk factors for misdiagnosis by EUS. In the subgroup analysis, however, the additive effect of EUS was found in the lesions with the differentiated-type cancers exhibiting SM2 invasion (submucosal invasion ≥ 500 μm) by CE and the undifferentiated-type EGCs meeting the expanded-indication criteria for ER.
EUS may not be necessary to determine the pretreatment staging of protruded (0-I)-type and the lesions with UL or absolute-indication criteria for ER; and EUS may be considered for the following lesions: (1) differentiated-type cancers diagnosed without UL and with invasion to SM2; and (2) undifferentiated-type cancers diagnosed as meeting the expanded-indication criteria for ER by CE.
EUS is a reliable method for predicting the invasion depth diagnosis of EGC. However, there is no need to perform EUS for the EGCs with the absolute-indication criteria, UL(+) or 0-I-type. The modality should be considered performing the limited lesions.
This is a good article to describe the indications for EUS staging of invasion depth in EGCs.
Manuscript source: Unsolicited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Japan
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arigami T, Sugimoto S, Zhang CW S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1895] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 2. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1325] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 3. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1148] [Article Influence: 47.8] [Reference Citation Analysis (4)] |
| 5. | Yamamoto H, Sekine Y, Higashizawa T, Kihira K, Kaneko Y, Hosoya Y, Ido K, Saito K, Sugano K. Successful en bloc resection of a large superficial gastric cancer by using sodium hyaluronate and electrocautery incision forceps. Gastrointest Endosc. 2001;54:629-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Miyamoto S, Muto M, Hamamoto Y, Boku N, Ohtsu A, Baba S, Yoshida M, Ohkuwa M, Hosokawa K, Tajiri H. A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc. 2002;55:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 506] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 8. | Sano T, Okuyama Y, Kobori O, Shimizu T, Morioka Y. Early gastric cancer. Endoscopic diagnosis of depth of invasion. Dig Dis Sci. 1990;35:1340-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Yanai H, Noguchi T, Mizumachi S, Tokiyama H, Nakamura H, Tada M, Okita K. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut. 1999;44:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011;73:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Akahoshi K, Chijiwa Y, Hamada S, Sasaki I, Nawata H, Kabemura T, Yasuda D, Okabe H. Pretreatment staging of endoscopically early gastric cancer with a 15 MHz ultrasound catheter probe. Gastrointest Endosc. 1998;48:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Hizawa K, Iwai K, Esaki M, Matsumoto T, Suekane H, Iida M. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy. 2002;34:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 13. | Yoshida S, Tanaka S, Kunihiro K, Mitsuoka Y, Hara M, Kitadai Y, Hata J, Yoshihara M, Haruma K, Hayakawa N. Diagnostic ability of high-frequency ultrasound probe sonography in staging early gastric cancer, especially for submucosal invasion. Abdom Imaging. 2005;30:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009;43:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010;42:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Pei Q, Wang L, Pan J, Ling T, Lv Y, Zou X. Endoscopic ultrasonography for staging depth of invasion in early gastric cancer: A meta-analysis. J Gastroenterol Hepatol. 2015;30:1566-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;CD009944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Kim JH, Song KS, Youn YH, Lee YC, Cheon JH, Song SY, Chung JB. Clinicopathologic factors influence accurate endosonographic assessment for early gastric cancer. Gastrointest Endosc. 2007;66:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Okada K, Fujisaki J, Kasuga A, Omae M, Yoshimoto K, Hirasawa T, Ishiyama A, Yamamoto Y, Tsuchida T, Hoshino E. Endoscopic ultrasonography is valuable for identifying early gastric cancers meeting expanded-indication criteria for endoscopic submucosal dissection. Surg Endosc. 2011;25:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Park JM, Ahn CW, Yi X, Hur H, Lee KM, Cho YK, Han SU. Efficacy of endoscopic ultrasonography for prediction of tumor depth in gastric cancer. J Gastric Cancer. 2011;11:109-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Mandai K, Yasuda K. Accuracy of endoscopic ultrasonography for determining the treatment method for early gastric cancer. Gastroenterol Res Pract. 2012;2012:245390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kim GH, Park DY, Kida M, Kim DH, Jeon TY, Kang HJ, Kim DU, Choi CW, Lee BE, Heo J. Accuracy of high-frequency catheter-based endoscopic ultrasonography according to the indications for endoscopic treatment of early gastric cancer. J Gastroenterol Hepatol. 2010;25:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Yamamoto S, Nishida T, Kato M, Inoue T, Hayashi Y, Kondo J, Akasaka T, Yamada T, Shinzaki S, Iijima H. Evaluation of endoscopic ultrasound image quality is necessary in endosonographic assessment of early gastric cancer invasion depth. Gastroenterol Res Pract. 2012;2012:194530. [PubMed] |
| 24. | Tsujii Y, Kato M, Inoue T, Yoshii S, Nagai K, Fujinaga T, Maekawa A, Hayashi Y, Akasaka T, Shinzaki S. Integrated diagnostic strategy for the invasion depth of early gastric cancer by conventional endoscopy and EUS. Gastrointest Endosc. 2015;82:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Tsuzuki T, Okada H, Kawahara Y, Nasu J, Takenaka R, Inoue M, Kawano S, Kita M, Hori K, Yamamoto K. Usefulness and problems of endoscopic ultrasonography in prediction of the depth of tumor invasion in early gastric cancer. Acta Med Okayama. 2011;65:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Lee JY, Choi IJ, Kim CG, Cho SJ, Kook MC, Ryu KW, Kim YW. Therapeutic Decision-Making Using Endoscopic Ultrasonography in Endoscopic Treatment of Early Gastric Cancer. Gut Liver. 2016;10:42-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Kida M, Tanabe S, Watanabe M, Kokutou M, Kondou I, Yamada Y, Sakaguchi T, Saigenji K. Staging of gastric cancer with endoscopic ultrasonography and endoscopic mucosal resection. Endoscopy. 1998;30 Suppl 1:A64-A68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 29. | Kobara H, Mori H, Fujihara S, Kobayashi M, Nishiyama N, Nomura T, Kato K, Ishihara S, Morito T, Mizobuchi K. Prediction of invasion depth for submucosal differentiated gastric cancer by magnifying endoscopy with narrow-band imaging. Oncol Rep. 2012;28:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Kikuchi D, Iizuka T, Hoteya S, Yamada A, Furuhata T, Yamashita S, Domon K, Nakamura M, Matsui A, Mitani T. Usefulness of magnifying endoscopy with narrow-band imaging for determining tumor invasion depth in early gastric cancer. Gastroenterol Res Pract. 2013;2013:217695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Roubein LD, Lynch P, Glober G, Sinicrope FA. Interobserver variability in endoscopic ultrasonography: a prospective evaluation. Gastrointest Endosc. 1996;44:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |