Published online Jul 10, 2016. doi: 10.4253/wjge.v8.i13.458
Peer-review started: February 16, 2016
First decision: March 25, 2016
Revised: May 13, 2016
Accepted: May 31, 2016
Article in press: June 2, 2016
Published online: July 10, 2016
Processing time: 138 Days and 1.8 Hours
AIM: To investigate the safety and utility of an electrocautery dilation catheter for endoscopic ultrasonography (EUS)-guided pancreatic fluid collection drainage.
METHODS: A single-center, exploratory, retrospective study was conducted between August 2010 and August 2014. This study was approved by the Medical Ethics Committee of our institution. Informed, written consent was obtained from each patient prior to the procedure. The subjects included 28 consecutive patients who underwent EUS-guided transmural drainage (EUS-TD) for symptomatic pancreatic and peripancreatic fluid collections (PFCs) by fine needle aspiration using a 19-gauge needle. These patients were retrospectively divided into two groups based on the use of an electrocautery dilation catheter as a fistula dilation device; 15 patients were treated with an electrocautery dilation catheter (electrocautery group), and 13 patients were treated with a non-electrocautery dilation catheter (non-electrocautery group). We evaluated the technical and clinical successes and the adverse events associated with EUS-TD for the treatment of PFCs between the two groups.
RESULTS: There were no significant differences in age, sex, type, location and diameter of PFCs between the groups. Thirteen patients (87%) in the electrocautery group and 10 patients (77%) in the non-electrocautery group presented with infected PFCs. The technical success rates of EUS-TD for the treatment of PFCs were 100% (15/15) and 100% (13/13) for the electrocautery and the non-electrocautery groups, respectively. The clinical success rates of EUS-TD for the treatment of PFCs were 67% (10/15) and 69% (9/13) for the electrocautery and the non-electrocautery groups, respectively (P = 0.794). The procedure time of EUS-TD for the treatment of PFCs in the electrocautery group was significantly shorter than that of the non-electrocautery group (mean ± SD: 30 ± 12 min vs 52 ± 20 min, P < 0.001). Adverse events associated with EUS-TD for the treatment of PFCs occurred in 0 patients and 1 patient for the electrocautery and the non-electrocautery groups, respectively (P = 0.942).
CONCLUSION: EUS-TD using an electrocautery dilation catheter as a fistula dilation device for the treatment of symptomatic PFCs appears safe and contributes to a shorter procedure time.
Core tip: Endoscopic ultrasonography-guided transmural drainage using an electrocautery dilation catheter as a fistula dilation device for the treatment of symptomatic peripancreatic fluid collections appears to be safe and contributes to a shorter procedure time.
- Citation: Kitamura K, Yamamiya A, Ishii Y, Nomoto T, Honma T, Yoshida H. Electrocautery vs non-electrocautery dilation catheters in endoscopic ultrasonography-guided pancreatic fluid collection drainage. World J Gastrointest Endosc 2016; 8(13): 458-465
- URL: https://www.wjgnet.com/1948-5190/full/v8/i13/458.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i13.458
Pancreatic and peripancreatic fluid collections (PFCs) are accumulations of liquid and/or necrotic tissue associated with acute pancreatitis, chronic pancreatitis, surgery, or abdominal trauma. The 1992 Atlanta Classification of acute pancreatitis was revised in 2012 to classify local complications caused by acute pancreatitis into the following 4 types: (1) acute peripancreatic fluid collection (APFC); within 4 wk of the onset of pancreatitis, without pancreatic and/or peripancreatic necrosis; (2) pancreatic pseudocyst (over 4 wk after the onset of pancreatitis without pancreatic and/or peripancreatic necrosis); (3) acute necrotic collection (ANC; within 4 wk of the onset of pancreatitis with pancreatic and/or peripancreatic necrosis); and (4) walled-off necrosis (WON; over 4 wk after the onset of pancreatitis with pancreatic and/or peripancreatic necrosis)[1].
Endoscopic transmural drainage is a minimally invasive procedure for PFC. Endoscopic drainage for PFCs is superior to percutaneous drainage or surgery in terms of the duration of hospital stay and cost[2,3]. Recently, endoscopic ultrasonography-guided transmural drainage (EUS-TD) for the treatment of PFCs has been widely accepted[4-6]. However, it has been reported that EUS-TD for PFCs causes adverse events, such as bleeding and perforation, at a rate of 0%-21%[7,8], and the establishment of a safe procedure is necessary.
Electrocautery or non-electrocautery dilation catheters are currently used as fistula dilation devices for EUS-TD; however, few studies have investigated the safety and advantages of electrocautery dilation catheters for EUS-TD.
The aim of this study was to evaluate the safety and utility of an electrocautery dilation catheter as a fistula dilation device for EUS-TD in the treatment of PFCs.
This study was conducted as an exploratory retrospective study at Showa University Hospital, was approved by the Medical Ethics Committee of our institution, and was registered at the UMIN Clinical Trials Registry (UMIN 000018352). Informed, written consent was obtained from each patient prior to the procedure.
Twenty-eight consecutive patients who underwent EUS-TD at our institution between August 2010 and August 2014 were retrospectively analyzed. All of these patients underwent EUS-TD using an electrocautery or a non-electrocautery dilation catheter as a fistula dilation device after undergoing PFC puncture with a 19-gauge fine needle aspiration needle.
We used a non-electrocautery dilation catheter from August 2010 to April 2012 for 13 patients. An electrocautery dilation catheter was used for 15 patients after May 2012, which is when the electrocautery dilation catheter became available as a treatment option at our institution. We conducted an exploratory retrospective study to compare the clinical outcomes between the electrocautery and the non-electrocautery groups. PFCs were classified into 4 types[1] according to the revised 2012 Atlanta Classification. We diagnosed PFCs using computed tomography, magnetic resonance imaging and EUS. We performed EUS-TD in patients with signs of infection, abdominal pain and an increase of PFC size (i.e., 6 cm or more). Signs of infection of PFCs were judged according to blood examination, blood culture examination and imaging results. We defined infected PFCs as those with bacteria present based on a culture examination of the PFC obtained by EUS-TD. Exclusion criteria of EUS-TD included coagulopathy, the interposition of blood vessels on the puncture tract for the PFC, and the inability to obtain informed consent.
A convex array echoendoscope, GF-UCT 240-AL5 (Olympus Medical Systems Corp, Tokyo, Japan), was used for transmural drainage of the PFCs. A 19-gauge fine needle aspiration needle EchoTip® Ultra Endoscopic Ultrasound Needle (Cook Medical Inc., Bloomington Indiana, United States), Expect™ Endoscopic Ultrasound Aspiration Needle (Boston Scientific, Natick, MA, United States) or EZ shot 2 (Olympus Medical Systems) was used to puncture the PFC.
As a guide wire (GW) for insertion into the PFC, a 0.035-inch Hydra Jagwire™ (Boston Scientific) and/or a 0.025-inch VisiGlide™ or a 0.025-inch VisiGlide 2™ (Olympus Medical Systems) was used.
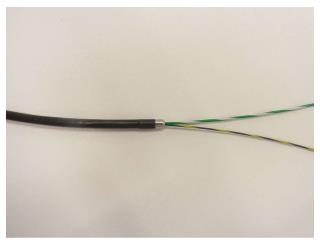
An electrocautery dilation catheter, 6-Fr and/or 8.5-Fr Cyst-Gastro-Set (Endo-Flex GmbH, Voerde, Germany), was used to dilate the puncture tract of the PFC. The electrocautery dilation catheter is a wire-guided dilation catheter with a distal electrocautery tip. An 8.5-Fr catheter allows for the insertion of two GWs of 0.035 and 0.025- inches (Figure 1).
An Erbotom ICC200 (ERBE Elektromedizin GmbH, Tubingen, Germany) was used for cautery with the Endocut; the effect 3 current was set at an output limit of 120 W, and the forced coagulation current was set at an output limit of 30 W.
For the use of non-electrocautery dilation catheters, an MTW cannula (MTW Endoscopy, Dusseldorf, Germany) and a 6-F-10-Fr Soehendra® Biliary Dilation Catheter (Cook Medical) and/or an 8 mm-diameter balloon Non-Slip Bile Duct Dilation Catheter (Sumitomo Bakelite Co. Ltd., Tokyo, Japan) were used.
As a drainage catheter and stent, a 6-Fr pigtail nasobiliary catheter (Create Medic Co. LTD., Tokyo, Japan) and/or a 7-Fr 4 cm Zimmon® Biliary Stent Set (Cook Medical) or a Double Pigtail Stent delivery system Through Pass (Gadelius Medical K.K., Tokyo, Japan) were used.
A convex array echoendoscope was used in all cases of EUS-TD for the treatment of PFCs. All patients underwent endoscopic procedures under deep sedation with benzodiazepines and/or pentazocine as analgesics. Carbon dioxide inflation was used after May 2011. Antibiotics were initiated on the procedure day and were continued until improvement of the infection.
PFCs were accessed from the stomach or duodenum using a 19-gauge fine needle aspiration needle with EUS guidance. When the needle punctured the PFC, a 0.035-inch GW was inserted through the needle and advanced into the PFC under fluoroscopic guidance.
The puncture tract was dilated over the length of the GW using a 6-Fr and/or an 8.5-Fr electrocautery dilation catheter. After an 8.5-Fr electrocautery dilation catheter was inserted into the PFC over the 0.035-inch GW, another 0.025-inch GW was inserted into the PFC through the catheter lumen under fluoroscopic guidance. After the 0.035-inch and 0.025-inch GWs were inserted into the PFC, a 7-Fr 4 cm double pigtail stent (as an internal drain) and a 6-Fr drainage catheter (as an external drain) were inserted as far as possible.
According to the method for using a non-electrocautery dilation catheter, the puncture tract was dilated using an endoscopic retrograde cholangiopancreatography cannula and biliary dilation catheter with a balloon dilatation catheter over the GW. After another GW was inserted into the PFC through the fistulous tract, a 7-Fr 4 cm double pigtail stent and a 6-Fr drainage catheter were inserted as far as possible.
The external drainage catheter was placed during the first 1 to 2 wk, and the infected PFC was washed with saline. The stent of the internal drain was maintained for 3 to 6 mo and was removed after the resolution of the PFC.
The primary endpoints were the technical and clinical successes of using an electrocautery dilation catheter as a fistula dilation device for EUS-TD in the treatment of PFCs.
Technical success was defined as good fistulous dilation and successful internal and/or external drain placement within the PFC.
Clinical success was defined as the resolution of the PFC and/or the improvement of the infected PFCs by the use of only the EUS-TD without the need for additional drainage or necrosectomy.
The secondary endpoints were the procedure time and the safety of EUS-TD for the treatment of PFCs using an electrocautery dilation catheter.
The procedure time was defined as the time from echoendoscopic insertion until the internal and/or external drain placement.
The safety of EUS-TD for the treatment of PFCs was evaluated according to the development of procedure-related adverse events, such as bleeding, perforation, stent migration, and free air in the abdomen.
Continuous variables are expressed as the mean ± SD. Statistical analyses were performed using StatMate III software (ATMS Co. Ltd., Tokyo, Japan). Data were analyzed using the Mann-Whitney U test and the χ2 test. Differences of P < 0.05 were considered significant.
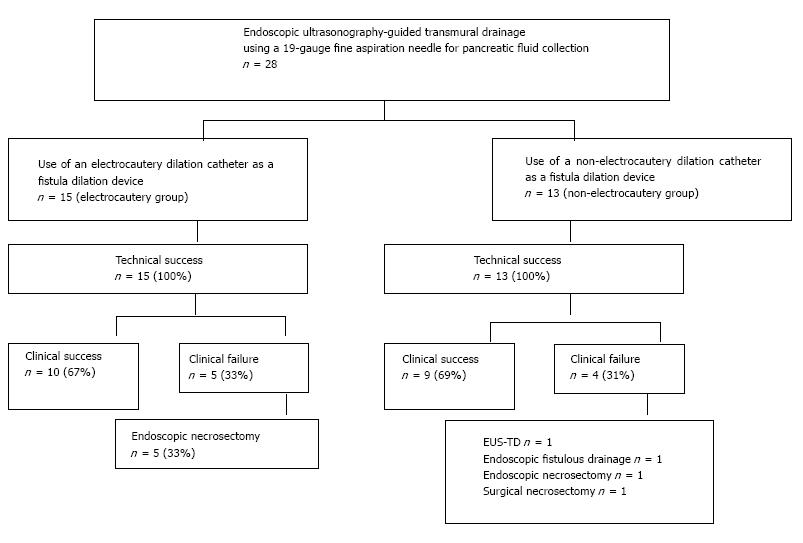
A total of 28 consecutive patients who underwent EUS-TD for the treatment of symptomatic PFCs by fine needle aspiration using a 19-gauge needle were investigated in this study. After we obtained permission to use an electrocautery dilation catheter at our institution in May 2012, we used the dilation catheter for these patients. Fifteen patients were treated with an electrocautery dilation catheter for the fistulous dilation of PFCs (electrocautery group), and 13 patients were treated with a non-electrocautery dilation catheter (non-electrocautery group) (Figure 2). The mean follow-up period of all patients was 1001 ± 463 d.
Patient characteristics are presented in Table 1. There were no statistically significant differences in age, sex, type of PFC based on the revised Atlanta Classification, location and diameter of PFCs between the groups. Thirteen patients (87%) in the electrocautery group and 10 patients (77%) in the non-electrocautery group presented with infected PFCs (Table 1).
| Electrocautery group (n = 15) | Non-electrocautery group (n = 13) | P value | |
| Age: mean ± SD, yr | 60 ± 14 | 64 ± 14 | 0.4891 |
| Sex: male/female, n | 12/3 | 11/2 | 0.8602 |
| Type of PFC, n (%) | 0.6152 | ||
| WON | 4 (27) | 6 (46) | |
| Pancreatic pseudocyst | 6 (40) | 4 (31) | |
| ANC | 4 (27) | 3 (23) | |
| APFC | 1 (6) | 0 | |
| Location of PFC, n (%) | 0.9762 | ||
| Head | 3 (20) | 3 (23) | |
| Body-tail | 11 (73) | 9 (69) | |
| Head-body-tail | 1 (7) | 1 (8) | |
| Diameter of PFC, mean ± SD, cm | 7.2 ± 3.1 | 9.9 ± 3.7 | 0.0611 |
| Infected PFC, n (%) | 13 (87) | 10 (77) | 0.8602 |
The technical success rates of EUS-TD for the treatment of PFCs were 100% and 100% for the electrocautery and non-electrocautery groups, respectively. Transgastric puncture was carried out in 14 patients (93%) in the electrocautery group and in 13 patients (100%) in the non-electrocautery group (P = 0.942). Internal and external drain placements for PFCs were performed on 14 patients (93%) in the electrocautery group and on 11 patients (85%) in the non-electrocautery group (P = 0.896).
The clinical success rates of EUS-TD for the treatment of PFCs without the need for additional transmural drainage or necrosectomy were 67% (10/15) and 69% (9/13) for the electrocautery and non-electrocautery groups, respectively (P = 0.794).
In the non-electrocautery group, 1 patient required additional EUS-TD for the treatment of a pancreatic pseudocyst that showed exacerbation of infection due to the dislocation of the external drain, and 1 patient required additional endoscopic internal drainage through the fistula for WON that showed exacerbation of infection after external drain withdrawal.
In the electrocautery group, 5 patients required endoscopic necrosectomy for infections associated with WON and ANC due to the lack of efficacy of EUS-TD. In the non-electrocautery group, 1 patient required endoscopic necrosectomy, and 1 patient underwent surgical necrosectomy for uncontrolled infected WON (Figure 2 and Table 2).
| Electrocautery group (n = 15) | Non-electrocautery group (n = 13) | P value | |
| Technical success, n (%) | 15 (100) | 13 (100) | |
| Puncture tract, n (%) | 0.9421 | ||
| Transgastric | 14 (93) | 13 (100) | |
| Transduodenal | 1 (7) | 0 | |
| Drainage method, n (%) | 0.8961 | ||
| Internal and external drainage | 14 (93) | 11 (85) | |
| External drainage | 1 (7) | 2 (15) | |
| Clinical success, n (%) | 10 (67) | 9 (69) | 0.7941 |
| Procedure time, mean ± SD, min | 30 ± 12 | 52 ± 20 | < 0.0012 |
| Adverse events, n | 0.9421 | ||
| Free air | 0 | 1 | |
| Procedure-related death, n | 0 | 0 | |
| Additional procedure, n | 0.7941 | ||
| EUS-TD | 0 | 1 | |
| Endoscopic fistulous drainage | 0 | 1 | |
| Endoscopic necrosectomy | 5 | 1 | |
| Surgical necrosectomy | 0 | 1 |
The procedure time for EUS-TD in the electrocautery group was significantly shorter than that of the non-electrocautery group (30 ± 12 min vs 52 ± 20 min, P < 0.001) (Table 2).
No adverse events occurred during the EUS-TD procedure in the electrocautery group. One patient in the non-electrocautery group presented with free air in the abdomen during the procedure but was relieved conservatively. There were no procedure-related deaths in either of the groups (Table 2).
Endoscopic drainage has recently replaced percutaneous or surgical drainage as the initial approach for the treatment of PFCs. Percutaneous drainage of PFCs allows for improved drainage by using a drainage tube with a larger diameter. A single-center, retrospective study reported that endoscopic drainage has a similar clinical success rate, fewer required re-interventions, a shorter hospital stay, and a decreased number of follow-up abdominal imaging studies for symptomatic pancreatic pseudocysts compared with percutaneous drainage[2]. In a randomized trial for pancreatic pseudocyst drainage comparing endoscopic and surgical cystogastrostomy, endoscopic drainage was associated with shorter hospital stays, better physical and mental health of the patients, and lower costs[3].
Recently, EUS-TD has been widely accepted as a minimally invasive procedure that allows the safe puncture of PFCs using a visualized approach. In a prospective randomized trial comparing EUS-TD and conventional endoscopic drainage for pancreatic pseudocysts, the technical success associated with EUS-TD was significantly greater than that of conventional endoscopic drainage, from which the authors concluded that EUS should be considered the first-line treatment modality for pancreatic pseudocysts[7].
A non-electrocautery dilation catheter has been used conventionally as a device for fistulous dilation for PFCs. However, sufficient dilation of the fistula site can be difficult using only a non-electrocautery dilation catheter. As a result, it may prove impossible to insert a drain catheter or a plastic stent into the cavities of PFCs using this method.
As an alternative to the non-electrocautery method, Azar et al[9] reported that 21 of 23 patients with PFCs underwent technically successful wire-guided pancreatic pseudocyst drainage using a modified needle knife. Additionally, Ahlawat et al[10] reported that 9 of 11 total PFC patients successfully underwent EUS-guided pseudocyst drainage using a single-step approach involving a cystotome to electrically enlarge the fistula site. However, misdirected PFC punctures may occur when using only a cystotome, as it is difficult to visualize the tip of an electrocautery catheter with EUS guidance. Our data show that the expansion of the puncture tract using dilator catheters after having punctured a PFC using a 19-gauge fine needle aspiration needle with EUS guidance is a safe and effective alternative procedure. An additional advantage of using a wire-guided electrocautery dilation catheter for EUS-TD in the treatment of PFCs is that we can penetrate the puncture tract without excessive resistance, which increases the efficiency of the procedure.
Prior to our study, few studies have compared the clinical effects of electrocautery and non-electrocautery dilation catheters as fistula dilation devices for EUS-guided transmural PFC drainage. Therefore, we retrospectively investigated the clinical outcomes and adverse events associated with the use of electrocautery and non-electrocautery dilation catheters for EUS-TD in the treatment of PFCs.
The technical success rate of EUS-TD for the treatment of symptomatic PFCs was 100% for the electrocautery and non-electrocautery groups. Moreover, the procedure time for EUS-TD in the electrocautery group was significantly shorter than that of the non-electrocautery group (30 ± 12 min vs 52 ± 20 min, P < 0.001). Median procedure times of EUS-TD using plastic stents for PFCs have been reported as 29.5 min (interquartile range: 23.5-42 min)[11] and 42.6 ± 14.2 min[12].
We used a 6-Fr and/or an 8.5-Fr wire-guided electrocautery dilation catheter under fluoroscopic guidance to dilate the puncture tract of PFCs. An 8.5-Fr electrocautery dilation catheter offers the advantage of accommodating the insertion of a 0.035-inch and a 0.025-inch GW, and the placement of internal and external drain catheters is possible at the same time.
The procedure time of EUS-TD for PFCs using an electrocautery dilation catheter was shorter than that of a non-electrocautery dilation catheter in part because we could easily create a large fistulous tract without changing devices, which allowed for the quick insertion of a drain catheter and a plastic stent into the PFC by simultaneously inserting two GWs through the catheter into the cavity.
Seewald et al[13] also reported that eight out of eight patients were successfully treated without complications using a one-step, simultaneous double-wire technique for the treatment of pancreatic pseudocysts and abscess drainage; the mean procedural time was 32.5 min (range 25-45 min).
The main adverse events for EUS-TD are bleeding and perforation. In this study, the use of an electrocautery dilation catheter resulted in no adverse events.
We hypothesized that using a wire-guided electrocautery dilation catheter under EUS and fluoroscopic guidance would allow us to safely expand the puncture tract.
The clinical success rates of EUS-TD for the treatment of PFCs without the need for additional treatments were 67% (10/15) in the electrocautery group and 69% (9/13) in the non-electrocautery groups, and there was no significant difference between the groups (P = 0.794).
The clinical success rate of EUS-guided or conventional transmural drainage for PFCs is related to the PFC type. In terms of clinical success rates of EUS-guided or conventional endoscopic transmural PFC drainage, the success rates for pancreatic pseudocysts are between 86% and 100%[7,14-18], whereas the success rate for WON with necrosis is between 25% and 72%[15,19-21]. A possible reason for the discrepancies in these results is that the drainage effects of EUS-TD treatment of WON is poor due to the inclusion of necrotic material, which increases the risk of catheter and plastic stent occlusion. Because a plastic stent can be easily occluded by necrotic material, a novel, fully covered and self-expandable metal stent has become available to create a large fistulous site and reduce stent occlusion[22,23]. More recently, Rinninella et al[24] reported that EUS-TD for the treatment of PFCs using a lumen-apposing metal stent on an electrocautery-enhanced delivery system is a safe, effective, and minimally invasive treatment.
In our study, clinical failure of EUS-TD for the treatment of PFCs occurred in 6 patients with WON, 2 patients with ANC, and 1 patient with a pancreatic pseudocyst. The clinical success rate of EUS-TD was 91% (10/11) for patients with pancreatic pseudocysts and APFC and 53% (9/17) for patients with WON and ANC. Approximately 50% of patients who experienced poor outcomes from EUS-TD treatment for WON and ANC, which include necrotic material, required additional treatment in our study.
Recently, endoscopic transmural necrosectomy has been accepted as a step–up approach in patients with infected necrotizing pancreatitis, in whom EUS-TD treatment is insufficient[25]. In this report, the authors declared that an endoscopic step-up approach reduces mortality, major complications, hospital stay and related costs compared with a surgical step-up approach in patients with infected necrotizing pancreatitis.
A limitation of this study is the single-center, small, and exploratory retrospective nature of the study. The difference in procedure time may be related to increased experience of the clinicians with the procedure. Multi-center, randomized, controlled trials are needed to confirm our findings.
In conclusion, EUS-TD using an electrocautery dilation catheter as a fistula dilation device for the treatment of PFCs appears to be safe and contributes to shorter procedure times.
We express our deepest appreciation to Professor Kenichi Matsui and Professor Eiji Uchida, Office for Promoting Medical Research Showa University, for the statistical review of descriptions of study design.
Endoscopic ultrasonography-guided transmural drainage (EUS-TD) has been widely accepted as a minimally invasive procedure for pancreatic and peripancreatic fluid collections (PFCs). However, EUS-TD for the treatment of PFCs may cause adverse events, such as bleeding and perforation; thus, the establishment of a safe procedure for EUS-TD is necessary. Few articles have investigated the clinical outcomes associated with the use of an electrocautery dilation catheter as a fistula dilation method for EUS-TD in the treatment of PFCs. The aim of this study was to evaluate the safety and efficacy of an electrocautery dilation catheter as a fistula dilation device for EUS-TD in the treatment of PFCs by fine needle aspiration using a 19-gauge needle.
Electrocautery or non-electrocautery dilation catheters are used as fistula dilation devices for EUS-TD in the treatment of PFCs; however, prior to this study, few studies have investigated the safety and efficacy of an electrocautery dilation catheter in this procedure.
The authors retrospectively compared the clinical outcomes between electrocautery and non-electrocautery dilation catheters for EUS-TD in the treatment of symptomatic PFCs. The results show that EUS-TD using an electrocautery dilation catheter as a fistula dilation device appears to be safe and contributes to shorter procedure times.
The results of this exploratory retrospective study suggest that EUS-TD for the treatment of PFCs using an electrocautery dilation catheter as a fistula dilation device appears to be safe and contributes to shorter procedure times. However, multi-center, randomized, controlled trials are needed to confirm these findings.
An electrocautery dilation catheter was used to dilate the puncture tract of PFCs. This device is a wire-guided dilation catheter with a distal electrocautery tip. An 8.5-Fr electrocautery dilation catheter allows the simultaneous insertion of 0.035-inch and 0.025-inch guide wires, and the placement of internal and external drain catheters is possible at the same time.
This manuscript is generally well written. The data are of interest, although it is a rather specialized topic.
P- Reviewer: Chow WK, Ding XW, Kayaalp C, Kleeff J S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4316] [Article Influence: 359.7] [Reference Citation Analysis (45)] |
| 2. | Akshintala VS, Saxena P, Zaheer A, Rana U, Hutfless SM, Lennon AM, Canto MI, Kalloo AN, Khashab MA, Singh VK. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc. 2014;79:921-928; quiz 983.e2, 983.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-590.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 331] [Article Influence: 27.6] [Reference Citation Analysis (1)] |
| 4. | Giovannini M, Bernardini D, Seitz JF. Cystogastrotomy entirely performed under endosonography guidance for pancreatic pseudocyst: results in six patients. Gastrointest Endosc. 1998;48:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Seifert H, Dietrich C, Schmitt T, Caspary W, Wehrmann T. Endoscopic ultrasound-guided one-step transmural drainage of cystic abdominal lesions with a large-channel echo endoscope. Endoscopy. 2000;32:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Braden B, Dietrich CF. Endoscopic ultrasonography-guided endoscopic treatment of pancreatic pseudocysts and walled-off necrosis: new technical developments. World J Gastroenterol. 2014;20:16191-16196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Binmoeller KF, Weilert F, Shah JN, Bhat YM, Kane S. Endosonography-guided transmural drainage of pancreatic pseudocysts using an exchange-free access device: initial clinical experience. Surg Endosc. 2013;27:1835-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Azar RR, Oh YS, Janec EM, Early DS, Jonnalagadda SS, Edmundowicz SA. Wire-guided pancreatic pseudocyst drainage by using a modified needle knife and therapeutic echoendoscope. Gastrointest Endosc. 2006;63:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Ahlawat SK, Charabaty-Pishvaian A, Jackson PG, Haddad NG. Single-step EUS-guided pancreatic pseudocyst drainage using a large channel linear array echoendoscope and cystotome: results in 11 patients. JOP. 2006;7:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Lee BU, Song TJ, Lee SS, Park do H, Seo DW, Lee SK, Kim MH. Newly designed, fully covered metal stents for endoscopic ultrasound (EUS)-guided transmural drainage of peripancreatic fluid collections: a prospective randomized study. Endoscopy. 2014;46:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Mukai S, Itoi T, Baron TH, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N. Endoscopic ultrasound-guided placement of plastic vs. biflanged metal stents for therapy of walled-off necrosis: a retrospective single-center series. Endoscopy. 2015;47:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Seewald S, Thonke F, Ang TL, Omar S, Seitz U, Groth S, Zhong Y, Yekebas E, Izbicki J, Soehendra N. One-step, simultaneous double-wire technique facilitates pancreatic pseudocyst and abscess drainage (with videos). Gastrointest Endosc. 2006;64:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Weckman L, Kylänpää ML, Puolakkainen P, Halttunen J. Endoscopic treatment of pancreatic pseudocysts. Surg Endosc. 2006;20:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts. Arq Gastroenterol. 2008;45:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Giovannini M, Pesenti C, Rolland AL, Moutardier V, Delpero JR. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 317] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 20. | Gardner TB, Chahal P, Papachristou GI, Vege SS, Petersen BT, Gostout CJ, Topazian MD, Takahashi N, Sarr MG, Baron TH. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc. 2009;69:1085-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc. 2012;75:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 23. | Yamamoto N, Isayama H, Kawakami H, Sasahira N, Hamada T, Ito Y, Takahara N, Uchino R, Miyabayashi K, Mizuno S. Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections. Gastrointest Endosc. 2013;77:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Rinninella E, Kunda R, Dollhopf M, Sanchez-Yague A, Will U, Tarantino I, Gornals Soler J, Ullrich S, Meining A, Esteban JM. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: a large retrospective study (with video). Gastrointest Endosc. 2015;82:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 25. | van Brunschot S, van Grinsven J, Voermans RP, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ. Transluminal endoscopic step-up approach versus minimally invasive surgical step-up approach in patients with infected necrotising pancreatitis (TENSION trial): design and rationale of a randomised controlled multicenter trial [ISRCTN09186711]. BMC Gastroenterol. 2013;13:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |