INTRODUCTION
A new paradigm is developing in the treatment of obesity and metabolic disease. Endoscopic procedures in development, in trials, and in use have the potential to bridge the gap between medical therapy and weight loss surgery. Obesity and its comorbidities - diabetes, hypertension, hyperlipidemia, and nonalcoholic fatty liver disease, have become a global epidemic[1]. Dietary modification, exercise, and pharmacologic therapy have been ineffective in arresting the spread of obesity at the population level. Bariatric surgery, which is effective and is utilized by hundreds of thousands of patients each year, can only be performed on a fraction of eligible patients given the current number of practicing surgeons[2]. Endoscopic therapies for weight loss are potentially less invasive, reversible, and lower cost; they may also be repeatable as necessary. These characteristics mean that various endoscopic procedures may play a role as primary therapy, as a bridge to bariatric surgery, or as a revisional procedure after bariatric surgery. Current primary endoscopic bariatric therapies can be classified as restrictive, bypass, space-occupying, or aspiration therapy. These procedures, as well as endoscopic revision of gastric bypass, are discussed herein.
RESTRICTIVE PROCEDURES AND DEVICES
Restrictive procedures remodel the stomach via suturing, stapling, or tissue anchor placement to reduce gastric volume.
Incisionless Operating Platform for Primary Obesity Surgery Endolumenal
The Incisionless Operating Platform (IOP) [USGI Medical, San Clemente, California (CA)] can perform full-thickness tissue plication. The platform of the IOP is the four-channel TransPort, which is steerable in four directions and has a 73 cm insertion length. A 4.9-mm endoscope is passed through one channel for endoscopic visualization. The g-Prox, which is capable of 360-degree rotation, has 33-mm stainless steel jaws at its tip to grasp tissue. A helix, called g-Lix, is passed through one channel to grasp tissue and pull it into the jaws of the g-Prox. The g-Cath is advanced through the g-Prox and used to deploy suture anchors. The g-Prox is able to cut suture. The device can be reloaded in vivo.
The device has been used to perform the Primary Obesity Surgery Endolumenal (POSE) procedure. To perform POSE, eight to ten plications are created in the gastric fundus (in retroflexion) in two parallel ridges until the fundic apex is brought down to the level of the gastroesophageal junction. The device is then straightened so that the distal gastric body is visualized. A tissue ridge is created with three or four plications in the distal gastric body across from the incisura. Care should be taken to avoid deep g-Lix insertion in this area, in order to avoid injury of adjacent viscera. After the procedure, patients advance from a clear liquid diet to soft pureed diet during the first month, and then to solid food by six weeks. A study of 45 patients with average body mass index (BMI) 36.7 ± 3.8 kg/m2 reported six-month weight loss of 16.3 ± 7.1 kg or 15.5% ± 6.1%[3]. BMI decreased by 5.8 ± 2.5 kg/m2 over six months. Adverse events associated with the procedure included one case of low-grade fever and one case of chest pain. POSE is currently being studied in the ongoing randomized sham-controlled ESSENTIAL trial.
OverStitch for endoscopic sleeve gastroplasty
The Apollo OverStitch (Apollo Endosurgery, Austin, TX) can place full-thickness stitches in a variety of interrupted or running patterns. Sutures can be reloaded without endoscope removal. The OverStitch includes a curved needle driver attached to the tip of the endoscope, a catheter-based suture anchor, and an actuating handle attached near the endoscope controls. A double-channel endoscope is necessary.
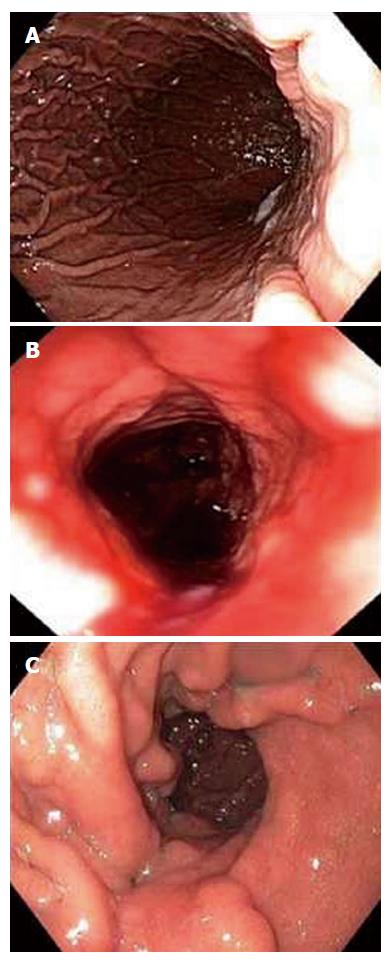
The OverStitch can be used to perform endoscopic sleeve gastroplasty (Figure 1). Initial human cases were performed in a three-center study: a pilot study of five patients to establish procedure technique, safety, and feasibility followed by 23 cases to study efficacy[4]. Gastroplasty was performed by placing running stitches in a triangular configuration starting in the antrum and working proximally. Each suture was used to create two conjoined triangles. Between 8 and 14 sutures were placed in this fashion. The procedure included fundic reduction in retroflexion. The sleeve was reinforced with interrupted stitches. BMI in the 23 patients studied for efficacy decreased from 34.2 ± 1.1 kg/m2 to 29.4 kg/m2. Gastroplasty using a different method was studied in a single-center pilot trial including four patients with average BMI of 35.9 ± 1.2 kg/m2[5]. This technique employed two parallel rows of interrupted plications to create a gastric sleeve. The trial established technical feasibility. The multicenter Primary Obesity Multicenter Incisionless Suturing Evaluation trial to study efficacy of endoscopic sleeve gastroplasty using OverStitch is ongoing in the United States.
Figure 1 Endoscopic sleeve gastroplasty using Apollo OverStitch: before (A), after (B), and at three months (C)[5].
EndoCinch for endoscopic gastroplasty
The EndoCinch [Davol, Murray Hill, New Jersey (NJ)] is a superficial-thickness endoscopic suturing system. EndoCinch uses suction to acquire tissue in a hollow capsule, and then passes a needle through the tissue. EndoCinch has been studied for endoscopic gastroplasty in adolescents and adults. A study of gastroplasty in 64 patients with average BMI of 39.9 kg/m2 reported no serious adverse events[6]. Weight loss of 58.1% ± 19.9% Excess Weight Loss (EWL) was reported after one year. A study of the same procedure in 21 adolescents (age 13-17) with average BMI of 36.2 kg/m2 reported 67.3% EWL after one year and 61.5% EWL after 18 mo[7]. The device was then modified and named the RESTORe (Davol, Murray Hill, NJ), and was capable of both full-thickness suturing and suture reloading in vivo. This device was studied in a two-site trial including 18 patients[8]. There were no significant adverse events. One-year mean weight loss was 11.0 ± 10 kg, or 27.7% ± 21.9% EWL. Half of the patients lost more than 30% of excess weight. Average waist circumference declined by 12.6 ± 9.5 cm. Blood pressure decreased significantly (systolic -15.2 mmHg, diastolic -9.7 mmHg). However, follow-up endoscopy revealed partial or complete release of plications in 13 of 18 patients.
TransOral GAstroplasty
The TransOral GAstroplasty device (TOGA; Satiety Inc, Palo Alto, CA) is a flexible endoscopic stapler capable of full-thickness tissue apposition. The device comprises a stapler and a restrictor. The sleeve stapler comprises a handle and a long but flexible shaft. It also has a short rigid capsule with stapler assembly, two vacuum pods, and a septum at the end. An 8.6 mm endoscope can be passed through the device and retroflexed to visualize the procedure. The stapler creates a vertical sleeve approximately 8 cm long and 2 cm in diameter along the lesser curvature. The restrictor has a long flexible shaft and a short rigid capsule with stapler. It reduces the sleeve outlet to 10-15 mm in diameter. The procedure begins with dilation of the esophagus to 60F with a Savary dilator[9]. The device is inserted into the stomach over a guidewire. Once in position, vacuum apposes the gastric walls, acquiring tissue into the device. Firing the stapler creates a 4.5 cm sleeve around the stapler using titanium staples. The device has to be removed for reloading, and the firing process is repeated once more distally, overlapping the first sleeve. The restrictor is inserted over the guidewire, with the endoscope adjacent to the device. Vacuum acquires tissue into the device at the distal sleeve, and firing the restrictor creates a 2.5 cm long stapled narrowing at the outlet of the sleeve.
TOGA has been studied for endoscopic gastroplasty (Figure 2). A study of 21 patients (average BMI 43.3 kg/m2) used the first-generation device[10]. There were no serious adverse events, although pain, nausea, vomiting, and temporary dysphagia were reported. Average 6-mo weight loss was 12 kg (24.4% EWL). Endoscopy at that time found staple line gaps in 13 patients, although every patient had at least a partial sleeve. The second-generation device was studied in 11 patients[11]. In this study, additional distal restrictions were created during retreatment if necessary. No significant adverse events were reported. Six-month weight loss was an average 24.0 kg, and average BMI decreased from 41.6 to 33.1 kg/m2. A multicenter study of 67 patients reported adverse events including respiratory insufficiency in one case and asymptomatic pneumoperitoneum in another[9]. At one year, patients with BMI ≥ 40 had 52.2% EWL and patients with BMI < 40 had 41.3% EWL. There were significant improvements in hemoglobin A1c (decline from 7.0% to 5.7%), HDL and triglycerides. A single-center study of 29 patients reported mean BMI decline from 41.7 kg/m2 to 35.5 kg/m2 over two years[12]. Average weight loss was 16.8 kg, or 14.9% total body weight loss.
Figure 2 Creation of sleeve using TransOral GAstroplasty[9].
ACE stapler
The ACE stapler (Boston Scientific Corporation, Natick, MA) is an endoscopic stapler with a head capable of both 360-degree rotation and complete retroflexion. A 5-mm endoscope enables visualization; the device is 16 mm in diameter. The stapler head acquires gastric tissue using vacuum suction; firing the stapler creates a full-thickness plication using a 10-mm plastic ring with 8 titanium staples. For gastric volume reduction, up to 8 plications are made in the fundus. Two plications are created in the antrum, which may delay gastric emptying. A prospective safety and feasibility study of gastric volume reduction in 17 patients (median BMI 40.2 kg/m2) reported median procedure time of 123 min[13]. The most common adverse event was abdominal pain (7 patients); sore throat, diarrhea, nausea, constipation, and vomiting were also reported. All were self-limited. Median EWL was 34.9% (interquartile range 17.8-46.6). Endoscopy performed at 12 mo (in 11/17 patients) revealed 6-9 plications in all participants, as well as durability of gastric volume reduction.
Transoral Endoscopic Restrictive Implant System
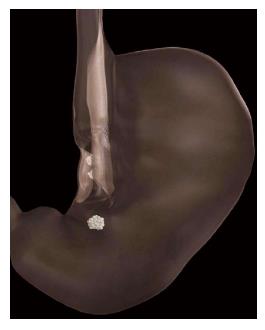
Unlike the aforementioned devices, Transoral Endoscopic Restrictive Implant System (TERIS) (Barosense, Menlo Park, CA) is an implanted device. A gastric pouch is created by implanting a diaphragm with a 10-mm orifice. This is attached to the cardia (Figure 3). For implantation, a 22-mm endogastric tube is inserted. A gastroscope with a stapling device is retroflexed, and a full-thickness plication is created in the cardia. An anchor is attached to the plication. This is repeated until five anchors have been implanted. The restrictive diaphragm is then attached to the anchors. A study of TERIS in 13 patients reported three adverse events: One gastric perforation and two cases of pneumoperitoneum[14]. The procedure was modified after these events, and no further adverse events occurred. In total, 12 of 13 implantation procedures were successful. Procedure time was 142 min on average. Weight loss at three-month follow-up was 16.9 kg or 22.2% EWL; median BMI fell from 42.1 to 37.9 kg/m2.
Figure 3 Transoral Endoscopic Restrictive Implant System restrictive diaphragm[14].
BYPASS DEVICES AND PROCEDURES
Bypass of the small intestine is thought to have a significant role in the weight loss and metabolic benefits experienced after certain bariatric surgeries. Animal models suggest that duodenal exclusion and accelerated arrival of partially-digested meals to mid-jejunum and ileum are partially responsible for the salutary effects of gastric bypass in diabetes and obesity. Endoscopically implanted devices have been developed to reproduce this effect.
EndoBarrier duodenal-jejunal bypass liner
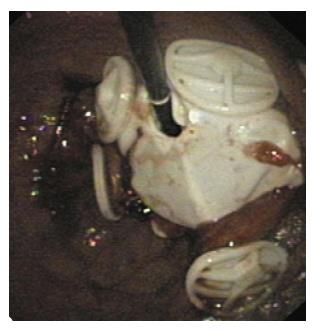
The EndoBarrier duodenal-jejunal bypass device (GI Dynamics, Lexington, MA) comprises a nickel-titanium implant attached to a 60 cm polymer sleeve (Figure 4). The sleeve extends from the duodenal bulb into the jejunum. It prevents food from contacting the mucosa of the small intestine, but allows pancreaticobiliary secretions to move along the outside of the device to the jejunum. Additionally, it allows food to reach the mid-jejunum earlier. The device is placed endoscopically, with fluoroscopic guidance, under general anesthesia. A guidewire is advanced into the duodenum. The sleeve and anchor are enclosed in a capsule, which is advanced over the guidewire. The sleeve is deployed in the intestine; once it is fully extended, the anchor is deployed in the duodenal bulb approximately 5 mm distal to the pylorus. Device removal is also performed under general anesthesia. A foreign body hood is placed at the tip of the endoscope, and the device is removed by securing the anchor with a procedure-specific grasping device.
Figure 4 EndoBarrier duodenal-jejunal liner[16].
A multicenter randomized trial compared 30 EndoBarrier patients (BMI 48.9 kg/m2) with 11 controls (BMI 47.4 kg/m2)[15]. No serious adverse events were reported. However, four of 30 EndoBarrier patients required removal due to migration, obstruction, pain, or anchor dislocation. The EndoBarrier group had significantly higher weight loss at three months, with BMI decrease of 5.5 kg/m2vs 1.9 kg/m2 in control patients. Notably, 7 of 8 diabetics in the EndoBarrier group had improvement in diabetes.
A multicenter randomized trial including 25 patients reported successful EndoBarrier implantation in 21 patients, with implantation failure in patients with small duodenal bulb[16]. Adverse events resulted in device explantation in seven of 21 implanted patients, including three cases of bleeding that presented as hematemesis. There was significantly more weight loss in the EndoBarrier group: (8.2 ± 1.3 kg vs 2.0 ± 1.1 kg).
A randomized trial of 39 patients assigned 25 patients to EndoBarrier and 14 patients to the control group[17]. At 3 mo, the EndoBarrier group had 22% EWL vs 5% EWL in controls. The adverse event rate, including bleeding, migration, and obstruction, was 20%.
A multicenter randomized controlled trial including 77 patients with obesity and type II diabetes included 31 patients who completed EndoBarrier therapy and 35 controls who completed dietary intervention[18]. The EndoBarrier group experienced 32.0% EWL vs 16.4% in the control group; the EndoBarrier group also had a significantly larger improvement in hemoglobin A1c (P < 0.05 for both). After the EndoBarrier had been removed for 6 mo, EWL was 19.8% vs 11.7% in controls (P < 0.05).
A one-year prospective open-label trial of 42 patients reported that 39 patients were successfully implanted[19]. Premature explantation was necessary in 15 patients due to anchor movement in 8 patients, device obstruction in 3 patients, abdominal pain in 2 patients, acute cholecystitis in 1 patient, and one patient request. Initial average BMI was 43.7 ± 5.9 kg/m2. At 1 year, the 24 patients with EndoBarrier in place experienced weight loss of 22.1 ± 2.1 kg or 47.0% ± 4.4% EWL, and BMI decline of 9.1 ± 0.9 kg/m2. Waist circumference decreased significantly, from 120.5 ± 6.8 cm to 96.0 ± 2.6 cm. Statistically significant improvements were also reported in blood pressure, hemoglobin A1c, cholesterol, low-density lipoprotein, triglycerides, and prevalence of metabolic syndrome.
A modified EndoBarrier with a 4-mm flow-restriction orifice was implanted in 10 patients with average BMI of 40.8 kg/m2[20]. Eight of 10 patients in the trial developed abdominal pain, nausea, and vomiting; they required balloon dilation of the restrictive orifice. Weight loss at three months was 16.7 ± 1.4 kg.
SPACE-OCCUPYING DEVICES
Space-occupying devices displace volume and induce gastric distention, but may also alter gastrointestinal motility, nutrient transit, and hormone levels[21]. One space-occupying device, the intragastric balloon, was described in 1982 and approved for American use in 1985[22]. In the intervening decades, balloons have built a track record of safety and efficacy in Europe, and are likely to reappear in the United States. The intragastric balloon has found a role as a bridge to bariatric surgery in patients with high risk for anesthesia, temporary use in patients eligible for bariatric surgery but unwilling to undergo it, and temporary use in patients not eligible for bariatric surgery as part of an integrated medical weight loss program[23]. Space-occupying devices other than balloons are in clinical trials.
Orbera intragastric balloon
The Orbera (formerly BioEnterics) intragastric balloon (Apollo Endosurgery, Austin, TX) is an endoscopically implanted spherical silicone elastomer device. The balloon is placed in the stomach and then filled with saline (and where allowed, methylene blue dye, which alters urine color in case of balloon perforation). The balloon is resistant to gastric acid, and is indicated for insertion for up to six months. The device is inflated in the gastric fundus during endoscopic visualization using 500-750 mL saline and 10 mL methylene blue.
Orbera balloon placement was studied in a meta-analysis of 3698 patients[24]. Early device removal was required in 4.2% of patients; reported adverse events included nausea, vomiting, bowel obstruction (0.8%), and gastric perforation (0.1%). Average weight loss after six months was 14.7 kg or 32.1% EWL, with drop in BMI of 5.7 kg/m2. The largest study in the meta-analysis, which included 2515 patients, reported average decrease in BMI of 9.0 kg/m2 over six months[25]. Notably, statistically significant improvement was reported in blood pressure, fasting glucose, and lipid profile. Significant decrease in or normalization of hemoglobin A1c was reported in 87.2% of the 488 diabetic patients in the study. Two instances of mortality were reported, both in patients with prior gastric surgery.
The long-term weight loss trend after removal of the Orbera balloon was studied in 500 patients[26]. Average BMI before therapy was 43.7 kg/m2. Success was defined as ≥ 20% EWL. At the time of balloon removal, 83% of patients had reached this threshold, with average loss of 23.9 ± 9.1 kg and BMI loss of 8.3 kg/m2. In the 41% of patients available five years after balloon removal, the successful group had average loss of 7.3 ± 5.4 kg and average BMI loss of 2.5 kg/m2.
The effectiveness of a second Orbera balloon placement was studied in a prospective trial of 118 patients[27]. The balloon was replaced immediately in 8 patients, replaced after a balloon-free interval in 11 patients, and not replaced in 99 patients. Those patients undergoing a second balloon placement with a balloon-free interval regained 13.6 kg on average during that interval. The second balloon therapy did result in weight loss, although its magnitude was smaller than that of the initial therapy (9.0 kg vs 14.6 kg, or 18.2% EWL vs 49.3% EWL). The effect of second balloon placement dissipated by the third year of follow-up. A study of 112 patients undergoing a second Orbera balloon placement within one month of removing the first balloon found average BMI loss of 2.5 kg/m2 with the second balloon in addition to BMI loss of 6.5 kg/m2 with the first balloon[23].
The utility of the Orbera balloon as a bridge to gastric bypass was studied in 60 consecutive super-super obese subjects with average BMI of 66.5 ± 3.4 kg/m2[28]. The balloon was placed in 23 patients, while 37 patients went to surgery without prior balloon therapy. In the Orbera group, the balloon was in place for 155 ± 62 d. The balloon group achieved BMI loss of 5.5 ± 1.3 kg/m2 at the time of gastric bypass, as well as statistically significant decreases in systolic blood pressure and gamma-glutamyl transpeptidase. The operative time for performance of gastric bypass was shorter in the Orbera group (146 ± 47 vs 201 ± 81 min). The Orbera group also experienced significantly fewer major adverse events (defined as conversion to laparotomy, ICU stay longer than 2 d, and total hospital stay longer 2 wk): 2 events vs 13 in patients who did not have balloon placement. Weight loss was similar between groups one year after gastric bypass.
The metabolic effects of Orbera balloon placement were examined in a prospective trial including 130 patients (average BMI 43.1 kg/m2)[29]. Premature balloon explantation was required in ten patients due to intolerance, abdominal pain, or vomiting. Patients were maintained on a 1000-1200 daily kilocalorie diet during the 6-mo balloon therapy period. Average weight loss was 13.1 kg, with decrease in prevalence of class IV obesity from 23% to 8%. Metabolic effects included decrease in the prevalence of hyperglycemia from 50% to 12%, and hypertriglyceridemia from 58% to 19%. Patients with decrease in BMI of greater than 3.5 kg/m2 experienced a significant decrease in the prevalence of severe hepatic steatosis from 52% to 4%. Weight regain occurred in 50% of the patients in the follow-up period (median 22 mo) after balloon removal.
Dietary counseling during Orbera balloon therapy was been found to be beneficial in a study of 28 patients[30]. Patients saw a dietitian weekly for two weeks, every two weeks for one month, and then monthly while the balloon was in place. BMI declined from 32.4 ± 3.7 kg/m2 to 28.5 ± 3.7 kg/m2 with therapy. Of the patients who achieved at least 20% EWL, 85% had attended at least half of dietitian appointments. Of patients failing to reach 20% EWL, 75% had missed at least half of dietitian appointments.
Orbera balloon therapy is associated with mental health benefits in patients with depression[31]. In this study, 100 consecutive female patients were characterized as depressed (65 patients) or non-depressed (35 patients) using the Beck Depression Inventory score. Other characteristics were similar between groups. Weight loss was similar between groups (39.3% EWL in depressed patients vs 36.1% EWL in non-depressed patients). The Depression Inventory score improved from 20.3 ± 8.5 to 7.9 ± 5.6 during balloon therapy. Resolution of depression occurred in 70.8% of the depressed patients, with a decrease in the prevalence of severe depression (27.7% to 1.5%).
Heliosphere BAG
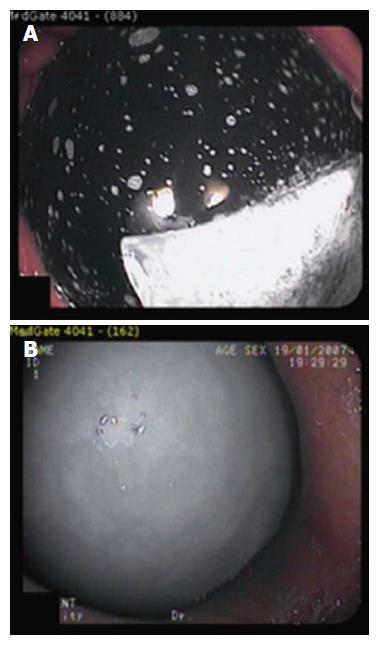
The Heliosphere BAG is filled with 950 mL of air rather than fluid. The Heliosphere BAG has been compared with the Orbera balloon (Figure 5)[32]. Sixty patients with average BMI of 46.3 kg/m2 were randomly assigned. The Heliosphere group achieved BMI decrease of 4.2 kg/m2, vs 5.7 kg/m2 in the Orbera group. The Heliosphere group had significantly longer extraction procedure time and significantly more discomfort during extraction.
Figure 5 The Orbera intragastric balloon (A) and Heliosphere BAG (B)[34].
A prospective study of 91 patients compared the Orbera balloon (73 patients) with Heliosphere BAG (18 patients, mean BMI 45.2 kg/m2)[33]. Balloons were implanted for six months, and 13.2% were removed early due to intolerance. Average weight reduction at six months was 13.3 kg, and BMI reduction was 5 kg/m2; 88% of weight reduction occurred in the first three months. Weight loss was similar between balloon types. The Heliosphere BAG deflated and passed spontaneously in 2 cases. Balloon extraction was difficult in 8 cases, and a rigid esophagoscope as required in 4 cases; laparoscopic surgery was required to remove BAG in 1 case. BAG was significantly more likely to result in retrieval complications.
A nonrandomized study compared Heliosphere BAG with the Orbera balloon in patients who failed six months of medical and dietary weight loss therapy[34]. The Orbera balloon was placed in 19 patients (BMI 45.6 ± 9 kg/m2), and the Heliosphere BAG was placed in 13 patients (BMI 45.0 ± 8 kg/m2). The Orbera balloon was more effective, with weight loss of 19.0 kg vs 13.0 kg for Heliosphere BAG. One patient with the Orbera balloon required removal for persistent nausea and vomiting at one month. There was one mortality in the Orbera group 13 d after placement.
Reshape Duo intragastric balloon
The Duo intragastric balloon (Reshape, San Clemente, CA) contains two silicone spheres filled with a total of 900 mL of saline, which prevents migration if one balloon deflates. A prospective trial of Duo included 30 patients at three centers (21 Duo vs 9 controls)[35]. Both groups received diet and exercise counseling. Four of the 21 Duo patients were readmitted for nausea, and two patients were found to have gastritis at the time of balloon removal. After 48 wk, 30% of the Duo patients achieved 25% EWL, vs 25% of the control patients.
Obalon intragastric balloon
The Obalon intragastric balloon (Obalon Therapeutics, Carlsbad, CA) is a 250-mL gas-filled balloon which is swallowed under fluoroscopic visualization rather than inserted endoscopically. The balloon is enclosed in a capsule. A catheter, which extends through the esophagus and outside the mouth, is used to fill the balloon with gas. The balloon is removed endoscopically; it is punctured and then grasped with forceps for extraction. If the balloon is tolerated and induces weight loss, a second balloon can be swallowed at 4 wk and a third balloon at 8 wk. A study including 17 patients with BMI ranging from 27 to 35 kg/m2 reported that 98% of balloons were swallowed successfully[36]. Abdominal pain (in 76%) and nausea (in 41%) were the most frequent adverse events. All balloons were removed endoscopically, under conscious sedation, at 12 wk.
Transpyloric Shuttle
The Transpyloric Shuttle (BAROnova, Goleta, CA) is made of a large spherical bulb attached to a smaller cylindrical bulb by a flexible tether. The cylinder is small enough to enter the duodenal bulb with peristalsis, and pulls the spherical bulb to the pylorus. The spherical bulb is too large to traverse the pylorus, but occludes it intermittently to reduce gastric emptying. The device is delivered transorally via catheter and removed endoscopically. A single-center nonblinded prospective trial of 20 patients with average BMI of 36.0 kg/m2 reported loss of 8.9 ± 5.2 kg, or 31.3% ± 15.7% EWL, at 3 mo[37]. Six-month weight loss was 14.6 ± 5.7 kg, or 50.0% ± 26.4% EWL. Two patients required early removal due to persistent ulcer.
SatiSphere
The SatiSphere (Endosphere, Columbus, OH) is made from a preformed memory wire with curled ends that conforms to the shape of the duodenum. The device anchors itself in the distal stomach and in the duodenum. Several mesh spheres are mounted along the wire. SatiSphere slows duodenal transit of food, which may alter satiety hormones levels and glucose metabolism. A trial of 31 patients with average BMI of 41.3 kg/m2 compared 21 SatiSphere patients with 10 controls[38]. Device migration was reported in 10 of 21 implanted patients. Emergency surgery was necessary in two patients. Of patients completing the trial, three-month weight loss was 6.7 kg in the SatiSphere group vs 2.2 kg in controls. SatiSphere was associated with delayed glucose absorption, delayed insulin secretion, and altered glucagon-like peptide-1 kinetics.
ENDOSCOPIC REVISION OF GASTRIC BYPASS
Roux-en-Y gastric bypass can induce 56.7%-66.5% EWL during the two years after surgery[40]. Comorbidities associated with obesity, including hypertension, diabetes, obstructive sleep apnea, and hyperlipidemia, often improve or resolve. It is postulated that small gastric pouch size and gastrojejunal anastomosis aperture create a restrictive effect. A weight plateau typically occurs as equilibrium in energy balance is reached 12 to 18 mo after gastric bypass[41]. However, approximately 20% of patients fail to achieve 50% EWL in the first year after gastric bypass. Additionally, 30% of patients regain weight by 18 to 24 mo after bypass; average regain of 18 kg has been reported at 2 years[42,43]. The long-term outcome of gastric bypass is affected by a number of factors, including preoperative BMI and postoperative diet and lifestyle[44]. Weight regain may be induced by neuroendocrine-metabolic dysregulation resulting in a starvation-like response[45,46]. Anatomic factors may also play a role: increased gastrojejunal anastomotic aperture may result in loss of restriction, and has been associated with weight regain in a linear fashion[5,47,48].
Surgical procedures, including reconstruction of the gastrojejunal anastomosis, placement of an adjustable gastric band over the gastric pouch, surgical revision of the pouch, and distal gastric bypass, are available to treat weight regain; however, few patients undergo surgical revision. Revision surgery is challenging in the context of older patients, altered anatomy, scarring, and adhesions; complication and mortality rates are higher than that of primary gastric bypass[49,50]. Endolumenal revision is an attractive option in this patient set. Endoscopic suturing, plication, and sclerotherapy are discussed here.
EndoCinch for transoral outlet reduction
The EndoCinch (Bard Davol, Murray Hill, NJ), as described above for endoscopic gastroplasty, is a superficial-thickness suturing device which uses suction to acquire tissue. The EndoCinch has been used to perform transoral outlet reduction (TORe), or endoscopic revision of gastric bypass. First, the entire gastric margin of the gastrojejunal anastomosis is ablated with argon plasma coagulation. The aperture of the gastrojejunal anastomosis is then reduced by placing interrupted stitches at the anastomotic margin, across the anastomotic opening. Cinching the sutures apposes the anastomotic margin, reducing the diameter of the anastomosis. The volume of the gastric pouch can be reduced by creating ridges and suturing them together.
Use of the EndoCinch for TORe was first reported in 2004[51]. The device was used in RESTORe, a randomized sham-controlled double-blinded multicenter trial which resulted in level 1 evidence for the effectiveness of endoscopic suturing in revision of gastric bypass[52]. Seventy-seven patients with gastrojejunal anastomosis aperture larger than 20 mm were randomized to TORe or to sham endoscopy. Average BMI was 47.6 kg/m2. Anastomotic aperture of < 10 mm was achieved in 89% of TORe patients. There was no difference in the adverse event rate between groups, and no perforations occurred. In the intent-to-treat analysis, total body weight loss was 3.8% in TORe patients vs 0.3% in the sham group (P = 0.02). Weight stabilization or weight loss was achieved in 96% of TORe patients during the 6-mo follow-up period.
OverStitch for TORe
Apollo OverStitch, as described in detail above for endoscopic sleeve gastroplasty, is reloadable in vivo and is capable of placing full-thickness sutures in a variety of stitch patterns. After TORe is performed to reduce the aperture of the gastrojejunal anastomosis, gastric pouch size can be reduced and fistulas can be closed during the procedure. TORe should be performed using general anesthesia, endotracheal intubation, and carbon dioxide insufflation. An overtube should be placed. Upper endoscopy is performed to ablate the margin of the gastrojejunal anastomosis. This can be performed using end-firing argon plasma coagulation (at 30 watts) to create a ring 5-10 mm thick around the margin of the anastomosis, or performance of endoscopic mucosal resection around the anastomosis. Anastomotic reduction can be performed using an interrupted technique, in which stitches are placed across the anastomosis and then cinched to appose its margins. Alternatively, a pursestring suture technique can be used (Figure 7). The pursestring technique potentially confers a number of benefits compared with the interrupted technique. It allows the use of a sizing balloon, which ensures precise control of final anastomosis aperture. It reinforces the entire circumference of the anastomosis against future dilation, and against transient compliant dilation during meals. In contrast, the interrupted technique closes part of the lumen entirely, but does not reinforce the remaining anastomotic margin, and the final anastomotic diameter cannot be precisely controlled. To perform anastomotic reduction using the pursestring technique, a running pursestring suture is placed around the anastomosis. A controlled radial expansion balloon is passed through the second channel of the endoscope and inflated to 8-10 mm. The pursestring is tightened around the balloon, and the suture is cinched. A second pursestring can be placed around the anastomosis for reinforcement.
Figure 7 Gastrojejunal anastomosis before (A), immediately after (B), and six months after (C) TORe using Apollo OverStitch[54].
Endoscopic revision of gastric bypass using OverStitch proved effective in a study of 25 patients[53]. Gastrojejunal anastomosis aperture was reduced from 26.4 mm to 6 mm on average. No significant adverse events were reported. Patients lost an average 11.7 kg during the 6-mo follow-up period, or 69.5% of regained weight. Endoscopic revision of gastric bypass using the superficial-thickness EndoCinch and full-thickness OverStitch were directly compared in a matched cohort study[54]. The interrupted stitch technique was used in both groups, and the technique used in the EndoCinch patients was the same technique used in the RESTORe trial. One hundred eighteen patients (59 in each group) were sequentially matched by gastrojejunal anastomosis aperture, then BMI, and then age. Average weight loss at six months was significantly higher in patients undergoing full-thickness suturing (4.4 ± 0.8 kg with EndoCinch vs 10.6 ± 1.8 kg with OverStitch, P < 0.01). One-year weight loss was also significantly higher in the OverStitch group (2.9 ± 1.0 kg with EndoCinch vs 8.6 ± 2.5 kg with OverStitch, P < 0.01).
IOP for Revision Obesity Surgery Endolumenal
The IOP (USGI Medical, San Clemente, CA), as described in detail above for the POSE procedure, is capable of performing full-thickness tissue plication by placement of tissue anchors. The platform has been optimized specifically for endoscopic revision of gastric bypass, called Revision Obesity Surgery Endolumenal (ROSE). ROSE entails reduction of dilated gastric pouch and gastrojejunal anastomosis aperture. A prospective study included 20 patients with weight regain[55]. The procedure was technically successful in 85%, with reduction of anastomotic aperture by an average of 65% and reduction of gastric pouch length by 36%. Anastomotic aperture was reduced to an average of 16 mm. Average weight loss was 8.8 kg after 3 mo. A subsequent iteration of the device was studied in five patients, with all five patients losing weight (average weight loss was 7.8 kg)[56]. A prospective multicenter trial of 116 patients with dilated gastrojejunal anastomosis and gastric pouch achieved technical success in 97%[57]. Gastrojejunal anastomosis aperture was reduced by an average of 50%, and the gastric pouch was shortened by an average of 44%. No significant procedural complications occurred; three patients had superficial esophageal tears, one of which required placement of an endoscopic clip. Pharyngitis was reported in 41% of patients, nausea and vomiting in 12%, and abdominal pain in 11%. During the 6-mo follow-up period, patients lost 32% of the weight regained after Roux-en-Y gastric bypass. Patients with anastomotic aperture of less than 10 mm at the end of the procedure experienced 24% EWL. The device has since been further optimized for revision of gastric bypass.
StomaphyX
StomaphyX (EndoGastric Solutions, Redmond, Washington) is a full-thickness tissue plication platform capable of endoscopic revision of gastric bypass. It uses vacuum to acquire a fold of the gastric pouch. Polypropylene H-fasteners are passed through the tissue to create full-thickness plications. Without removal of the device, 3 to 4 rows with 4 to 6 plications each (a total of 12-24) are created circumferentially around the margin of the anastomosis.
A study of StomaphyX in 39 patients with average BMI of 39.8 kg/m2 reported no adverse events[58]. EWL was 13.1% after 3 mo and 19.5% after 1 year. A subsequent study of 64 patients with average BMI of 39.5 kg/m2 reported placement of an average 23 plications, resulting in reduction of anastomotic diameter from 22 mm to 9 mm[59]. One patient had bleeding that did not require transfusion; no other significant adverse events were reported. During follow up (average 5.8 mo), patients lost an average 7.6 kg. A retrospective study of 59 patients with mean BMI of 36.1 kg/m2 reported mean weight loss of 3.8 kg and 11.5% EWL after 6 mo[60]. However, endoscopy in 12 patients at an average of 18 mo after revision showed no sustained reduction in pouch or anastomosis size. Mean follow-up duration was 41 mo, with average loss of 1.7 kg; 35.8% of patients had actually gained weight by this point. A randomized sham-controlled single-blind trial of StomaphyX revision with SerosFuse fasteners was terminated prematurely due to failure to reach preliminary efficacy targets. There was one adverse event in the StomaphyX group, and laparoscopic exploration and repair were necessary. A total of 45 StomaphyX patients and 29 sham patients completed 1-year follow-up. Of these, 22.2% of the StomaphyX patients and 3.4% of the sham patients achieved 15% excess BMI loss (P < 0.01). The StomaphyX group had significantly more weight loss at 6 and 12 mo (P≤ 0.05).
Endoscopic sclerotherapy
Endoscopic sclerotherapy entails injection of a sclerosant, such as sodium morrhuate, around the gastrojejunal anastomosis to reduce compliance and aperture. The procedure can be performed under conscious sedation in many patients. The anastomotic aperture should be measured prior to injection, as measurement afterwards will be inaccurate due to transient edema. A test dose of the sclerosing agent should be injected at the rim of the anastomosis, and the patient should be monitored for an adverse reaction before further injection. Approximately 2 mL should be injected into the submucosa at the margin of the gastrojejunal anastomosis until a bleb forms. Several such injections are performed around the anastomotic margin, for a total of 10-25 mL[61]. Overinjection is indicated by dark red or black discoloration and subsequent overt bleeding. Intravenous ciprofloxacin should be given as prophylaxis prior to the procedure, followed by a five-day course of liquid ciprofloxacin or trimethoprim-sulfamethoxazole. The patient should can start a liquid diet the day after the procedure and advance to a regular diet during the month after the procedure. Sclerotherapy can be repeated every 3-6 mo until the anastomosis aperture has reached a target of 12 mm; two or three sessions are often necessary[62]. The development of scar tissue after each sclerotherapy session can eventually make submucosal injection difficult.
Endoscopic sclerotherapy has proven effective in arresting weight regain after gastric bypass. One study including 28 patients reported that most patients (64%) lost more than 75% of regained weight[62]. An average of 2.3 sessions was required. Notably, patients with anastomotic aperture larger than 15 mm did not benefit. A study of 32 patients reported arrest or reversal of weight regain in 91.6% of patients at 1 year[61]. A study of 71 patients reported arrest or reversal of weight regain in 72% of patients after 1 year[63]. A recent study of 48 patients undergoing sclerotherapy reported average loss of 1.45 kg during a follow-up period averaging 22 mo[64]. Although weight regain was arrested, weight loss after sclerotherapy was not significant. The largest published series included 231 consecutive patients with mean anastomosis diameter of 19 mm undergoing 575 sclerotherapy sessions[65]. Weight regain was arrested in 78% of patients at one year after sclerotherapy. Average weight loss at six months was 4.5 kg. Bleeding occurred in 2.4%, with 57% of those requiring endoscopic clip placement. Transient elevation in blood pressure was observed in 15%, and was associated with higher injection volume. Small ulcerations were found on follow-up endoscopy in 1%.