Published online Jun 10, 2015. doi: 10.4253/wjge.v7.i6.606
Peer-review started: September 24, 2014
First decision: December 17, 2014
Revised: February 22, 2015
Accepted: March 16, 2015
Article in press: March 18, 2015
Published online: June 10, 2015
Processing time: 268 Days and 14.1 Hours
Biliary tract diseases are the most common complications following liver transplantation (LT) and usually include biliary leaks, strictures, and stone disease. Compared to deceased donor liver transplantation in adults, living donor liver transplantation is plagued by a higher rate of biliary complications. These may be promoted by multiple risk factors related to recipient, graft, operative factors and post-operative course. Magnetic resonance cholangiopancreatography is the first-choice examination when a biliary complication is suspected following LT, in order to diagnose and to plan the optimal therapy; its limitations include a low sensitivity for the detection of biliary sludge. For treating anastomotic strictures, balloon dilatation complemented with the temporary placement of multiple simultaneous plastic stents has become the standard of care and results in stricture resolution with no relapse in > 90% of cases. Temporary placement of fully covered self-expanding metal stents (FCSEMSs) has not been demonstrated to be superior (except in a pilot randomized controlled trial that used a special design of FCSEMSs), mostly because of the high migration rate of current FCSEMSs models. The endoscopic approach of non-anastomotic strictures is technically more difficult than that of anastomotic strictures due to the intrahepatic and/or hilar location of strictures, and the results are less satisfactory. For treating biliary leaks, biliary sphincterotomy and transpapillary stenting is the standard approach and results in leak resolution in more than 85% of patients. Deep enteroscopy is a rapidly evolving technique that has allowed successful treatment of patients who were not previously amenable to endoscopic therapy. As a result, the percutaneous and surgical approaches are currently required in a minority of patients.
Core tip: One third of liver transplant recipients are affected by biliary tract complications which are the major source of morbidity in these patients. Biliary-biliary (as opposed to bilio-enteric) anastomoses are first treated by endoscopy, with resolution of > 85% and > 75% of cases in deceased and living-donor transplant recipients, respectively. New stenting protocols and new designs of fully covered self-expandable metal stents are at the frontline of efforts aiming to reduce patient burden during treatment. Here, we discuss the latest developments in the endoscopic approaches to these complications.
- Citation: Macías-Gómez C, Dumonceau JM. Endoscopic management of biliary complications after liver transplantation: An evidence-based review. World J Gastrointest Endosc 2015; 7(6): 606-616
- URL: https://www.wjgnet.com/1948-5190/full/v7/i6/606.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i6.606
Liver transplantation (LT) has become a standard of care in patients with end-stage liver disease. After LT, approximately one third of patients are affected by biliary tract complications and these result in significant morbidity and decreased patient survival[1]. Due to the scarcity of organ donors and the increasing number of patients waiting for LT, living donor liver transplantation (LDLT) has emerged as an alternative to deceased donor liver transplantation (DDLT). Even though surgical techniques are constantly improving, biliary complications are more frequent following LDLT compared with DDLT[2]; LDLT also remains characterized by its technical complexity and ethical controversies.
Biliary complications following LT include biliary leaks, strictures, choledocholithiasis and other less common conditions[3,4]. Approaches commonly used for treating biliary complications involve endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangiography (PTC) and surgery. ERCP is commonly regarded as the first choice treatment modality in most circumstances; if it fails PTC is often used, reserving surgery for severe complications or refractory conditions not manageable by less invasive techniques[5-8].
Here we review the literature focusing on the endoscopic management of biliary complications, the different strategies for treating strictures and biliary leaks and summarize their outcomes.
It is essential for endoscopists to have a clear comprehension of the different types of surgical reconstruction during LT. Biliary reconstruction is performed at the end of LT, once all vascular anastomoses have been completed. An end-to-end choledoco-choledocal anastomosis is the first choice procedure in most institutions following whole organ LT in patients with healthy native bile ducts of suitable caliber[6,9]. This technique produces physiological bilioenteric continuity, preserves the function of the sphincter of Oddi and allows for potential future endoscopic treatment of biliary complications. Bilioenteric reconstruction (Roux-en-Y hepaticojejunostomy) is performed in cases of previous biliary tract disease (e.g., sclerosing cholangitis, biliary atresia), large disparity in size or small caliber of the bile ducts, and may be preferred in cases of retransplantation because of inadequate recipient duct length[10]. Due to the shortage of cadaveric livers, LDLT has gained popularity in adult patients. With LDLT, the living donor’s right or left lobe or the left lateral segment is transplanted. Ductal anastomoses are more difficult to perform than in DDLT due to the small caliber of the intrahepatic ducts. In reduced size split-liver transplantation, a liver from a dead donor is splitted into two organs to permit two recipients to receive a graft; the anastomoses of both right and left lobe are alike to those of LDLT.
Biliary complications may be promoted by multiple risk factors related to recipient, graft, operative factors and post-operative course: (1) among recipient-related factors, advanced recipient age and more advanced liver function impairment contribute to the development of biliary complications[11,12]; (2) among graft-related factors, prolonged cold and warm ischemia time, extended donor criteria grafts and donation after cardiac death, as opposed to brain death, are associated with a higher incidence of ischemic-type biliary lesions (ITBL)[13,14]. Nonetheless, a recent report by Vanatta et al[15] showed that, by carefully selecting donors and recipients, overall patient and graft survival as well as the incidence of ITBL were similar following donation after cardiac vs brain death[15]; (3) operative risk factors are different for DDLT and LDLT for various reasons: LDLT by itself is an important risk factor for biliary complications due to the small duct size, the presence of multiple biliary duct outlets and the devascularization of the bile ducts during hilar dissection of the graft[16-18]. In DDLT, T-tube placement for duct to duct (DD) reconstruction allows minimizing the incidence of anastomotic strictures[19] and it is unequivocally recommended by some authors[20]; however, this results in biliary leakage following T-tube removal in 5%-33% of cases[19]; (4) during the postoperative course, early hepatic artery thrombosis may lead to the severest forms of non-anastomotic strictures, at multiple sites of the donor biliary system, because blood supply to the bile ducts is fragile. This may result in partial or total biliary necrosis with the formation of typical biliary casts and multiple intraluminal filling defects at cholangiography[5,21]; and (5) other documented factors, including ABO incompatibility, cytomegalovirus infection and chronic/acute rejection episodes have been reported to be potential risk factors for biliary complications in historical publications; more recently these factors have been strongly associated with non-anastomotic, rather than anastomotic, complications[22-24].
The clinical presentation of biliary complications varies considerably; patients could present no symptom at all, jaundice, abdominal pain, biliary leak or cholangitis. In asymptomatic LT recipients, a biliary complication usually is first suspected because of elevations of serum bilirubin, alkaline phosphatase, and/or gamma-glutamyl transferase levels. In the case of cholestasis, the initial diagnostic step is to discriminate obstructive vs nonobstructive causes, like LT rejection (acute or chronic), recurrence of primary disease and drug-induced cholestasis.
The initial evaluation should include a liver ultrasound (US) with a Doppler evaluation of the hepatic vessels, due to the frequent association of biliary complications with the presence of hepatic artery thrombosis or stenosis[6,25]. If hepatic artery stenosis or occlusion is suspected by Doppler US, multidetector computed tomography should be used as the second-line modality of choice for the rapid assessment of major vascular complications requiring pre-treatment confirmation. If hepatic artery thrombosis is confirmed, angiographic intervention should be performed urgently to re-establish hepatic artery flow[26,27]. Magnetic resonance cholangiopancreatography (MRCP) has substantially facilitated the accurate recognition of biliary tract complications (sensitivity and specificity of 93%-97% and 92%-98%, respectively, compared with ERCP as the reference standard)[28-31]. MRCP provides the endoscopist with a map of the whole biliary tract and, unlike ERCP, consistently demonstrates ducts even upstream from a tight stricture, therefore it is especially useful for hilar or intrahepatic anastomotic strictures. When findings at MRCP were compared to other approaches, including ERCP, PTC, and surgery to diagnose post-LT biliary complications, the sensitivity, specificity, positive predictive value, and negative predictive of MRCP were 98%, 94%, 94%, and 98%, respectively[31]. Its main disadvantages include a low sensitivity in the case of sludge or small stones (< 5 mm). MRCP is noninvasive and is the technique of choice for diagnosing post-LT biliary complications.
Post-LT biliary strictures are usually classified as anastomotic strictures (ASs) or non-anastomotic strictures (NASs), also called ischemic type biliary strictures (ITBS)[32-34]. Biliary strictures complicate around 2%-14% of LT and can be categorized in to early or late (occurring within or after the first month following LT, respectively). Strictures which appear soon after LT are commonly referable to technical problems, whereas late strictures are generally attributable to vascular insufficiency and problems with healing and fibrosis. In a recent systematic review, 1844 (12.8%) of 14359 LT patients had biliary strictures. The appearance of a stricture varies widely, from 7 d to 11 years after LT[35].
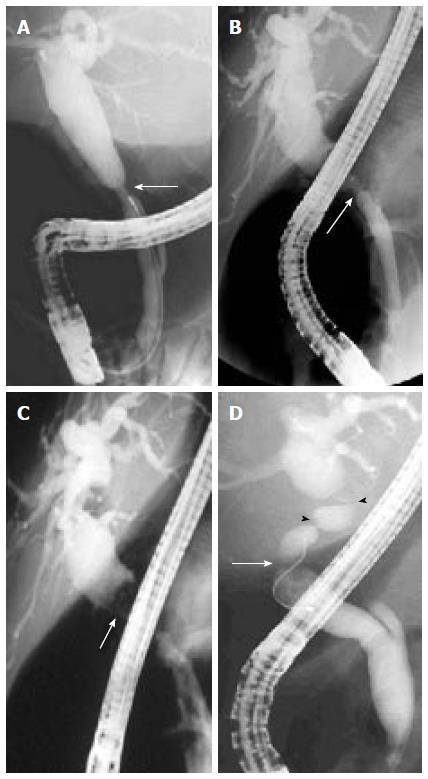
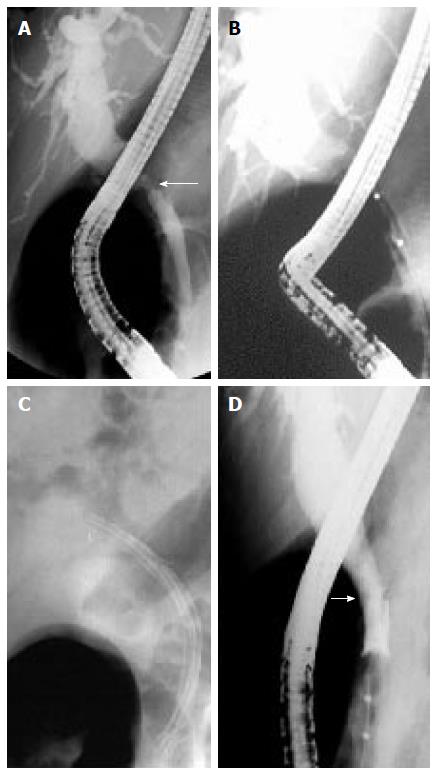
Anastomotic strictures: ASs can present at any time after transplantation but most of them are diagnosed within one year following LT with a mean interval between LT and diagnosis of 5-8 mo. ASs complicate around 6%-12% and 34% of deceased and living donor LT procedures, respectively[33,36,37]. ASs pathogenesis is believed to include inadequate mucosa-to-mucosa anastomosis, local tissue ischemia, and the fibrotic nature of the healing process[33,38]. ASs are solitary and short in length (Figure 1A and B). They may involve a choledocho-jejunostomy or a choledocho-choledochostomy; they are considered clinically relevant only if cholestasis or cholangitis are present. A slight and transient narrowing of the biliary lumen occurs frequently within the first one to two months following biliary anastomosis due to postoperative edema and inflammation, but it is uncertain how many of these cases progress to clinically significant ASs (Figure 1A)[33]. ASs can generally be effectively treated by endoscopic means and do not decrease graft or patient survival.
Non-anastomotic strictures: Post-LT strictures are classified as NASs if they are located more than 5 mm proximal to the anastomosis (Figure 1D). They account for 10% to 25% of all strictures complicating LT, with an incidence in the range of 0.5% to 10%[19,38-40]. NASs are considered to derive from ischemic damage to the duct as it may occur following hepatic artery thrombosis. Conditions associated with NASs include a prolonged ischemia time (cold and warm), transplantation after cardiac death donation, prolonged vasopressor support for the donor, ABO-type incompatibility, primary sclerosing cholangitis, autoimmune hepatitis or hepatitis C virus infection in the recipient[41-48]. Furthermore, nowadays a wider acceptance of older and extended criteria donors has been suggested to contribute to an increased incidence of NASs[19]. True NASs, usually referred to as ITBSs, characteristically are diffuse and include the hilum and sectorial or segmental intrahepatic branches. The treatment of NASs is technically more difficult than that of ASs and, in the case of hepatic artery thrombosis, the endoscopic treatment is mostly ineffective if the arterial blood flow cannot be restored.
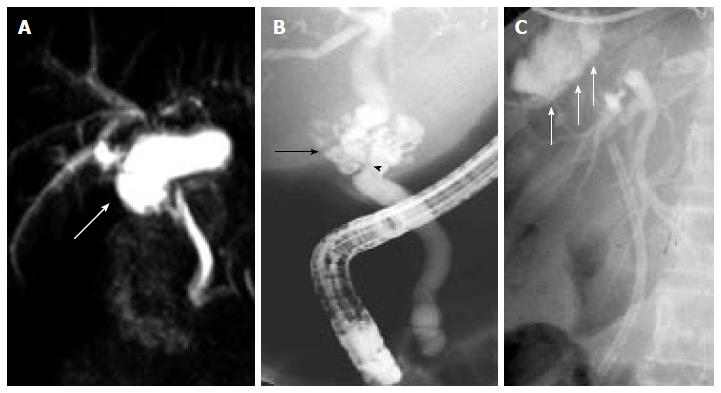
Biliary leakage is the second most common complication after LT, with an incidence of 2%-21%[19,49,50]. In a recent meta-analysis, the rate of biliary leakage after LT was 8.2%, without significant difference between DDLT (7.8%) and LDLT (9.5%)[35]. Leakage may develop at the level of the anastomotic site, from the cystic duct remnant, from the cut surface of partial liver grafts in the case of LDLT, and following T-tube removal (Figure 2). Bile leaks can be classified into two categories: early bile leaks, which present within 4 wk following LT (these usually occur at the anastomotic site and are often related to technical issues, not to the type of biliary reconstruction), and late bile leaks, which present beyond this time (they are usually related to T-tube removal, resulting from delayed T-tube tract maturation possibly related to immunosuppression). A bile leak should be suspected in any patient who develops abdominal pain, fever or any sign of peritonitis following LT, especially after T-tube removal. Bile leaks can derive in collections of fluids and abscesses that might be related to strictured or disconnected ducts. Depending on the size of the leakage and the clinical presentation, bile leaks can be managed conservatively, nonsurgically or surgically[4,51].
Stones, sludge and casts occur in approximately 5% of patients after LT, with stones accounting for 70% of the cases. Biliary stone disease is associated with disorders that can reduce the flow of bile such as ASs or NASs. In addition, medications such as cyclosporine may play a role in bile lithogenicity by inhibiting bile secretion and promoting functional biliary stasis. Sludge is described as a thick collection of mucus, calcium bicarbonate and cholesterol crystals, which, when left untreated, can transform into biliary stones (Figure 3A).
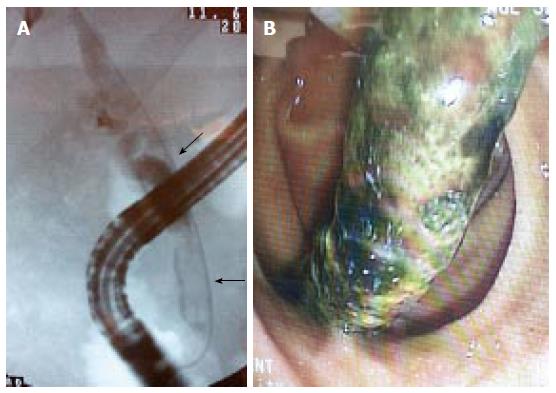
Casts refer to the presence of multiple hard pigmented dark material that mold the bile ducts (Figure 4). These are thought to develop due to bile duct mucosal damage related to obstruction, ischemia, or bacterial infection. A history of hepatic artery thrombosis and a prolonged cold ischemia time are associated with debris formation[52-54]. This disorder occurs in 2.5% to 18.0% of LT recipients[32,54]. Casts are associated with increased morbidity, graft failure, retransplantation and mortality.
Sphincter of oddi dysfunction (SOD) describes a clinical syndrome of biliary or pancreatic functional obstruction that may be responsible for cholestasis, pain, or pancreatitis.
It is hypothesized that, in the post-LT setting, denervation of the ampulla (secondary to surgical intervention) might generate a hypertonic sphincter, resulting in increased intraductal biliary pressure. This complication has been reported in 2% to 7% of patients who have undergone LT[55,56]. Typically, patients present with cholestasis, dilation of the distal bile duct and no obstacle detected at cholangiography.
Managing post-LT biliary complications needs a multidisciplinary team involving transplant surgeons, hepatologists, endoscopists, and interventional radiologists. Endoscopic therapy is the first line therapy in most cases with a duct-to-duct anastomosis. With recent developments in enteroscopy, many patients with Roux-en-Y hepaticojejunostomy can also be treated endoscopically[57], with PTC being mostly reserved for the salvage of failures. The spectrum of endoscopic therapies includes biliary sphincterotomy, balloon dilation of strictures, basket and balloon extraction of stones, sludge, and casts, and the placement of one or multiple, side-by-side, biliary plastics stents. Additionally, cholangioscopy allows the characterization of strictures by observation and tissue sampling, and therapy of difficult casts or stones by intraductal lithotripsy[58-62]. Endoscopic therapy is usually highly successful and has a low incidence of procedure-related complications, reserving surgery as a last option intervention if endoscopic and/or percutaneous treatment is not feasible or is ineffective.
Traditionally, post-LT biliary leaks have been treated surgically with anastomotic revision or conversion to a Roux-en-Y hepaticojejunostomy if a duct-to-duct anastomosis is not technically feasible. With advances in endoscopic therapy, ERCP has now become the initial therapeutic option in the management of biliary leaks. Usually the leakage of bile is treated through biliary sphincterotomy followed by the placement of a transpapillary stent (Figure 2C) for 2 to 3 mo (in contrast to post-cholecystectomy leaks, where the stent can be removed in 4 to 6 wk) with the aim of ensuring the proper healing of the leaks. Prolonged stenting is advised because healing may be delayed by immunosuppressors. If the leak is associated with a biliary stricture, this can be prudently dilated before inserting one or more plastic stents upstream from both the stricture and the leak[63]. Biliary stenting provides faster leak resolution than sphincterotomy alone and it is equally effective whether sphincterotomy is performed or not. At the time of stent removal, a careful anatomical evaluation should be performed and duct cleansing should always be performed because biliary abnormalities (mostly sludge, stones, or persistent leak) can be found at this time in a significant proportion of patients[64]. Endoscopic therapy solves the leakage of bile in more than 85% of patients[38,63-66]. Recently, fully covered self-expandable metal stents (FCSEMS) have been used in a pilot study of 17 LT recipients with biliary leaks[67]. FCSEMS offered minimally invasive and low-morbidity short-term control of leaks but it resulted in a relatively high stricture rate. In this series of 17 patients, 8 (47%) patients developed common bile duct strictures following FCSEMS removal; of these, 6 (35%) required repeat endoscopic treatment for a clinically significant stricture, therefore the use of current FCSEMS models cannot be recommended in the post-LT population. In specific situations, endoscopic therapy can be impossible or fail, for example, in the case of large anastomotic leaks associated with hepatic artery compromise or surgically altered anatomy (Roux-en-Y anastomosis). These patients will most often require surgical management.
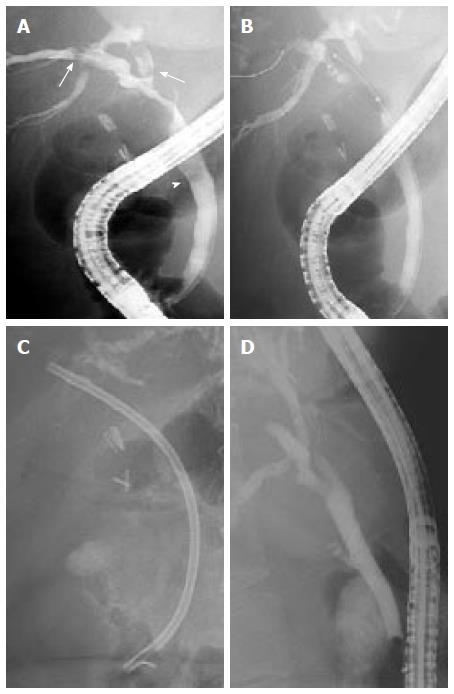
Anastomotic strictures: No standard protocol has emerged for the endoscopic therapy of ASs. By analogy with the more frequent postcholecystectomy biliary stricture, endoscopic therapy of ASs usually requires biliary sphincterotomy plus balloon dilatation (BD) and stent placement (Figure 5). The use of BD alone in early onset anastomotic strictures (the first 2 mo following LT) may be effective. However, despite good initial success, BD alone led to a high rate of recurrent stricture formation[68]. Therefore, the combination of BD and stenting is a more adequate approach[33,65,68-71].
Multiple 10-Fr plastic stents are usually maintained until stricture resolution or for a minimum of 12 mo, with stent exchange scheduled every 3-4 mo to reduce the chance of stent blockage and cholangitis. In a recent systematic review that included 440 LT-related ASs treated with multiple simultaneous plastic stents[72], the mean AS resolution rate was approximately 85% for early as well as late ASs. Higher ASs resolution rates (97% vs 78%) and lower ASs recurrence rates (1.5% vs 14%) have been reported with stenting durations > 12 mo vs < 12 mo. This was observed despite the fact that shorter stenting durations were applied for early vs late ASs. Most cases of ASs recurrence were successfully managed with repeat plastic stenting.
Recently, different strategies of AS treatment have been described to decrease patient burden: (1) long-term maximal stent therapy with stent exchange only when signs or symptoms of biliary obstruction are detected: this strategy has allowed minimizing the number of ERCPs needed to treat ASs without compromising success or patient safety. With this protocol, complete AS resolution was reached in 94% of patients and recurrence rate at a median follow-up of 11 mo was 3%[73]. The authors reported in a total of 83 patients 2 cases of post-ERCP pancreatitis, 2 cases of periprocedural bacteremia but no episodes of cholangitis caused by stent occlusion; (2) stent exchange every 2 wk: ERCP with rapid-sequence balloon dilation followed by stenting with multiple stents over a short time period[74]. With this approach, mean stenting duration was 107 d and long-term stricture resolution was achieved in 33 (87%) of 38 patients; ERCP-related complications occurred in 2 (5%) patients. During a mean follow-up of one year after stent removal, 5 (13%) patients had a stricture recurrence, successfully retreated by endoscopic means in 4 cases; and (3) temporary placement of covered self-expandable metal stent (SEMSs). Covered SEMSs offer the advantage of longer stent patency and larger nominal diameter compared with a single plastic stent. Covered SEMSs should be maintained in place for a minimum of 3 mo as shorter stenting durations result in lower ASs resolution (72% vs 90%)[75-79]. In the systematic review cited above[72], covered SEMSs had a much higher stent migration rate (16%) compared with simultaneous multiple plastic stenting. Furthermore, covered SEMS carry a low but real risk of tissue ingrowth and stent impaction. Therefore, the authors concluded that current evidence does not suggest a clear advantage of SEMS use over multiple simultaneous plastic stenting in the management of ASs. In a large prospective study that was not included in the systematic review[80], the AS resolution rate using FCSEMSs was 68% of 42 LT patients and the migration rate was 17% and 75% at 3 and 6 mo, respectively. In this study, cholangitis was reported in 24% of patients with LT-related ASs and it was strikingly associated with stent migration. Finally, a recent randomized trial compared a new design of FCSEMS vs multiple simultaneous plastic stenting in 20 patients with LT-related ASs[81]. ASs resolution rates were similar with both stent models but complication rate and hospital stay duration were non-significantly higher with the plastic stent vs FCSEMS, suggesting that some FCSEMS designs that effectively prevent stent migration might be a cost-effective alternative to plastic stenting.
Endoscopic management of ASs seemed to be more challenging in LDLT vs DDLT due to the complexity of duct-to-duct anastomosis. However, using an aggressive strategy of maximal endoscopic stent placement, two studies reported high (75%-100%) AS resolution rates in LDLT patients[82,83]. The long-term resolution rates of biliary leaks and/or strictures reported in selected retrospective studies are summarized in Table 1[37,82-87]. Factors identified as independent predictors of failed endoscopic treatment of LDLT-related ASs include higher LT recipient age, longer operation duration, and a pouched morphology of the AS[84,88]. Recurrent ASs occur in approximately 21% of patients and may be retreated by endoscopy[83]. PTC plays an important role when a guide wire cannot be inserted through the anastomotic stricture at the time of ERCP (e.g., disconnected duct, some refractory angulated or twisted strictures). For these patients, the rendez-vous technique (PTC + ERCP) may be useful to insert a stent above the stricture. This approach has been demonstrated to be feasible and relatively safe for the management of biliary strictures complicating LDLT with duct-to-duct anastomosis[89]. The endoscopic treatment of some ASs can be unsuccessful and may need long-term stenting or surgical hepaticojejunal anastomosis[87,90].
| Ref. | Patients (n) | Stenting (m) | Success (%) | F/U(m) | Relapse (%) |
| Yazumi et al[37] (2006)1 | 75 | 6 | 68 | 20 (1-50) | 10 |
| Gómez et al[84] (2009) | 10 | NR | 20 | 30.5 (2-23) | NR |
| Seo et al[87] (2009) | 29 | 3-6 | 64.5 | 31 | 30 |
| Chang et al[86] (2010) | 113 | 3-6 | 26.5 | 33 (3-96) | NR |
| Kim et al[85] (2011) | 112 | 12.7 | 36 | 42.8 ± 15.2 | 11.5 |
| Chan[82] (2013) | 8 | NR | 75 | 18 ± 8.7 | NR |
| Hsieh[83] (2013)2 | 38 | 5.3 | 100 | 74 | 21 |
Non-anastomotic strictures: The endoscopic therapy of NASs or ITBSs often involves the hilum and intrahepatic ducts and is notably more demanding than the therapy of ASs. The stenosis at the level of the sectorial or segmental branch ducts can result in a cholangiographic appearance that simulates primary sclerosing cholangitis. It is challenging to make general recommendations for managing NASs and treatment should be individualized. Treatment success depends upon stricture grade, number, and location. Extra-hepatic strictures generally respond better to therapy and altogether, in the few published reports of endoscopic treatment, the success rates ranged between 50% and 70%[50,91]. Finally, a few patients (especially the ones with complex ischemic intrahepatic strictures) may need surgical revision or retransplantation.
In patients who have undergone Roux-en-Y hepaticojejunostomy, a potential alternative to PTC is the use of various techniques of enteroscopy. In 25 pediatric patients with hepaticojejunal anastomoses, the bilioenteric anastomosis could be reached in 17 patients, a stent could be placed in 9 patients and AS resolution was obtained in 5 (20%) patients, showing the difficulty of this procedure[92]. In a series of 44 adults with choledochojejunal AS following various hepato-biliary-pancreatic surgery, temporary stenting (including stent removal) was achieved in 32 (73%) patients and restenosis occurred in 7/32 patients[93].
Biliary stones, sludge and casts: In LT recipients, the endoscopic management of stones is similar to that performed in the nontransplant setting although the approach may be complicated by the presence of a stricture downstream from the stone. In such circumstances, delayed stone extraction (following biliary stenting) or advanced endoscopic techniques like intraductal lithotripsy or direct choledocoscopy may be required to achieve stone removal. In patients with serious coagulation disorders or thrombocytopenia where sphincter ablation may be relatively contraindicated, balloon dilatation of the intact sphincter can be applied.
For biliary casts, the endoscopic approaches are alike to those utilized in stone disease. However, the success rate is significantly lower owing to the multiplicity of filling defects located in intrahepatic bile ducts[39]. Treatment usually requires multiple ERCPs, possibly complemented with PTC and it may require retransplantation in a significant proportion of cases[39,94] Cholangioscopy might aid to discriminate biliary casts from strictures[59].
As for SOD in the non-LT setting, biliary sphincterotomy is the common treatment and provides a high success rate[39]. The question of whether these patients are at similar risk of post-ERCP pancreatitis as those who are affected in the non-LT setting has not been formally studied; however it seems reasonable to consider prophylactic pancreatic stenting in addition to standard rectal administration of NSAIDs when performing sphincterotomy in these patients[95].
Biliary complications remain a burden in LT patients and continue in some cases to be a challenging aspect of the multidisciplinary care of LT patients. As biliary complications are the most frequent complication following LT, the index of suspicion for requesting further investigations should be low. MRCP is the most useful examination to establish the diagnosis, especially because the low sensitivity of US may be more detrimental in LT as compared to the average patient. Successful endoscopic treatment is achieved in most cases, with the notable exceptions of ASs in LDLT patients, NASs and biliary casts. For ASs, temporary simultaneous multiple plastic stenting for a minimum of 12 mo (except in some cases of early AS) remains the standard of care; FCSEMS have yielded disappointing results up to now. In patients with choledocojejunostomy, deep enteroscopy techniques may allow successful treatment but success rates are lower. Nowadays PTC and surgery are reserved for a small minority of patients.
P- Reviewer: Boucek C, Uchiyama H S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Duffy JP, Kao K, Ko CY, Farmer DG, McDiarmid SV, Hong JC, Venick RS, Feist S, Goldstein L, Saab S. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg. 2010;252:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2014;20:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Ayoub WS, Esquivel CO, Martin P. Biliary complications following liver transplantation. Dig Dis Sci. 2010;55:1540-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Thuluvath PJ, Atassi T, Lee J. An endoscopic approach to biliary complications following orthotopic liver transplantation. Liver Int. 2003;23:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Arain MA, Attam R, Freeman ML. Advances in endoscopic management of biliary tract complications after liver transplantation. Liver Transpl. 2013;19:482-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, Todo S, Fung JJ, Starzl TE. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40-45. [PubMed] |
| 7. | Kochhar G, Parungao JM, Hanouneh IA, Parsi MA. Biliary complications following liver transplantation. World J Gastroenterol. 2013;19:2841-2846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 186] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 8. | Lopez RR, Benner KG, Ivancev K, Keeffe EB, Deveney CW, Pinson CW. Management of biliary complications after liver transplantation. Am J Surg. 1992;163:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Verran DJ, Asfar SK, Ghent CN, Grant DR, Wall WJ. Biliary reconstruction without T tubes or stents in liver transplantation: report of 502 consecutive cases. Liver Transpl Surg. 1997;3:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Vallera RA, Cotton PB, Clavien PA. Biliary reconstruction for liver transplantation and management of biliary complications: overview and survey of current practices in the United States. Liver Transpl Surg. 1995;1:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Qian YB, Liu CL, Lo CM, Fan ST. Risk factors for biliary complications after liver transplantation. Arch Surg. 2004;139:1101-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Welling TH, Heidt DG, Englesbe MJ, Magee JC, Sung RS, Campbell DA, Punch JD, Pelletier SJ. Biliary complications following liver transplantation in the model for end-stage liver disease era: effect of donor, recipient, and technical factors. Liver Transpl. 2008;14:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Jay CL, Skaro AI, Ladner DP, Wang E, Lyuksemburg V, Chang Y, Xu H, Talakokkla S, Parikh N, Holl JL. Comparative effectiveness of donation after cardiac death versus donation after brain death liver transplantation: Recognizing who can benefit. Liver Transpl. 2012;18:630-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Merion RM, Pelletier SJ, Goodrich N, Englesbe MJ, Delmonico FL. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 2006;244:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Vanatta JM, Dean AG, Hathaway DK, Nair S, Modanlou KA, Campos L, Nezakatgoo N, Satapathy SK, Eason JD. Liver transplant using donors after cardiac death: a single-center approach providing outcomes comparable to donation after brain death. Exp Clin Transplant. 2013;11:154-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ishiko T, Egawa H, Kasahara M, Nakamura T, Oike F, Kaihara S, Kiuchi T, Uemoto S, Inomata Y, Tanaka K. Duct-to-duct biliary reconstruction in living donor liver transplantation utilizing right lobe graft. Ann Surg. 2002;236:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Lee KW, Joh JW, Kim SJ, Choi SH, Heo JS, Lee HH, Park JW, Lee SK. High hilar dissection: new technique to reduce biliary complication in living donor liver transplantation. Liver Transpl. 2004;10:1158-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Ohkubo M, Nagino M, Kamiya J, Yuasa N, Oda K, Arai T, Nishio H, Nimura Y. Surgical anatomy of the bile ducts at the hepatic hilum as applied to living donor liver transplantation. Ann Surg. 2004;239:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Riediger C, Müller MW, Michalski CW, Hüser N, Schuster T, Kleeff J, Friess H. T-Tube or no T-tube in the reconstruction of the biliary tract during orthotopic liver transplantation: systematic review and meta-analysis. Liver Transpl. 2010;16:705-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | López-Andújar R, Orón EM, Carregnato AF, Suárez FV, Herraiz AM, Rodríguez FS, Carbó JJ, Ibars EP, Sos JE, Suárez AR. T-tube or no T-tube in cadaveric orthotopic liver transplantation: the eternal dilemma: results of a prospective and randomized clinical trial. Ann Surg. 2013;258:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Buckel EG, Steers JL, Wiesner RH, Krom RA. Diagnostic features and clinical outcome of ischemic-type biliary complications after liver transplantation. Hepatology. 1993;17:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 156] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Fisher A, Miller CH. Ischemic-type biliary strictures in liver allografts: the Achilles heel revisited. Hepatology. 1995;21:589-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 25. | Zajko AB, Campbell WL, Logsdon GA, Bron KM, Tzakis A, Esquivel CO, Starzl TE. Cholangiographic findings in hepatic artery occlusion after liver transplantation. AJR Am J Roentgenol. 1987;149:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Meng XC, Huang WS, Xie PY, Chen XZ, Cai MY, Shan H, Zhu KS. Role of multi-detector computed tomography for biliary complications after liver transplantation. World J Gastroenterol. 2014;20:11856-11864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Girometti R, Como G, Bazzocchi M, Zuiani C. Post-operative imaging in liver transplantation: state-of-the-art and future perspectives. World J Gastroenterol. 2014;20:6180-6200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Beltrán MM, Marugán RB, Oton E, Blesa C, Nuño J. Accuracy of magnetic resonance cholangiography in the evaluation of late biliary complications after orthotopic liver transplantation. Transplant Proc. 2005;37:3924-3925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Boraschi P, Donati F, Gigoni R, Volpi A, Salemi S, Filipponi F, Falaschi F. MR cholangiography in orthotopic liver transplantation: sensitivity and specificity in detecting biliary complications. Clin Transplant. 2010;24:E82-E87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Linhares MM, Gonzalez AM, Goldman SM, Coelho RD, Sato NY, Moura RM, Silva MH, Lanzoni VP, Salzedas A, Serra CB. Magnetic resonance cholangiography in the diagnosis of biliary complications after orthotopic liver transplantation. Transplant Proc. 2004;36:947-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Novellas S, Caramella T, Fournol M, Gugenheim J, Chevallier P. MR cholangiopancreatography features of the biliary tree after liver transplantation. AJR Am J Roentgenol. 2008;191:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Porrett PM, Hsu J, Shaked A. Late surgical complications following liver transplantation. Liver Transpl. 2009;15 Suppl 2:S12-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 34. | Verdonk RC, Buis CI, Porte RJ, van der Jagt EJ, Limburg AJ, van den Berg AP, Slooff MJ, Peeters PM, de Jong KP, Kleibeuker JH. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl. 2006;12:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 35. | Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 36. | Gastaca M. Biliary complications after orthotopic liver transplantation: a review of incidence and risk factors. Transplant Proc. 2011;44:1545-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Yazumi S, Yoshimoto T, Hisatsune H, Hasegawa K, Kida M, Tada S, Uenoyama Y, Yamauchi J, Shio S, Kasahara M. Endoscopic treatment of biliary complications after right-lobe living-donor liver transplantation with duct-to-duct biliary anastomosis. J Hepatobiliary Pancreat Surg. 2006;13:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Rerknimitr R, Sherman S, Fogel EL, Kalayci C, Lumeng L, Chalasani N, Kwo P, Lehman GA. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc. 2002;55:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Pfau PR, Kochman ML, Lewis JD, Long WB, Lucey MR, Olthoff K, Shaked A, Ginsberg GG. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Thethy S, Thomson BNj, Pleass H, Wigmore SJ, Madhavan K, Akyol M, Forsythe JL, James Garden O. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Fujita S, Fujikawa T, Mizuno S, Reed AI, Kim RD, Howard RJ, Firpi RJ, Nelson DR, Hemming AW. Is early recurrence of hepatitis C associated with biliary anastomotic stricture after liver transplantation. Transplantation. 2007;84:1631-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Suárez F, Otero A, Solla M, Arnal F, Lorenzo MJ, Marini M, Vázquez-Iglesias JL, Gómez M. Biliary complications after liver transplantation from maastricht category-2 non-heart-beating donors. Transplantation. 2008;85:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Fujikawa T, Fujita S, Mekeel KL, Reed AI, Foley DP, Kim RD, Howard RJ, Hemming AW. Effect of early recurrence of hepatitis C on late biliary anastomotic stricture after liver transplantation. Transplant Proc. 2006;38:3661-3662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 45. | Kowdley KV, Fawaz KA, Kaplan MM. Extrahepatic biliary stricture associated with cytomegalovirus in a liver transplant recipient. Transpl Int. 1996;9:161-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Halme L, Hockerstedt K, Lautenschlager I. Cytomegalovirus infection and development of biliary complications after liver transplantation. Transplantation. 2003;75:1853-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Koneru B, Sterling MJ, Bahramipour PF. Bile duct strictures after liver transplantation: a changing landscape of the Achilles’ heel. Liver Transpl. 2006;12:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Maheshwari A, Maley W, Li Z, Thuluvath PJ. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 49. | Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 50. | Thuluvath PJ, Pfau PR, Kimmey MB, Ginsberg GG. Biliary complications after liver transplantation: the role of endoscopy. Endoscopy. 2005;37:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Righi D, Franchello A, Ricchiuti A, Breatta AD, Versace K, Calvo A, Romagnoli R, Fonio P, Gandini G, Salizzoni M. Safety and efficacy of the percutaneous treatment of bile leaks in hepaticojejunostomy or split-liver transplantation without dilatation of the biliary tree. Liver Transpl. 2008;14:611-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Sheng R, Sammon JK, Zajko AB, Campbell WL. Bile leak after hepatic transplantation: cholangiographic features, prevalence, and clinical outcome. Radiology. 1994;192:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Londoño MC, Balderramo D, Cárdenas A. Management of biliary complications after orthotopic liver transplantation: the role of endoscopy. World J Gastroenterol. 2008;14:493-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Gor NV, Levy RM, Ahn J, Kogan D, Dodson SF, Cohen SM. Biliary cast syndrome following liver transplantation: Predictive factors and clinical outcomes. Liver Transpl. 2008;14:1466-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Douzdjian V, Abecassis MM, Johlin FC. Sphincter of Oddi dysfunction following liver transplantation. Screening by bedside manometry and definitive manometric evaluation. Dig Dis Sci. 1994;39:253-256. [PubMed] |
| 56. | Clavien PA, Camargo CA, Baillie J, Fitz JG. Sphincter of Oddi dysfunction after liver transplantation. Dig Dis Sci. 1995;40:73-74. [PubMed] |
| 57. | Saleem A, Baron TH. Successful endoscopic treatment of biliary cast syndrome in an orthotopic liver transplant patient with a Roux-en-Y anastomosis via balloon enteroscopy. Liver Transpl. 2010;16:527-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Parsi MA, Guardino J, Vargo JJ. Peroral cholangioscopy-guided stricture therapy in living donor liver transplantation. Liver Transpl. 2009;15:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Navaneethan U, Venkatesh PG, Al Mohajer M, Gelrud A. Successful diagnosis and management of biliary cast syndrome in a liver transplant patient using single operator cholangioscopy. JOP. 2011;12:461-463. [PubMed] |
| 60. | Tsukui D, Yano T, Nakazawa K, Osawa H, Kawano Y, Mizuta K, Kawarasaki H, Yamamoto H. Rendezvous technique combining double-balloon endoscopy with percutaneous cholangioscopy is useful for the treatment of biliary anastomotic obstruction after liver transplantation (with video). Gastrointest Endosc. 2008;68:1013-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Petersen BT. Cholangioscopy for special applications: primary sclerosing cholangitis, liver transplant, and selective duct access. Gastrointest Endosc Clin N Am. 2009;19:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Gürakar A, Wright H, Camci C, Jaboour N. The application of SpyScope® technology in evaluation of pre and post liver transplant biliary problems. Turk J Gastroenterol. 2010;21:428-432. [PubMed] |
| 63. | Johnston TD, Gates R, Reddy KS, Nickl NJ, Ranjan D. Nonoperative management of bile leaks following liver transplantation. Clin Transplant. 2000;14:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Dumonceau JM, Tringali A, Blero D, Devière J, Laugiers R, Heresbach D, Costamagna G. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44:277-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 65. | Morelli J, Mulcahy HE, Willner IR, Cunningham JT, Draganov P. Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest Endosc. 2003;58:374-379. [PubMed] |
| 66. | Solmi L, Cariani G, Leo P, Miracolo A, Nigro G, Roda E. Results of endoscopic retrograde cholangiopancreatography in the treatment of biliary tract complications after orthotopic liver transplantation: our experience. Hepatogastroenterology. 2007;54:1004-1008. [PubMed] |
| 67. | Phillips MS, Bonatti H, Sauer BG, Smith L, Javaid M, Kahaleh M, Schmitt T. Elevated stricture rate following the use of fully covered self-expandable metal biliary stents for biliary leaks following liver transplantation. Endoscopy. 2011;43:512-517. [PubMed] |
| 68. | Zoepf T, Maldonado-Lopez EJ, Hilgard P, Malago M, Broelsch CE, Treichel U, Gerken G. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl. 2006;12:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 69. | Zoepf T, Maldonado de Dechêne EJ, Dechêne A, Malágo M, Beckebaum S, Paul A, Gerken G, Hilgard P. Optimized endoscopic treatment of ischemic-type biliary lesions after liver transplantation. Gastrointest Endosc. 2012;76:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Kulaksiz H, Weiss KH, Gotthardt D, Adler G, Stremmel W, Schaible A, Dogan A, Stiehl A, Sauer P. Is stenting necessary after balloon dilation of post-transplantation biliary strictures Results of a prospective comparative study. Endoscopy. 2008;40:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Costamagna G, Tringali A, Mutignani M, Perri V, Spada C, Pandolfi M, Galasso D. Endotherapy of postoperative biliary strictures with multiple stents: results after more than 10 years of follow-up. Gastrointest Endosc. 2010;72:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 72. | Kao D, Zepeda-Gomez S, Tandon P, Bain VG. Managing the post-liver transplantation anastomotic biliary stricture: multiple plastic versus metal stents: a systematic review. Gastrointest Endosc. 2013;77:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 73. | Tabibian JH, Asham EH, Han S, Saab S, Tong MJ, Goldstein L, Busuttil RW, Durazo FA. Endoscopic treatment of postorthotopic liver transplantation anastomotic biliary strictures with maximal stent therapy (with video). Gastrointest Endosc. 2010;71:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 74. | Morelli G, Fazel A, Judah J, Pan JJ, Forsmark C, Draganov P. Rapid-sequence endoscopic management of posttransplant anastomotic biliary strictures. Gastrointest Endosc. 2008;67:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Hu B, Gao DJ, Yu FH, Wang TT, Pan YM, Yang XM. Endoscopic stenting for post-transplant biliary stricture: usefulness of a novel removable covered metal stent. J Hepatobiliary Pancreat Sci. 2011;18:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Kahaleh M, Brijbassie A, Sethi A, Degaetani M, Poneros JM, Loren DE, Kowalski TE, Sejpal DV, Patel S, Rosenkranz L. Multicenter trial evaluating the use of covered self-expanding metal stents in benign biliary strictures: time to revisit our therapeutic options. J Clin Gastroenterol. 2013;47:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Traina M, Tarantino I, Barresi L, Volpes R, Gruttadauria S, Petridis I, Gridelli B. Efficacy and safety of fully covered self-expandable metallic stents in biliary complications after liver transplantation: a preliminary study. Liver Transpl. 2009;15:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | García-Pajares F, Sánchez-Antolín G, Pelayo SL, Gómez de la Cuesta S, Herranz Bachiller MT, Pérez-Miranda M, de La Serna C, Vallecillo Sande MA, Alcaide N, Llames RV. Covered metal stents for the treatment of biliary complications after orthotopic liver transplantation. Transplant Proc. 2010;42:2966-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Sauer P, Chahoud F, Gotthardt D, Stremmel W, Weiss KH, Büchler M, Schemmer P, Weitz J, Schaible A. Temporary placement of fully covered self-expandable metal stents in biliary complications after liver transplantation. Endoscopy. 2012;44:536-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 80. | Devière J, Nageshwar Reddy D, Püspök A, Ponchon T, Bruno MJ, Bourke MJ, Neuhaus H, Roy A, González-Huix Lladó F, Barkun AN. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology. 2014;147:385-395; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 81. | Kaffes A, Griffin S, Vaughan R, James M, Chua T, Tee H, Dinesen L, Corte C, Gill R. A randomized trial of a fully covered self-expandable metallic stent versus plastic stents in anastomotic biliary strictures after liver transplantation. Therap Adv Gastroenterol. 2014;7:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 82. | Chan CH, Donnellan F, Byrne MF, Coss A, Haque M, Wiesenger H, Scudamore CH, Steinbrecher UP, Weiss AA, Yoshida EM. Response to endoscopic therapy for biliary anastomotic strictures in deceased versus living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2013;12:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Hsieh TH, Mekeel KL, Crowell MD, Nguyen CC, Das A, Aqel BA, Carey EJ, Byrne TJ, Vargas HE, Douglas DD. Endoscopic treatment of anastomotic biliary strictures after living donor liver transplantation: outcomes after maximal stent therapy. Gastrointest Endosc. 2013;77:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 84. | Gómez CM, Dumonceau JM, Marcolongo M, de Santibañes E, Ciardullo M, Pekolj J, Palavecino M, Gadano A, Dávolos J. Endoscopic management of biliary complications after adult living-donor versus deceased-donor liver transplantation. Transplantation. 2009;88:1280-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 85. | Kim TH, Lee SK, Han JH, Park do H, Lee SS, Seo DW, Kim MH, Song GW, Ha TY, Kim KH. The role of endoscopic retrograde cholangiography for biliary stricture after adult living donor liver transplantation: technical aspect and outcome. Scand J Gastroenterol. 2011;46:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Chang JH, Lee IS, Choi JY, Yoon SK, Kim DG, You YK, Chun HJ, Lee DK, Choi MG, Chung IS. Biliary Stricture after Adult Right-Lobe Living-Donor Liver Transplantation with Duct-to-Duct Anastomosis: Long-Term Outcome and Its Related Factors after Endoscopic Treatment. Gut Liver. 2010;4:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 87. | Seo JK, Ryu JK, Lee SH, Park JK, Yang KY, Kim YT, Yoon YB, Lee HW, Yi NJ, Suh KS. Endoscopic treatment for biliary stricture after adult living donor liver transplantation. Liver Transpl. 2009;15:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Chok KS, Chan SC, Cheung TT, Sharr WW, Chan AC, Fan ST, Lo CM. A retrospective study on risk factors associated with failed endoscopic treatment of biliary anastomotic stricture after right-lobe living donor liver transplantation with duct-to-duct anastomosis. Ann Surg. 2014;259:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Chang JH, Lee IS, Chun HJ, Choi JY, Yoon SK, Kim DG, You YK, Choi MG, Choi KY, Chung IS. Usefulness of the rendezvous technique for biliary stricture after adult right-lobe living-donor liver transplantation with duct-to-duct anastomosis. Gut Liver. 2010;4:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 90. | Kyoden Y, Tamura S, Sugawara Y, Matsui Y, Togashi J, Kaneko J, Kokudo N, Makuuchi M. Incidence and management of biliary complications after adult-to-adult living donor liver transplantation. Clin Transplant. 2010;24:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Tsujino T, Isayama H, Sugawara Y, Sasaki T, Kogure H, Nakai Y, Yamamoto N, Sasahira N, Yamashiki N, Tada M. Endoscopic management of biliary complications after adult living donor liver transplantation. Am J Gastroenterol. 2006;101:2230-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 92. | Sanada Y, Mizuta K, Yano T, Hatanaka W, Okada N, Wakiya T, Umehara M, Egami S, Urahashi T, Hishikawa S. Double-balloon enteroscopy for bilioenteric anastomotic stricture after pediatric living donor liver transplantation. Transpl Int. 2011;24:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Sakakihara I, Kato H, Muro S, Noma Y, Yamamoto N, Harada R, Horiguchi S, Tsutsumi K, Okada H, Yamamoto K. Double-balloon enteroscopy for choledochojejunal anastomotic stenosis after hepato-biliary-pancreatic operation. Dig Endosc. 2015;27:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Srinivasaiah N, Reddy MS, Balupuri S, Talbot D, Jaques B, Manas D. Biliary cast syndrome: literature review and a single centre experience in liver transplant recipients. Hepatobiliary Pancreat Dis Int. 2008;7:300-303. [PubMed] |
| 95. | Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C, Testoni PA. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46:799-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (0)] |