Published online May 16, 2015. doi: 10.4253/wjge.v7.i5.510
Peer-review started: November 17, 2014
First decision: December 12, 2014
Revised: January 16, 2015
Accepted: February 9, 2015
Article in press: February 11, 2015
Published online: May 16, 2015
Processing time: 182 Days and 1.6 Hours
Peroral cholangioscopy (POC) is an important tool for the management of a selected group of biliary diseases. Because of its direct visualization, POC allows targeted diagnostic and therapeutic procedures. POC can be performed using a dedicated cholangioscope that is advanced through the accessory channel of a duodenoscope or via the insertion of a small-diameter endoscope directly into the bile duct. POC was first described in the 1970s, but the use of earlier generation devices was substantially limited by the cumbersome equipment setup and high repair costs. For nearly ten years, several technical improvements, including the single-operator system, high-quality images, the development of dedicated accessories and the increased size of the working channel, have led to increased diagnostic accuracy, thus assisting in the differentiation of benign and malignant intraductal lesions, targeting biopsies and the precise delineation of intraductal tumor spread before surgery. Furthermore, lithotripsy of difficult bile duct stones, ablative therapies for biliary malignancies and direct biliary drainage can be performed under POC control. Recent developments of new types of conventional POCs allow feasible, safe and effective procedures at reasonable costs. In the current review, we provide an updated overview of POC, focusing our attention on the main current clinical applications and on areas for future research.
Core tip: Peroral cholangioscopy is a rapidly developing endoscopic technique that provides the possibility to directly explore the bile duct, thereby increasing diagnostic accuracy in selected cases. Less expensive and safer than in the past, the field of applications of peroral cholangioscopy, through the development of new dedicated accessories, has been recently expanded and includes several therapeutic options such as the lithotripsy of difficult bile duct stones, ablative therapies for biliary malignancies and direct biliary drainage.
- Citation: Ghersi S, Fuccio L, Bassi M, Fabbri C, Cennamo V. Current status of peroral cholangioscopy in biliary tract diseases. World J Gastrointest Endosc 2015; 7(5): 510-517
- URL: https://www.wjgnet.com/1948-5190/full/v7/i5/510.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i5.510
Since the 1970s, the dream of all biliopancreatic endoscopists was the ability to directly explore the bilio-pancreatic tree. The first cholangioscopic mother-baby scope system appeared to realize this ambition; however, the technique was too rudimentary, cumbersome, labor intensive and time-consuming because the scopes were very fragile, and two highly skilled endoscopists were required to perform the procedure. Therefore, the effect on clinical practice was marginal and was strictly confined to the research field[1].
In 2005, the advent of new types of peroral cholangioscopes led to renewed interest in endoscopic visualization of the biliary tree. Several technical improvements were introduced, and the leading one was the single-operator system. Furthermore, the endoscopic image quality was progressively improved, and the size of the working channel increased. All these technical improvements have led to an increased diagnostic accuracy.
The aim of the current review is to provide an updated overview on peroral cholangioscopy, focusing our attention on the main current clinical applications and on areas for future research.
Currently, two different systems for the direct visualization of the biliary tree are available. The first one, the so-called indirect peroral cholangioscopy, is based on a catheter with an optical probe inside that is inserted within the duodenoscope. SpyGlass® (Boston Scientific, Natick, MA, United States) is the most frequently used and widely diffused probe; the second system is based on an ultraslim upper endoscope (direct peroral cholangioscopy).
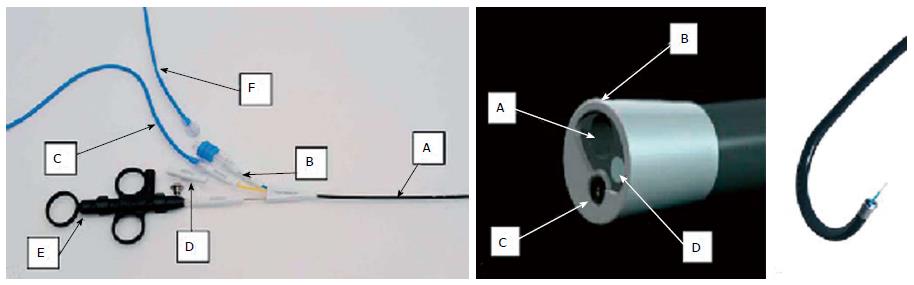
The SpyGlass® system is inserted through the instrument channel of the duodenoscope, and the previous placement of a guidewire into the biliary tree is generally recommended. The insertion of the cholangioscope into the bile duct is one of the most challenging aspects of the technique because it can damage the cholangioscope. Once inside, the SpyScope has two dials that allow for four-way tip deflection. The SpyScope has a 10 French outer diameter, is 230 cm in length, and houses four channels: a 1.2-mm instrument channel, two 0.6-mm independent air and irrigation channels, and the 0.9-mm channel used for the fiberoptic probe. This latter channel is a 6000 pixel, reusable probe with a camera in its distal portion that conducts light and acquires and transmits images. The quality of the endoscopic images can be adjusted by moving the probe forward and backward throughout the procedure. The working channel allows the passage of biopsy forceps (SpyBite®) and dedicated accessories, such as the Holmium laser, for the intraductal fragmentation of non-removable stones (Figure 1).
Similar to the SpyGlass scope is the Polyscope® (Polyscope system; Polydiagnost, Pfaffenhofen, Germany), which consists of a detachable flexible endoscope system available in 8 Fr (185 cm length) with separate optical, working/irrigation (1.2 mm), illumination, and steering channels (Figure 2). There are few differences between the two systems, as summarized in Table 1, but potentially the most important one lies in the image quality because the optical fiber has 10000 pixels of definition; however, the angle of view (70°) is the same as in the SpyGlass scope.
| Characteristics | Spyglass | Polyscope |
| Optics resolution | 6000 Pixel | 10000 Pixel |
| Working channel | 1.2 mm | 1.2 mm |
| Viewing angle | 70° | 70° |
| Outer diameter | 10 Fr | 8 Fr |
| Re-useable | Yes | Yes |
| Optical channel hermetically close | No | Yes (The optical fiber doesn't need to be sterilized; this prolongs its life cycle) |
| Steerability | 4 way | 1 way (With locking of the bending and rotating of the tip) |
| Compatibility with existing endoscopy tower | No (You have to buy a complete endoscopy tower system) | Yes (You can use, through adapters an existing endoscopy tower in the Hospital) |
Ultraslim endoscopes present larger outer diameters, generally 5-6 mm; therefore, they can be used only after a large endoscopic sphincterotomy and/or sphinteroplasty. The use of this system is definitely more challenging because of the significant difficulties that can be encountered in the initial insertion into the biliary tree as a result of the looping and in remaining anchored inside the duct. Therefore, a 0.025-0.035 inch diameter super-stiff guidewire previously placed within the intrahepatic duct is mandatory to introduce the scope into the acute angle of the biliary system from the second part of the duodenum. Once the duodenoscope is removed, the ultraslim endoscope is then advanced over the guidewire. Large loop development is common, particularly within the gastric fundus and the deep portion of the second part of the duodenal lumen. Hence, several accessories may be useful to successfully advance the ultraslim scope into the biliary tree. Recently, two techniques for an ultraslim endoscopic peroral cholangioscopy (POC) have been reported. The implementation of an intraductal 5 French balloon catheter that is inserted and fixed within a branch of the intrahepatic duct or proximally to a stricture has been proposed[2]. This technique facilitates the advancement of the ultraslim scope and the cannulation of the bile duct; however, it presents several drawbacks: the balloon must be withdrawn from the working channel of the scope for interventional procedures, and this maneuver can provide technical difficulties in maintaining the desired position; in addition, the balloon placement within the intraductal branches is not always easily reached. The implementation of the balloon doubles the success rate of direct peroral cholangioscopy from 45.5% with only the guide-wire in place to greater than 95% using a 5 F balloon catheter.
Notably, an anchoring balloon produced by Cook Medical (Winston-Salem, NC, United States) was removed from the market after a fatal complication because of an air embolus[3]. This development represents the most worrisome complication that can develop during a cholangioscopy; therefore, the use of CO2 rather than room air is mandatory.
An overtube balloon-assisted cholangioscopy has also been proposed with successful results[4-9]. This device is generally adapted from the overtube of either a single or double-balloon enteroscope; however, these overtubes are too large in diameter for an ultraslim scope, making the manipulation cumbersome[4,10].
An overtube allows the right position to be obtained by securing the endoscope and preventing loop formation during advancement; it also provides an easier access to the papilla. Several techniques have been described to achieve easier cannulation of CBD, including the inflation of the overtube balloon in the distal gastric antrum rather than in the duodenal bulb, which may or may not make a J-turn maneuver right in front of the papilla[11].
Twenty-five years ago, endoscopic retrograde cholangiopancreatography (ERCP) was the gold standard for the diagnosis of biliary diseases. Currently, that role has been almost completely replaced by other imaging modalities, including endoscopic ultrasound, magnetic resonance (MRI) and computed tomography (CT). However, the accuracy of these methodologies does not always allow for a definitive diagnosis. Therefore, the necessity of direct viewing and tissue sampling has always been claimed as a demanding goal in selected cases. Both direct and indirect cholangioscopy offer great advantages in terms of diagnostic and therapeutic options, as reported in the details in Table 2. Nevertheless, the main field of application of cholangioscopy is the work-up of indeterminate biliary strictures and, less frequently, the treatment of difficult bile duct stones.
| Diagnostic applications | Therapeutic applications | ||
| Common | Uncommon | Common | Uncommon |
| Indeterminate biliary strictures | Biliary cyst evaluation | Lithotripsy for choledocholithiasis | Biliary guidewire placement |
| Verification of bile duct stone clearance | Bile duct ischemia evaluation (post-liver-transplant) | Transpapillary gallbladder drainage | |
| Staging of cholangiocarcinoma | Ductal involvement in ampullary adenoma Hemobilia | Foreign body removal (e.g., stent) | |
Direct visualization of biliary strictures is one of the most interesting applications of cholangioscopy, and it allows the physician to improve the diagnosis to plan the most suitable treatment. Indeterminate biliary strictures, in which a diagnosis has not been reached after standard procedures have been performed (i.e., CT, RMN, or ERCP with brushing), is the initial and natural field of application of cholangioscopy. Currently, the visual criteria for malignancy are not fully standardized, and clinical experience interpreting cholangioscopic visual findings is still limited[12].
Criteria highly suggestive for malignancy include dilated and tortuous “tumor vessels” (also known as “capillary signs”), intraductal nodular or papillary masses, and oozing and irregular vascular patterns with an irregular surface. A benign condition should be considered when a smooth or fine granular surface structure without neovascularization or intraductal mass is observed[13,14]. The diagnostic accuracy of the “tumor vessel” sign for malignancy has been evaluated in 63 patients with indeterminate strictures, reporting a sensitivity of 61% and a specificity of 100% with excellent interobserver agreement (100%)[15].
A definitive diagnosis requires histological assessment. Several prospective trials have shown enthusiastic diagnostic accuracy results achieved with cholangioscopic-direct tissue sampling. Draganov et al[16] compared three sampling techniques during the ERCP: standard cytology brushing vs standard forceps biopsies vs SpyBite miniforceps biopsies. The authors enrolled 26 patients with biliary strictures, and the sample quality was adequate in 25 of 26 of the cytology brushings (96%), in 26 of 26 of the standard forceps biopsies (100%) and in 25 of 26 of the SpyBite miniforceps biopsies (96%). Three high-quality prospective trials showed a diagnostic accuracy of SpyBite forceps biopsy for indeterminate biliary lesions ranging from 72% to 85%, with a sensitivity of 49% to 82%, a specificity of 82% to 100%, a positive predictive value of 100% and a negative predictive value of 69% to 100% (Table 3)[12,16,17].
Although the high values of both the positive predictive value and specificity did not differ from those observed with traditional sampling techniques (i.e., brushing and standard forceps biopsies), the interesting finding was the high sensitivity and negative predictive value, likely because of the possibility of directly targeting the altered mucosa. Although the SpyBite miniforceps biopsy showed expected disappointing results for extrinsic lesions, with a sensitivity of only 8%, the sensitivity of the SpyGlass visual impression alone was less severely compromised (62%)[12].
Concerning extrinsic compression, the specificity is unavoidably reduced when direct visualization is solely used because it can be secondary to benign conditions, and in the case of several benign intraductal diseases, such as primary sclerosing cholangitis (PSC), it can present irregular biliary mucosa without harboring malignancy[18].
Nevertheless, it should be noted that in a prospective trial enrolling 53 patients with PSC and dominant stenosis, cholangioscopy, which was performed using a 9 Fr cholangioscope, was found to be significantly superior to ERCP for detecting malignancy in terms of its sensitivity (92% vs 66%), specificity (93% vs 51%), PPV (79% vs 29%) and NPV (97% vs 84%), respectively[19]. In patients with PSC, the main limitation is that the small diameter of their ducts frequently does not allow endoscope passage[19,20].
Image-enhanced cholangioscopy techniques have been proposed to improve diagnostic accuracy, particularly through new techniques that are currently being investigated, including chromocholangioscopy and narrow band imaging. Only limited experiences with chromocholangioscopy have been reported[21,22]. Hoffman et al[22] prospectively enrolled 55 patients who underwent chromoendoscopic cholangioscopy for biliary strictures or filling defects as a result of various etiologies (orthotopic liver transplantation, PSC, idiopathic). After the initial inspection of the bile duct, 15 mL of methylene blue (0.1%) was administered via the working channel of a Pentax “baby” cholangioscope, and the lesions were judged according to the macroscopic type and staining features. The authors identified characteristic surface and staining patterns in chronic inflammation, dysplasia and ischemic-type biliary lesions; in particular, they found that homogeneous staining predicted the presence of normal mucosa, the absence of staining predicted circumscribed lesions, and the diffused staining of such lesions represented neoplastic changes or inflammation. Unfortunately, these findings have not been confirmed by other studies, and their clinical usefulness remains limited.
Narrow band imaging (NBI) was developed by the Olympus medical system and is based on narrowing the bandwidth of spectral transmittance, resulting in optical color separation. In particular, the shorter band (415 nm) is thought to provide information regarding the capillary and pit patterns of the superficial mucosa, whereas the longer band (540 nm) provides more information regarding thicker capillaries in slightly deeper tissues. NBI is available on a few models of cholangioscopes. The literature concerning NBI application in cholangioscopy is limited to case reports and small case series[23-25].
Based on these preliminary experiences, it appears that the addition of NBI to the usual inspection with conventional white light cholangioscopies increases the ability to identify unknown strictures and might be helpful in differentiating benign from malignant strictures. Azeem et al[26] recently published the results of a prospective study conducted on a total of 30 patients with PSC using NBI and high-resolution peroral video cholangioscopy with NBI-directed biopsies of suspicious lesions. The goal was the early detection of cholangiocarcinoma and high-grade dysplasia and the identification of candidates for liver transplantation. Even if there was a 48% increase in suspicious lesions biopsied with NBI compared to white-light imaging, the NBI-directed biopsies did not improve the dysplasia detection rate. Additional experience is required to assess the exact role of NBI in detecting dysplasia.
Theoretically, systems with a higher image quality definition should allow a better identification of such alterations; however, comparative studies focusing on this issue have not been conducted. In 2012, we published a case-series describing the clinical usefulness of peroral cholangioscopy that implements a new type of cholangioscope, the Polyscope®, which enhances image quality as a result of the 10000 pixel definition[27]. Peroral cholangioscopy was performed in 12 patients with different indications: 4 patients with strictures that developed after orthotopic liver transplantation and were suspected of being ischemic biliary lesions; three patients in which the indication was indeterminate biliary strictures, three patients in which retained bile duct stones were suspected, and finally two cases in which a cholangioscopy was performed for evaluating the intraductal spread of adenomatous tissue after ampullectomy. All the peroral cholangioscopies were successful, no procedure-related morbidity was reported and a correct diagnosis was reached in all the patients.
The diagnosis of biliary stones is easily obtained using imaging techniques that are routinely available. However, these techniques are often insufficient because small stones can be missed and larger stones can block a duct, thus preventing the passage of contrast and avoiding detection during an ERCP. Indeed, it has been shown that previous ERCPs failed to correctly identify choledocholithiasis in 8%-16% of cases that were referred for a SpyGlass choledochoscopy[17,28]. In a study conducted in patients with primary sclerosing cholangitis, stones were not detectable in a cholangiography in approximately 30% of cases (7 out of 23 patients)[20]. In a multicenter study, stones were missed in 29% of cases that underwent an ERCP for different indications[12].
The most interesting feature of cholangioscopy is the possibility of fragmenting difficult-to-remove stones for which conventional techniques have failed. The “difficult stones” may result from several factors related to size, shape, texture or position. In these cases, intraductal electro-hydraulic (EHL) or laser lithotripsy (LL) under direct vision may be performed. Probes that pass through the accessory channels of cholangioscopies for EHL or LL are commercially available. These probes must be positioned close to the stones to increase effectiveness and reduce possible complications, thereby avoiding potentially dangerous shock waves delivered to the bile duct wall. Several studies have reported high success rates in clearing the bile ducts of stones after a cholangioscopic EHL or LL, ranging from 80% to 100%; these results are frequently achieved in only one session[12,29]. In the case of intrahepatic stones, the thinner LL probe is generally preferred to the EHL probe, whereas the EHL is the most widely used technique, particularly with the SpyGlass system, because of the dedicated irrigation channel providing the flowing water that is required to perform the EHL.
Several infrequent applications of cholangioscopy have been described, such as the study of cystic lesions of the biliary tree[30], the evaluation of ductal involvement in ampullary neoplasms[27], the diagnosis and treatment of cases of hemobilia as a result of rare causes[31,32], the identification of biliary varices in patients suffering from portal hypertension[33] and the use of different ablative therapies for intraductal tumor lesions, such as Nd-YAG laser photo-ablation, argon plasma coagulation or brachiotherapy for mucin-producing bile duct tumors[24]. Anecdotal cases of the cholangioscopy-assisted removal of stents that migrated proximally, targeted placements of guide-wires, transpapillary gallbladder drainage in cholecystitis and foreign body extractions have also been reported.
One interesting field of application of cholangioscopy is the evaluation of the biliary tract lesions in liver transplant patients or the treatment of liver complications after surgical resection or anti-tumoral therapies (i.e., transarterial chemoembolization, TACE). In a study of 20 liver transplant patients, direct cholangioscopy helped identify the biliary stricture etiologies, such as ischemia, scar tissue, intraductal clots and retained suture material, that were otherwise missed by the ERCP[34].
The usefulness of cholangioscopy in the management of complications after the anti-tumoral treatment of hepatocarcinoma has also been reported by our group. In 2011, we described a choledochoscope-assisted percutaneous fibrin glue sealing of a bile leak complicating a TACE of a nodule of hepatocellular carcinoma after conventional ERCP treatments had failed[35]. An inverse rendezvous procedure was successfully performed, allowing the insertion of the percutaneous wire-guided choledochoscope (Polyscope system) into the biloma and the injection of fibrin glue around the distal opening of the bile leak, allowing the direct closure of a fistula.
Peroral cholangioscopy is generally considered a safe procedure; however, cases of cholangitis, pancreatitis, bleeding and infection have been reported[15,36,37]. In particular, the most commonly reported complication is cholangitis, which is reported in up to 14% of cases[36]; hence, prophylactic antibiotics are mandatory. Chen et al[12] conducted a large, prospective, international, multicenter study using the SpyGlass system and reported an overall complication rate of 7.5% (17/226); the most frequent adverse event was early onset cholangitis. All the episodes were resolved without sequelae. Aspiration pneumonia is theoretically possible because of the large amount of normal saline generally used. Air embolism is a rare but fatal complication associated with direct peroral cholangioscopy[3]; therefore, CO2 insufflation is strongly recommended rather than room air.
Cholangiocarcinoma has one of the worst prognoses of virtually all tumors, with a 5-year survival rate lower than 5%[38]. The early diagnosis of biliary preneoplastic lesions could reasonably identify patients who are at an increased risk of developing cholangiocarcinoma. Biliary duct pathology may be evaluated by intraductal endoscopy, but feasibility studies are required to test the diagnostic accuracy of cholangioscopy in the identification of preneoplastic lesions of the extrahepatic biliary tree. The improvement of image quality and the development of dedicated accessories will further promote the diffusion of peroral cholangioscopy.
Endoscopic drainage can be performed by direct cholangioscopy using a 5-Fr catheter or stent inserted in the 2.0-mm working channel, particularly in patients who require “ultra” selective guidewire access, such as for the orifice of the cystic duct or major intrahepatic branches[39]. Additionally, proximally migrated biliary stents, which cannot be removed via conventional ERCP, can be removed using 5-F baskets or other accessories under direct visual cholangioscopic control[40].
Cholangioscopy have been reported for the evaluation of recurrent pancreatitis because of T-tube remnants in the cystic duct stump that were previously not detected via ERCP, CT or MRI[41]. Although there are no published data on the therapeutic applications of cholangioscopy for the resection of a biliary lesion, a biliary polypoid lesion could be removed using a 5-F snare.
The introduction of peroral cholangioscopy has constituted a turning point for biliary endoscopy. In particular, the single-operator systems are able to address a new, enthusiastic approach to biliary tract diseases, with great advantages in everyday practice. The greatest interest has centered on the evaluation of indeterminate stenosis, in which a diagnosis has not been reached after standard procedures; this is the main field of application of POC. The development of standardized criteria for the differential diagnosis between benign and malignant strictures is the main goal for the future. Prospective multicenter studies are required to define criteria with high intra- and inter-observer agreements and adequate diagnostic accuracy. Peroral cholangioscopy currently remains a challenging and expensive technique in expert hands. However, the renewed interest of researchers, clinicians and the medical device industries, and the substantial technological improvements in image quality and dedicated accessories, might contribute in the near future to the dissemination of this technique.
P- Reviewer: Pauli E, Syam AF, Zambudio N S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Nguyen NQ, Binmoeller KF, Shah JN. Cholangioscopy and pancreatoscopy (with videos). Gastrointest Endosc. 2009;70:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Moon JH, Ko BM, Choi HJ, Hong SJ, Cheon YK, Cho YD, Lee JS, Lee MS, Shim CS. Intraductal balloon-guided direct peroral cholangioscopy with an ultraslim upper endoscope (with videos). Gastrointest Endosc. 2009;70:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Efthymiou M, Raftopoulos S, Antonio Chirinos J, May GR. Air embolism complicated by left hemiparesis after direct cholangioscopy with an intraductal balloon anchoring system. Gastrointest Endosc. 2012;75:221-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Choi HJ, Moon JH, Ko BM, Hong SJ, Koo HC, Cheon YK, Cho YD, Lee JS, Lee MS, Shim CS. Overtube-balloon-assisted direct peroral cholangioscopy by using an ultra-slim upper endoscope (with videos). Gastrointest Endosc. 2009;69:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Itoi T, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Ikeuchi N, Moriyasu F, Kasuya K, Tsuchida A. Diagnostic and therapeutic peroral direct cholangioscopy in patients with altered GI anatomy (with videos). Gastrointest Endosc. 2012;75:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Takaoka M, Shimatani M, Ikeura T, Koyabu M, Kusuda T, Fukata N, Matsushita M, Okazaki K. Diagnostic and therapeutic procedure with a short double-balloon enteroscope and cholangioscopy in a patient with acute cholangitis due to hepatolithiasis. Gastrointest Endosc. 2009;70:1277-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Matsushita M, Shimatani M, Takaoka M, Okazaki K. Effective peroral direct cholangioscopy with an ultraslim gastroscope in combination with a short double-balloon enteroscope for altered GI anatomy. Gastrointest Endosc. 2012;76:1075; author reply 1075-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Matsushita M, Shimatani M, Ikeura T, Takaoka M, Okazaki K. Peroral direct cholangioscopy with an ultraslim gastroscope in combination with a short double-balloon enteroscope for reconstructed biliary anatomy. Endoscopy. 2011;43:1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Matsushita M, Shimatani M, Takaoka M, Okazaki K. Effective device for peroral direct cholangioscopy: double-balloon enteroscope or ultra-slim gastroscope? Endoscopy. 2009;41:730; author reply 731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Waxman I, Dillon T, Chmura K, Wardrip C, Chennat J, Konda V. Feasibility of a novel system for intraductal balloon-anchored direct peroral cholangioscopy and endotherapy with an ultraslim endoscope (with videos). Gastrointest Endosc. 2010;72:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Tsou YK, Lin CH, Tang JH, Liu NJ, Cheng CL. Direct peroral cholangioscopy using an ultraslim endoscope and overtube balloon-assisted technique: a case series. Endoscopy. 2010;42:681-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Chen YK, Parsi MA, Binmoeller KF, Hawes RH, Pleskow DK, Slivka A, Haluszka O, Petersen BT, Sherman S, Devière J. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos). Gastrointest Endosc. 2011;74:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Fukuda Y, Tsuyuguchi T, Sakai Y, Tsuchiya S, Saisyo H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest Endosc. 2005;62:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000;52:635-638. [PubMed] |
| 16. | Draganov PV, Chauhan S, Wagh MS, Gupte AR, Lin T, Hou W, Forsmark CE. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. 2012;75:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Darisetty S, Sekaran A, Rao GV. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc. 2011;74:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc. 2007;65:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 285] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Tischendorf JJ, Krüger M, Trautwein C, Duckstein N, Schneider A, Manns MP, Meier PN. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy. 2006;38:665-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Awadallah NS, Chen YK, Piraka C, Antillon MR, Shah RJ. Is there a role for cholangioscopy in patients with primary sclerosing cholangitis? Am J Gastroenterol. 2006;101:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Hoffman A, Kiesslich R, Moench C, Bittinger F, Otto G, Galle PR, Neurath MF. Methylene blue-aided cholangioscopy unravels the endoscopic features of ischemic-type biliary lesions after liver transplantation. Gastrointest Endosc. 2007;66:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Hoffman A, Kiesslich R, Bittinger F, Galle PR, Neurath MF. Methylene blue-aided cholangioscopy in patients with biliary strictures: feasibility and outcome analysis. Endoscopy. 2008;40:563-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Moriyasu F, Gotoda T. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos). Gastrointest Endosc. 2007;66:730-736. [PubMed] |
| 24. | Lu XL, Itoi T, Kubota K. Cholangioscopy by using narrow-band imaging and transpapillary radiotherapy for mucin-producing bile duct tumor. Clin Gastroenterol Hepatol. 2009;7:e34-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Brauer BC, Fukami N, Chen YK. Direct cholangioscopy with narrow-band imaging, chromoendoscopy, and argon plasma coagulation of intraductal papillary mucinous neoplasm of the bile duct (with videos). Gastrointest Endosc. 2008;67:574-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Azeem N, Gostout CJ, Knipschield M, Baron TH. Cholangioscopy with narrow-band imaging in patients with primary sclerosing cholangitis undergoing ERCP. Gastrointest Endosc. 2014;79:773-779.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Cennamo V, Luigiano C, Fabbri C, Maimone A, Bazzoli F, Ceroni L, Morace C, Jovine E. Cholangioscopy using a new type of cholangioscope for the diagnosis of biliary tract disease: a case series. Endoscopy. 2012;44:878-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Fishman DS, Tarnasky PR, Patel SN, Raijman I. Management of pancreaticobiliary disease using a new intra-ductal endoscope: the Texas experience. World J Gastroenterol. 2009;15:1353-1358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Piraka C, Shah RJ, Awadallah NS, Langer DA, Chen YK. Transpapillary cholangioscopy-directed lithotripsy in patients with difficult bile duct stones. Clin Gastroenterol Hepatol. 2007;5:1333-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Warmann S, Meier PN, Kardorff R, Fuchs J. Cystic echinococcosis with perforation into the biliary tract in an eight-year-old girl. Eur J Pediatr Surg. 2002;12:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Hayashi S, Baba Y, Ueno K, Nakajo M. Small arteriovenous malformation of the common bile duct causing hemobilia in a patient with hereditary hemorrhagic telangiectasia. Cardiovasc Intervent Radiol. 2008;31 Suppl 2:S131-S134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Prasad GA, Abraham SC, Baron TH, Topazian MD. Hemobilia caused by cytomegalovirus cholangiopathy. Am J Gastroenterol. 2005;100:2592-2595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Albert JG, Friedrich-Rust M, Elhendawy M, Trojan J, Zeuzem S, Sarrazin C. Peroral cholangioscopy for diagnosis and therapy of biliary tract disease using an ultra-slim gastroscope. Endoscopy. 2011;43:1004-1009. [PubMed] |
| 34. | Siddique I, Galati J, Ankoma-Sey V, Wood RP, Ozaki C, Monsour H, Raijman I. The role of choledochoscopy in the diagnosis and management of biliary tract diseases. Gastrointest Endosc. 1999;50:67-73. [PubMed] |
| 35. | Cennamo V, Fuccio L, Giampalma E, Terzi E, Eusebi LH, Mosconi C, Piscaglia F. Choledochoscope-assisted percutaneous fibrin glue sealing of bile leak complicating transarterial chemoembolization of hepatocellular carcinoma after liver transplantation. Endoscopy. 2011;43 Suppl 2 UCTN:E238-E239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Sethi A, Chen YK, Austin GL, Brown WR, Brauer BC, Fukami NN, Khan AH, Shah RJ. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: a single-center experience. Gastrointest Endosc. 2011;73:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Hammerle CW, Haider S, Chung M, Pandey A, Smith I, Kahaleh M, Sauer BG. Endoscopic retrograde cholangiopancreatography complications in the era of cholangioscopy: is there an increased risk? Dig Liver Dis. 2012;44:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | McLean L, Patel T. Racial and ethnic variations in the epidemiology of intrahepatic cholangiocarcinoma in the United States. Liver Int. 2006;26:1047-1053. [PubMed] |
| 39. | Moon JH, Terheggen G, Choi HJ, Neuhaus H. Peroral cholangioscopy: diagnostic and therapeutic applications. Gastroenterology. 2013;144:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Lee YN, Moon JH, Choi HJ, Min SK, Kim HI, Lee TH, Cho YD, Park SH, Kim SJ. Direct peroral cholangioscopy using an ultraslim upper endoscope for management of residual stones after mechanical lithotripsy for retained common bile duct stones. Endoscopy. 2012;44:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Parsi MA, Sanaka MR, Dumot JA. Iatrogenic recurrent pancreatitis. Pancreatology. 2007;7:539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |