Published online May 16, 2015. doi: 10.4253/wjge.v7.i5.460
Peer-review started: October 21, 2014
First decision: November 27, 2014
Revised: January 8, 2015
Accepted: January 30, 2015
Article in press: February 2, 2015
Published online: May 16, 2015
Processing time: 211 Days and 19.5 Hours
Advances in stents design have led to a substantial increase in the use of stents for a variety of digestive diseases. Initially developed as a non-surgical treatment for palliation of esophageal cancer, the stents now have an emerging role in the management of malignant and benign conditions as well as in all segments of the gastrointestinal tract. In this review, relevant literature search and expert opinions have been used to evaluate the key-role of stenting in gastrointestinal benign and malignant diseases.
Core tip: Endoscopic stenting plays an indispensable role in the treatment of benign and malignant digestive strictures and leaks. In this review, we summarize data from randomized clinical trials or prospective studies together with meta-analytical data, when applicable; to present the most updated recommendations in stenting of gastrointestinal diseases.
- Citation: Mangiavillano B, Pagano N, Arena M, Miraglia S, Consolo P, Iabichino G, Virgilio C, Luigiano C. Role of stenting in gastrointestinal benign and malignant diseases. World J Gastrointest Endosc 2015; 7(5): 460-480
- URL: https://www.wjgnet.com/1948-5190/full/v7/i5/460.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i5.460
Stenting has become an optimal option for the treatment of a variety of gastrointestinal malignant and benign diseases, which plays a vital role in alleviating obstructive symptoms such as dysphagia, pain, and improving patients’ quality of life.
Over the past 30 years, dramatic changes have occurred in the composition and design of stents and their application to digestive disorders.
For example, stent composition began with plastic, evolved into self-expandable metal stents (SEMSs) and may soon evolve into biodegradable stents. At the same time, indications for stenting that began with esophageal cancer now include benign and malignant disorders involving a variety of sites in the gastrointestinal tract.
This paper will outline the indications and outcomes of stenting, the techniques of placement, composition and design of stents and prospects for new and improved stents.
A stent is a cylindrical medical device used to widen a narrow or stenosed lumen in order to maintain the patency of the lumen. The first stents were made of hard plastic and were used for obstructive esophageal cancers. Whereas early stents were mostly composed of plastic, the majority of contemporary stents are metal stents that are composed of either nitinol or stainless steel.
Nitinol mesh has improved the quality of the stents, replacing the other materials. This nickel-titanium shape-memory alloy is soft and flexible, with smoother wire ends, reducing the risk in and overgrowth.
Metal stents are available as uncovered, partially covered (PC), or fully covered (FC). An uncovered SEMS consists of a mesh that is bare and expands into the stenosis. A FCSEMS consists of a mesh stent that is covered by a membrane throughout its length. A PCSEMS consists of a stent with a membrane covering and uncovered proximal and distal ends of the stent.
Recently, FC self expanding plastic stent (SEPS) and biodegradable stent was developed. SEPS is made of woven plastic strands, while biodegradable stent is made from commercially available polydioxanone absorbable surgical suture material. Polydioxanone is a semicrystalline, biodegradable polymer. It degrades by hydrolysis of its molecule ester bonds, which is accelerated by low pH. The amorphous regions of the matrix deteriorate first and the crystalline portion deteriorates later.
Most of the metal stents on the market are mounted on a delivery system that consists of two coaxial tubes, but there is also a type of metal stent mounted on a delivery system with a user-friendly braided-suture release mechanism and it is deployed by pulling a ring attached to the suture string, thereby unraveling the string and slowly releasing the stent (Ultraflex esophageal or colonic stents, Boston Scientific/Microvasive, Natick, Massachusetts).
There are 2 types of delivery systems: through the scope (TTS), able to pass through the operative channel of the endoscope, and over the wire (OTW) that does not pass through the operative channel of the endoscope. The main differences between the delivery systems are the design of the handles, the lengths and the diameter, which determines the means of deployment.
Although the majority of deployment systems release the stent initially at the distal end of the catheter, there are some types of gastrointestinal stents available in both a proximal and distal release system (i.e., Ultraflex esophageal stent NG/Boston Scientific and the esophageal Nit-S®/TaeWoong). In contrast to most SEMSs, which are sold in a constrained packing, the SEPS requires mounting onto the delivery catheter just before use. One important aspect of deployment is the variable degree of foreshortening that occurs with a majority of SEMSs and SEPS during the transition from the compressed to fully expanded state. The endoscopist must anticipate and allow for this foreshortening to ensure appropriate placement.
Before stenting firstly the lesion should be endoscopically or radiologically evaluated, the proximal and distal aspects of the lesion identified and a guide-wire advanced through the lesion, and the stent positioned across and then deployed under fluoroscopic and/or endoscopic guidance by release of the constraining mechanism.
| Producer | Model | Material | Diameter mm (body/flare) | Length (cm) | Type and characteristics |
| Merit Endotek | Alimaxx-E® | Nitinol | 18/22 | 7-10-12 | Fully-covered with anti-migration system |
| Merit Endotek | EndoMAXX® | Nitinol | 19/24-23/28 | 7-10-12-15 | Fully-covered with anti-migration system |
| Boston Scientific | Ultraflex® | Nitinol | 18/23-23/28 | 10-12-15 | Uncovered and partially-covered Possibility of distal or proximal release |
| Boston Scientific | Flamingo Wallstent® | Stainless steel | 20/30 | 12-14 | Partially-covered |
| Boston Scientific | Wallflex® | Nitinol | 18/23-23/28 | 10-12-15 | Partially and fully-covered |
| Boston Sientific | Polyflex® | Polyester | 16/20-18/23 | 9-12-15 | Plastic stent |
| Cook Endoscopy | Evolution® | Nitinol | 20/25 | 8-10-12.5-15 | Partially and fully-covered |
| Ella-CS | SX-ELLA® HV | Nitinol | 20/25 | 8.5-11-13.5-15 | Fully-covered with collar anti-migration system |
| Ella-CS | SX-ELLA® Flexella | Nitinol | 20/25 | 8.5-11-13.5-15 | Fully-covered Possibility of distal or proximal release Possibility of anti-reflux valve |
| Ella-CS | FerX-ELLA® Boubella | Stainless steel | 20/25 | 9-10.5-12-13.5-15- 16.5-19.5-21 | Fully-covered Possibility of anti-reflux valve |
| Ella-CS | SX-ELLA® Danis | Nitinol | 25/30 | 13.5 | Fully-covered (with balloon/specific for variceal bleeding) |
| Ella-CS | SX-ELLA® Danis Seal | Nitinol | 25/30 | 13.5 | Fully-covered (specific for leaks) |
| Ella-CS | Ella BD stent® | Biodegradable polymer | 18/23-20/25-23/28-25/31 | 6-8-10-13.5 | - |
| Endochoice | Bonastent® ER | Nitinol | 18/24 | 6-8-10-12-14-16 | Fully-covered Possibility of anti-reflux valve |
| M.I. Tech | Hanarostent® | Nitinol | 18/24-20/26-22/28 | 8-9-10-11-12- 14-15-16-17 | Partially and fully-covered Possibility of double covered configuration, anti-reflux valve and asymmetrical configuration |
| M.I. Tech | Hanarostent® ECBB | Nitinol | 36-30-20-26 | 18-21-24 | Fully-covered (Bariatric surgery) |
| Micro-Tech | MT® Esophageal stent | Nitinol | (Diameter central/ extremities) 18/24-20/26-22/28 | 8-10-12 | Uncovered, partially and fully covered Possibility of anti-reflux valve and radioactive system |
| Micro-Tech | MT® Cardia stent | Nitinol | 16/22-18/24-20/26-22/28-24/30 | 9-10-11-12-13 | Partially and fully-covered |
| Micro-Tech | MT® Retrievable stent | Nitinol | 14/20-16/22-18/24-20/26-22/28-24/30 | 7-8-9-10-11-12 | Fully-covered |
| TaeWoong Medical | Beta-Stent Niti-S® | Nitinol | 18/24-20/26-22/28 | 10-12-14-15-16-18-20 | Fully-covered (Fistula after bariatric surgery) |
| TaeWoong Medical | Mega-Stent Niti-S® | Nitinol | 18/24-20/26-22/28 | 10-12-14-15-16-18-20 | Fully-covered (Stricures or fistula after sleeve gastrectomy) |
| TaeWoong Medical | Niti-S Conio® | Nitinol | 10/12-12/14-14/16 | 6-8-10-12-14-15 | Fully-covered (Hypopharyngeal strictures) |
| TaeWoong Medical | Niti-S Cervical® | Nitinol | 16/18-18/20 | 6-8-10-12-14-15 | Fully-covered (Upper esophageal strictures) Possibility of distal or proximal release |
| TaeWoong Medical | Niti-S® | Nitinol | 16/24-18/26-20/28 | 6-8-10-12-14-15 | Partially and fully-covered Possibility of distal or proximal release for over-the-wire stent Possibility of TTS 10.5 Fr delivery system |
| TaeWoong Medical | Niti-S® double layer | Nitinol | 16/24-18/26-20/28 | 6-8-10-12-14-15 | Fully-covered with additional uncovered nitinol mesh Possibility of distal or proximal release Possibility of anti-reflux valve |
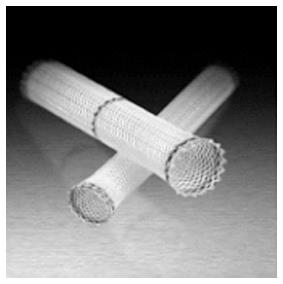
The SEPS Polyflex® (Boston Scientific) is a stent of polyester braid completely covered in silicone membrane. The stent need to be loaded prior to insertion into a large diameter delivery device (36-42 Fr) and is available in different sizes (diameters of 16, 18, and 21 mm and lengths of 9, 12 or 15 cm). Are available with proximal flare diameters of 20, 23 and 25 mm, the proximal end is flared for preventing distal migration with radio-opaque markers at the ends and in the middle for a more precise placement (Figure 1).
The most common used esophageal FC-SEMSs are: the Wallflex® (Boston Scientific), Niti-S® (TaeWoong), Evolution® (Cook Medical), Alimaxx-E® (Alveolus, Charlotte, NC, United States), SX-ELLA® stent Esophageal HV (Ella-CS, Hradec Kralove, Czech Republic) and the Hanaro® (M.I. Tech stent) and most of these stents are also available PC.
All these stents are made in nitinol and released OTW, the Alimaxx, Wallflex, Hanaro, SX-ELLA® and Evolution have a distal release, while Niti-S has both distal and proximal release.
The Alimaxx, Niti-S, Hanaro and Evolution are covered in silicone, while the Wallflex stent has the covering made of Permalume®, a particular type of silicone that diminish the food impaction. Actually the only FCSEMS with a possibility of a delivery-system TTS is the Niti-S.
The Wallflex, Alimaxx, Niti-S and Hanaro presents the same OTW release mechanism (the delivery system consists of a coaxial tubing assembly that constrains the stent on the delivery catheter shaft until the stent is released), an innovation, allowing a best control of the release, was recently projected by Cook Medical for the Evolution. The delivery system is composed by a “plastic handgun”. With one hand and a squeeze of the trigger, the handle gives a precise control over stent deployment and recapturability. To shift between stent release and recapture, it needs switch the “directional button”. There is furthermore a “point-of-no-return” reference mark that alerts when stent recapture is no longer possible.
The Ultraflex (Boston Scientific/Microvasive, Natick, Massachusetts) esophageal stent is another type of prosthesis that has a flared proximal end (23 or 28 mm), available uncovered and PC (the PCSEMS version have 1.5 cm of bare nitinol configured into wire loops at each end, thus sharp elements are absent) and is marketed with both a proximal and distal release design. This stent is highly flexible and is mounted on a delivery system with a user-friendly braided-suture release mechanism, deployed by pulling a ring attached to the suture string, thereby unraveling the string and slowly releasing the stent.
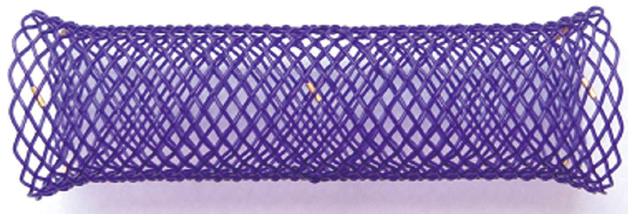
The Taewoong Medical produce specifically designed Niti-S stents. The Niti-S Conio Stent (Taewoong Medical, Seoul, South Korea) is a stent for hypopharyngeal stenosis. The stent have a body with a diameter of 12, 14 or 16 mm, and only the proximal crown has a diameter greater than the body of only 2 mm (14, 16 and 18 mm), to prevent distal migration and reduce the feeling foreign body (Figure 2). The Niti-S Beta stent (Taewoong Medical, Seoul, South Korea) is a newly design OTW stent with distal release with two rings in the body, for the anti-migration mechanism, produced for the treatment of complications of bariatric surgery. The biodegradable implant (ELLA BD stent) (Ella®-CS, Hradec Kralove, Czech Republic) is composed of polydioxanone which is a semi-crystalline polymer biodegradable (Figure 3).
The OTW delivery system has a diameter of 9 mm, the diameter of the stent is 25 mm, while the length varies between 6 and 13.5 cm. After the release in the esophageal lumen, the complete expansion of the stent occurs in 24-48 h. The degradation of the implant begins after 4 wk and the ninth week the radial force (RF) has been reduced by half, therefore the stent does not have to be removed.
The best way to release an esophageal stent is the combined endoscopic and fluoroscopic approach, expecially in presence of a leak, despite some expert endoscopist, only in selected cases, place the stent only with endoscopic control.
| Producer | Model | Material | Diameter (mm) (body/flare) | Length (cm) | Type and characteristics |
| Boston Scientific | Wallstent® | Elgiloy | 20/22 | 6-9 | Uncovered |
| Boston Scientific | Wallflex® | Nitinol | 22/27 | 6-9-12 | Uncovered |
| Cook Endoscopy | Evolution® | Nitinol | 22/27 | 6-9-12 | Uncovered |
| Ella-CS | SX-ELLA® Eneterella | Nitinol | 20-22-25 (no flare) | 8.2-9-11.3-13.5 | Uncovered |
| Endochoice | Bonastent® P | Nitinol | 20 | 6-8-10 | Uncovered |
| M.I. Tech | Hanarostent® | Nitinol | 20/25-20/26 | 8-9-11-14 | Uncovered and partially-covered |
| Micro-Tech | MT® Duodenal stent | Nitinol | 20/26 | 6-8-10 | Uncovered, partially and fully-covered |
| TaeWoong Medical | Niti-S® D-type unflared | Nitinol | 18-20-22-24 | 6-8-10-12-14-15 | Uncovered |
| TaeWoong Medical | Niti-S® S-type flared | Nitinol | 18/26-20/28- 22/30-24/32- 26/34-28/36 | 6-8-10-12-14-15-16 | Fully-covered |
| TaeWoong Medical | Niti-S® Comvi unflared | Nitinol | 18-20-22 | 6-8-10-12 | Partially-covered |
Different types of enteral stents are actually in use, all with a delivery system TTS (10 Fr) which needs a working scope channel of 3.8 mm.
All the commercialized stent are made in Nitinol, except for the Enteral Wallstent, known as a stainless steel stent, made with a mix of materials called Elgiloy® (Eligoy Inc., Elgin, IL, United States) (cobalt, chromium, nickel, iron, molybdenum, manganese). The Enteral Wallstent is characterized of an excellent RF but with the tendency of straightening, increasing the risk of stent impaction in the angulated sites.
An important role in the gastro-duodenal obstruction is played by two relevant features of the stents: the RF and the axial force (AF).
RF is the expanding force. AF is a force that maintains the stent straight after its placement. Combination of the two forces is more effective than only RF or AF, respectively. The AF straightens the stent, and plays a fundamental role in covered stents. The nature of the nitinol confers to these stents an optimal AF and RF.
Almost all of the TTS SEMS allow re-sheathing of the stent and TTS delivery systems also necessitate a kinking-resistant guide-wire. Delivery systems are available in different lengths (135, 180 and 230 cm). An important characteristic of duodenal stents is the diameter. For obtaining an adequate food transit has to be used stents with a diameter > 20 mm. Some stents have distal flared extremity to improve the anchorage and can be covered and uncovered. The choice of a covered vs an uncovered stent depends by the endoscopist, evaluating fatures and site of the lesion. Uncovered SEMS are generally used, in this site, because of the low risk of migration. The flexibility of the uncovered stents allows following duodenal angulations, despite could exercitate high pressure on the angulated strictures. The mesh pressure on the mucosa induces epithelial regeneration, that leads to ingrowth, can contribute to stent occlusion. Then, the placement of a covered-SEMS is preferable in non-surgical patients, or patients with a high risk of mortality and morbidity, with a life expectance > 2-3 mo. Covered stent are generally indicated in the treatment of the tissue ingrowth inside an uncovered stent.
Materials used for covered stent are polyurethane, silicone, and expanded polytetrafluoroethylene. Covered SEMS are conceived to prevent tumor ingrowth and for closing fistula, if present; the only disadvantage of these stent is the tendency to migrate.
Two stents are currently marketed for the closure of fistulas post-sleeve gastrectomy, both over-the-wire (OTW): the Beta-stent® (Niti-S - TaeWoong), with lengths of 15, 18 and 23 cm and a diameter of 24 and 28 mm, and the Hanaro® stent (M.I. Tech) with lengths of 18, 21 and 24 cm and a width of 30 mm diameter. Both the two stents must be placed under fluoroscopic vision, after placing a stiff guide-wire in the duodenum, and present the proximal tourniquet on the crown for removal.
Duodenal stricture evaluation with X-ray enema before the endoscopy is generally not required. The passage of orally administered water soluble contrast through the duodenal stricture is generally delayed because of the gastrectasia, presence of residual food and delayed stomach emptying.
Furthermore, assessment of concomitant proximal jejunal strictures is not satisfactory due to the small amount of contrast that can pass the duodenal stricture. For these reasons stricture assessment during duodenal stenting procedure is preferred. Computed tomography is helpful to exclude the presence of peritoneal carcinosis.
To avoid aspiration, insertion of a naso-gastric tube 24 h before the procedure to empty the stomach, and the prone position during the procedure, are recommended.
Commercially available TTS SEMS require an operative endoscope (3.8 mm diameter working channel). Operative duodenoscopes offer a better visualization of the duodenal stricture lumen, the elevator helps the orientation of the catheter, grips of the guidewire, and the delivery system. Duodenoscopes are also useful for the treatment of a concomitant biliary stricture.
Duodenal stenting placement is performed under fluoroscopic guidance because both endoscopic and radiological controls are preferable. Stricture study is performed by contrast injection above the stenosis to assess diameter and length of the stricture; when the duodenal stricture is passed with an ERCP catheter, with or without a wire, contrast is injected downstream for evaluating the patency of the GI lumen, distally to the stenosis. Another accessory, that could be useful in the angulated stricture, is the sphincterotome. Balloon dilation before stent placement is not necessary (it increases the risk of perforation), and a stiff or super-stiff guide-wire is generally preferred. When the guide-wire is correctly in place distal to the stricture the stent catheter is advanced OTW. It is important the choice of a stent few centimeters longer than the stricture to be sure of a correct stenting of the stricture. If possible, the stricture can be measured by a centimeter guide-wire. The presence of a possible angulation of the bowel, immediately after the stricture, has to be considered. In this case the length of the stent should be chosen to avoid stent impaction on the gut wall. In presence of short stenosis of the upper duodenal genu, it is a 6 cm length stents, with the proximal extremity deployed through the pylorus, has to be chosen, avoiding covering the papilla for a possible further ERCP. The majority of the delivery stent systems allow re-sheathing of a partially deployed stent, permitting further adjustments, before the release, if the position is not correct. If the stent is accidentally released beneath the stricture, it should be immediately replaced with a tooth-rat forceps. If the placed stent is shorter than the stricture, a second stent can be released, with the proximal part inside the first.
The correct position of the stent has to be radiologically documented immediately after the deployment, injecting contrast inside the stent. Completely stent expansion is generally obtained at 48 h.
| Producer | Model | Material | Delivery system and diameter | Diameters (mm) (body/flares- flanges) | Length (cm) | Type and characteristics |
| Boston Scientific | WallFlex® | Nitinol | TTS, 10 Fr | 25 (body)-30 (proximal flange) 22 (body)-27 (proximal flange) | 6, 9, 12 | Uncovered |
| Boston Scientific | Ultraflex precision® | Nitinol | OTW, 16 Fr | 25 (body)-30 (proximal flange) | 5.7, 8.7, 11.7 | Uncovered |
| Boston Scientific | Wallstent® | Stainless steel | TTS, 10 Fr | 20 (22/minimal to no flare) | 6, 9, 12 | Uncovered |
| Cook Endoscopy | Evolution® | Nitinol | TTS, 10 Fr | 25 (body) 30 (both ends flanged) | 6, 8, 10 | Uncovered |
| EndoChoice | BONASTENT® | Nitinol | TTS, 10 Fr and 12 Fr | 22, 24, 26 (minimal flare) | 6, 8, 10 | Uncovered and partially covered |
| Ella-CS | SX-ELLA® Enterella | Nitinol | TTS, 10 Fr | 22, 25 (no flare) | 7.5, 8, 9, 11, 13.5 | Uncovered and fully covered |
| Ella-CS | SX-ELLA® Enterella | Nitinol | OTW, 15 Fr and 18Fr | 22, 25, 30 (no flare) | 8.2, 9, 11.3, 13.5 | Uncovered and fully covered |
| Endochoice | Bonastent® C | Nitinol | TTS, 10 Fr | 22, 24, 26 | 6-8-10 | Uncovered and fully covered |
| Leufen Medizintechnik | Aixstent® | Nitinol | OTW, 24 Fr | 30 (body)-36 (both ends flared) | 8, 10 | Uncovered and partially covered |
| Micro-Tech | MT® Colon and rectum stent | Nitinol | TTS, 10 Fr | 25 (body)-30 (both ends flanged) | 8, 10 | Uncovered |
| Micro-Tech | MT® Colon and rectum stent | Nitinol | OTW, 24 Fr | 30 (body)-36 (both ends flanged) | 8, 10, 12 | Uncovered and partially covered |
| Micro-Tech | MT® Rectum stent | Nitinol | OTW, 24 Fr | 20, 26, 30 (body)-24, 21, 36 (both ends flanged) | 6 | Fully covered |
| M.I.Tech | Hanarostent® | Nitinol | TTS, 10.2 Fr and 10.5 Fr | 20, 22, 24 (body)-26, 28, 30 (both ends flared) (flanged and symmetric and asymmetric) | 6-16 | Uncovered and fully covered |
| M.I.Tech | Hanarostent® | Nitinol | OTW, 24 Fr | 20, 22, 24 (body)-26, 28, 30 (both ends flared) (flanged and symmetric and asymmetric) | 6-16 | Uncovered and fully covered |
| M.I.Tech | Choostent® | Nitinol | OTW, 24 Fr | 22, 24 (body)-30, 32 (both ends flanged) (symmetric and asymmetric) | 6-16 | Fully covered |
| S&G Biotech | EGIS® colorectal | Nitinol | TTS, 10 Fr and 12 Fr | 18, 20, 22, 24, 26, 28, 30 (no flare) | 6, 8, 10, 12 | Uncovered and partially covered |
| Taewoong Medical | Niti-S® D-Type | Nitinol | TTS, 10.5 Fr | 18, 20, 22, 24 (no flare) | 6, 8, 10, 12, 14, 15 | Uncovered |
| Taewoong Medical | Niti-S® D-Type | Nitinol | OTW, 16 Fr and 18 Fr | 18, 20, 22, 24, 26, 28, 30 (no flare) | 6, 8, 10, 12 | Uncovered |
| Taewoong Medical | Niti-S® S-Type | Nitinol | TTS, 10.5 Fr | 18, 20 (body)-24, 28 (both ends flanged) | 6, 8, 10, 12 | Fully covered |
| Taewoong Medical | Niti-S® S -Type | Nitinol | OTW, 16 Fr, 20 Fr and 22 Fr | 18, 20, 22, 24, 26, 28 (body) 24, 26, 28, 30, 32, 34 (both ends flanged) | 6, 8, 10, 12, 14, 15 | Fully covered |
| Taewoong Medical | ComVi Niti-S® | Nitinol | TTS, 10.5 Fr | 18, 20, 22 (no flare) | 6, 8, 10 | Partially covered |
| Taewoong Medical | ComVi Niti-S® | Nitinol | OTW, 14 Fr, 16 Fr and 18 Fr | 18, 20, 22, 24, 26, 28, 30 (no flare) | 6, 8, 10 | Partially covered |
Over the years have been progressively introduced various types of stent. Material was initially steel (Z-stent® Cook Medical) or Elgiloy (Wallstent®, Boston Scientific) and subsequently the nitinol. The stents actually available are all in nitinol and can differentiate between them for the shape, the size, the type of mesh, the presence or absence of coverage, the catheter carrier (TTS or OTW) and the release system. The stent OTW, typically have a 16 Fr catheter and can be used, in consideration of the length and rigidity of the catheter carrier, only for strictures of the rectum or sigmoid typically within 30 cm from the anus. For stenosis located further upstream in the bowel, for anatomical reasons, it needs to use TTS stents. The Wallflex® (Boston Scentific) and Niti-S® (TaeWoong) are the most used stents to date and on which there are more data in the literature. The Evolution® stent (Cook) (Figure 4) instead is the most recently introduced on the market and which presents a delivery system that allows a best control of the various phases of the stent placement.
In the study of an occluded patient the diagnostic steps should go include the execution of a contrast medium CT scan that can be extremely useful both to evaluate the seat, the extension and nature of the stenosis, both to obtain a more comprehensive assessment of the situation abdominal (cecal diameter, exclusion of a bowel perforation or evaluation of liver metastases or peritoneal carcinomatosis). In general the onset of symptoms, their severity, and the distension of the cecum are the elements allowing the assessment in how many hours must be executed an attempt of endoscopic stent decompression. In most situations the endoscopic intervention can be performed within 6-8 h from the evaluation, for which reason even if the patient were to arrive later in the evening in the emergency room the stent placement could be deferred to the next morning. Logistically, it is necessary to have an X-ray room, a doctor and one or two experienced nurses or two doctors and a nurse, endoscopic instruments of different sizes to meet all anatomical situations and accessories needed for stent placement (guidewires, catheters, sphincterotome).
Stent TTS have a delivery catheter with a diameter of 10 Fr for which they pass in the channels of 3.8 mm diameter endoscope (standard colonscope, operative gastroscope and duodenoscope). Before placement of the stent is recommended the execution of a rectal enemas toileting which serve to clean the intestinal tract below the stenosis, thus facilitating the endoscopic exploration and identification of the stenosis. The procedure is almost always very well tolerated with minimal sedation with low doses of benzodiazepines. The anesthesia care should be required only for patients very sufferers or those with medical critical conditions.
For the possibility of ab-ingestis polmonitis, it is suggested to leave the stomach in a naso-gastric tube. It is also prudent to work with a very low level of endoscopic insufflation to avoid excessive distension of the colon upstream of the stenosis that could jeopardize the success of the maneuver.
It is preferable the patient lie supine to facilitate the radiological anatomy and identification of markers for the positioning of the stent. After reaching the stenosis, the next step is to cross the stricture with a guide-wire and an ERCP catheter. If the stenosis is very tight, long or very difficult to pass, it is suggested the use of a hydrophilic guide-wire, maybe with curved tip to avoid the risk of making false roads with a rigid (stiff) wire. In some occasional situations, in which the position of the stenosis is very lateralized respect to the endoscopic view for which the catheter fails to be directed in the stenosis, could be use a sphincterotome or, finally, the duodenoscope, having a different viewing angle, which allows to direct the tip of the catheter to the site of stenosis and facilitate the progression of the guide. Once passed the stenosis under radiological assistance, the wire must be withdrawn, injecting contrast medium to identify the correct position of the catheter, check the anatomy of the stricture and colon upstream. After that, a stiff guide-wire will be advanced across the catheter, far beyond the stenosis. This is useful to obtain a sufficient amount of guide-wire beyond the stenosis for all the maneuvers of the wire handling to give tension and straighten to the stent facilitating the advancement on the stricture. Dilation of the stricture before the release of the stent should be avoided because is a risk factor for intestinal perforation.
The choice of the stent will have to keep in mind the location and lenght of the stenosis. It is suggested to use stent with a length of at least 3-4 cm greater than that of the stenosis to allow a good adaptation of the stent also in very angled position where a stent too short might tend to straighten the curve inside and get in tension on the contralateral bowel wall, increasing the risk of dislocation and perforation. Once advanced the stent in the stenosis, the release should be supervised radiological and endoscopic (in the case of stents OTW is suggested to advance a small-caliber endoscope in parallel to the catheter carrying the stent). It is advisable to release the stent in a slow and gradual to always have full control of the maneuver. In the case of TTS stent is important that the endoscopist tends to progressively retract the catheter outside of the stent. Once completed the release, a last check radiological and endoscopic will be necessary to assess the proper expansion of the stent within the stenosis and evaluate the passage of air and fecal material through the stent.
The SEMSs have acquired a well-defined role in the palliation of dysphagia in patients affected by esophageal malignant strictures, with a technical success greater than 95% with regard to their positioning and the ability to quickly resolve the dysphagia in almost all of the patients, reducing the rate of complications during the positioning phase thanks to the small diameter of the delivery catheter, and this, together with the use of an pediatric endoscope, makes unnecessary the preliminary expansion[1,2].
There are several types of esophageal SEMSs actually available for the treatment of malignant and benign esophageal stenosis[3].
The esophageal SEMSs are currently available uncovered, PC (only in the extremes do not have coverage) that FC[4].
The latter have attracted the interest of clinical researchers to evaluate their role in benign stenosis, esophageal mainly because complete coverage would allow their extraction after a certain time. In addition to the FCSEMS is necessary to consider the other two implants which are used in benign esophageal disease, the SEPS (Polyflex®, Boston-Scientific, Natick, MA, United States) and the biodegradable stent (Ella®-CS, Hradec Kralove, Czech Republic).
Esophageal strictures: Before starting the endoscopic treatment is necessary to establish the real not malignancy of the stenosis, performing multiple biopsies and, when necessary, using endoscopic ultrasound (EUS) or other radiologic techniques. Essential is the classification of the dysphagia, so as to record the variation in the course of treatment (Table 4)[5].
| Grade of dysphagia | Symptoms |
| 0 | No dysphagia |
| 1 | Occasional dysphagia for solid foods |
| 2 | Dysphagia for solid foods |
| 3 | Dysphagia for semi-solid food |
| 4 | Dysphagia for liquids |
The benign strictures are relatively frequent finding in clinical practice. In the past peptic strictures were prevalent, but are currently most commonly encountered those caused by caustic and radiotherapy. Esophageal strictures have been recently described in endoscopic mucosal resection, when circumferential and after endoscopic submucosal dissection[6].
Generally most of stenosis responds to a few[7] sessions of endoscopic dilatation, but from 25% up to 30 % requires a larger number of dilatation[8]. There are, however, “complex” strictures that do not respond to dilatation therapy, even if repeated over time (Table 5).
| Length > 2 cm |
| Angulated |
| Irregular edges |
| Very low diameter |
The anastomotic strictures, post-caustic ingestion stenosis and stenosis resulting from radiotherapic treatments, have a low rate of response to endoscopic therapy: more than 40% tend to recur[9,10]. The most difficult to treat are the hypopharyngeal strictures, generally refractory.
It was moreover proposed the definition of “refractory stenosis” as: (1) absence of inflammation or motility disorders in presence of stricture; (2) impossibility of maintaining ≥ 14 mm diameter after 5 sessions of dilation performed with a interval of 2 wk (refractory stenosis); and (3) impossibility of maintaining ≥ 14 mm diameter for 4 wk after reaching a 14 mm diameter (recurrent stenosis)[11].
In addition to the expansion, have also been proposed other treatments, such as injection, at the level of the four quadrants of triamcinolon and, where appropriate, in particular in the anastomotic stenosis, the incisions of the fibrotic ring with a diathermic needle[12,13]. When these measures fail and dysphagia persists it is mandatory to evaluate the possibility to place a stent that, in addition to determining a laceration of the scarred submucosal layer and muscle of the esophageal wall, it maintains a constant pressure for the entire duration of its stay in the esophagus.
Total gastrectomy and esophagectomy are generally associated with high rate of morbidity and mortality even in specialized centres. The National Esophagogastric Cancer Audit of England and Wales published a rates of 8.3% of anastomotic leak after esophagectomy and 5.9% after total gastrectomy[14].
Although the improvements in the anastomotic techniques, anastomotic intrathoracic leak is generally associated with bacterial contamination, abscesses, and successive fistulas into pleural cavities. The continuous leakage of gastric juices and saliva into the pleural and mediastinal cavities can be life-threatening, with 30%-40% of post-surgical deaths[15].
Different treatments are described for the management of the esophago-gastric and the esophago-jejunal anastomotic leak. Some authors suggested the surgical treatment, but others prefrer a conservative approach with perianastomotic drainage, parenteral support and nasogastric decompression, and iv antibiotic therapy. All of these patients should be treated in appropriate critical care units[16].
The fibrin glue associated to metallic clips has been successfully used in small esophageal leaks[17,18]. An endoscopic stenting remains an attractive option, and SEPS and PC or FC-SEMS ensure good results, although the loading kit device and the delivery of the plastic stent can be difficult in non-expert hands. The leak closure rates ranges from 60% to 100% with an healing rates > 90%[19,20]. Leak closure should be confirmed by contrast medium injected during the endoscopic procedure. Stents removal is planned at different times, depending by the size of the leak, but the stents are generally removed from 14 to 28 d from the placement with a previous clinical evaluation of healing of the absence or sepsis, radiologically documented.
The majority of the published studies suggest the stent placement immediately after the diagnosis of the leak for minimizing the contamination of the mediastinal cavity[21]. In some cases, the delayed placement can result in the healing of the anastomotic leak. Patients in which a covered stent is placed present an earlier oral intake (11 d vs 23 d), short ICU stay (25 d vs 47 d), and a less hospital stay (35 d vs 57 d). The in hospital mortality ranges from 0% to 20%, in different published series, lower if compared to the groups treated conservatively[22].
Esophageal perforation is a life-threatening condition generally requiring surgical intervention. The management of the esopgaheal acute perforation (iatrogenic or spontaneous) is be divided into two groups: conservative and operative. Because of the rarity, the literature on this issue is based mainly on small series and case reports. Surgical repair seems to be the treatment of choice when an early diagnosis is made. The reported mortality in literature ranges from 0% (when the treatment starts within 24 h) to 30% if the treatment is delayed[23].
The conservative treatment is based on broad-spectrum iv antibiotic, parenteral nutrition and percutaneous drainage of the collections, when present. Ivey et al[24] showed as conservative therapy is appropriate only in presence of a perforation > 5 d; absence of sepsis; wide cavity at radiological imaging draining back into the esophageal lumen and absence of contamination of the pleural space[24]. A recent review advices that conservative treatment is feasible, with a survival rates of 60%-70% if the perforation are promptly diagnosed, but the need of the surgery is mandatory in case of failure[25].
Griffin et al[23] showed as in the management of spontaneous esophageal perforation (Boherhaave Syndrom’s) they did not use stents. They suppose that the stent may prevent adequate drainage of sepsis, but can be subject to dislocation in absence of a stricture. Moreover, Authors recommend a non-operative management only in selected cases, especially when unfit for surgery[23].
Only three case reports are present in literature about the treatment of spontaneous esophageal perforations with the placement of the SEPS. In the first case the SEPS was placed at 24 h, in another case after 3 wk, in association of chest drainage, broad-spectrum iv antibiotic therapy and fibrin-glue injection, and, in the last case, the stent was placed 10 d later. All of the patients survived, and the immediate radiological study after stent placement did not show contrast medium outside from the esophageal lumen[26,27]. Oral intake was started in all of the three patients within 7 d, and were discharged between 7 and 21 d after stent placement.
The stent removal was scheduled between 5 and 10 wk. In two of the three treated patients, the stent was found into the gastric cavity.
Self-expandable plastic stent: The SEPSs (Polyflex®) are usually placed with fluoroscopic assistance, but, in selected cases, deployment only under endoscopic view has been reported. A stent longer from 2 to 4 cm than the stenosis should be used for allowing a 1 to 2 cm extension above the edges of the lesion. Based on the RF of the SEPS, the completely expansion of the stent is obtained from hours to days[28,29].
The delivery device of the SEPS is larger and more rigid, if compared to other delivery system, with a non flexible tip. The assembly of the delivery device can sometimes be difficult in less well trained centres with low volume of cases, than these characteristics increase the challenging of the SEPS placement. The retraction rate of the SEPS is about 18% of the stent length before the release. The delivery system not allows the recapture. Because of the rigidity of the delivery system, it is suggested the neck hyperextension using a super-stiff wire. This may increase the risk of perforation, especially in presence of angulated strictures. This has been demonstrated in a prospective randomized trial (RCT) comparing 3 types of stents (Ultraflex, Niti-S and Polyflex); although dysphagia relief was achieved with all three types of stents, technical problems during stent release are encountered generally during SEPS placement than the other two SEMSs[30].
The SEPS presents some advantages if compared with PC-SEMS, as an easier removal and a less migration[31]. The soft material confers to the stent a well-balanced RF, adapting it to the wall of the esophagus, with a more probability of leak closure. The fully silicone covering does not allow granulation tissue ingrowth with minor overgrowth. It results in a possible successive easier repositioning and removal. The SEPS is available in different diameters and lengths, the exact diameter of stent has to be chosen on the basis of the size and site of the stricture (associated or not with a leak). There is no published evidence that the placement of large-diameter SEPS reduce the migration rate[32].
The SEPS presents other several drawbacks such as: the release takes place very fast, leading to the onset of severe sternal pain which can sometimes persist even 1 wk and the diameter of the introducer system that is excessive, especially in the presence of a “complex stenosis”.
The first study of the placement of SEPS in 15 patients reported a technical success, clinical success and a migration rates of 100%, 80% and 6.6% respectively[33].
Dua et al[34] in prospective study including 40 patients reported a clinical success of 40% and a migration rate of 22%, with a death due to a bleeding caused by erosion of the esophageal wall by SEPS[34].
A recent systematic review of the literature that has considered 10 studies with a total of 130 patients evidenced a technical and clinical success of 98% and 52%, respectively. The rate of migration (< 4 wk) was 24%, while complications were observed in 9% of patients with one death (0.8%)[35]. There is no consensus on the time to remove SEPS, but generally it is advisable the retrieval the stent after 6 wk, to prevent the onset of serious complications.
These stent should be not used in benign pathology because of the proliferation of granulation tissue through the proximal and distal uncovered mesh makes their removal difficult. One study that included 29 patients with benign esophageal strictures, reported the appearance of new stenosis the ends of the prosthesis in 41% of cases, migration in 31%, retrosternal pain and reflux in 21%, trachea-esophageal fistula in 6%[36]. Sometimes, in special cases where it cannot be used a stent completely covered for the high risk of migration, you can insert a PC-SEMS PC. To render its extraction after 6 wk, you can resort to the method indicated by Hirdes et al[37] aimed at eliminating the granulomatous tissue ingrowth present at the ends of the prosthesis: in it is placed a SEMS completely covered with similar diameter and length and leaving it up to a maximum of 2 wk. In this way the pressure of the stent will determine the necrosis of granulation tissue between the meshes, thus making possible the extraction of both stents[37].
The complete coverage of the stent facilitates the extraction after a predetermined period of time, but could increase the risk of migration. This problem can be reduced if the endoscopist is able to perform an appropriate stent choice, in length and size. The capability of auto-conforming and the diameter should be considered in each individual case. A larger diameter will oppose effectively the migration and in presence of the fistula, and the perfect adhesion of the proximal crown to the wall to effective impermeability to liquids.
Eloubeidi et al[38] in 7 patients with benign strictures placed the Alimaxx-E (Alveolus, Charlotte, NC, United States) FCSEMS. The resolution of dysphagia was observed in 29%, while the migration occurred in 36% of the cases, half of the patients developed an ulcer distally to the FCSEMS and the 23% proximally, however these lesions were solved after the removal of the stent[38]. Additional studies have reported a migration rate to 37% up to 50%, a resolution of dysphagia to 21% up to 100 % of cases and the extraction of the stent was possible in all of the cases[39,40].
A meta-analysis that compared SEPS and FCSEMS in esophageal refractory strictures, included 8 studies with a total of 199 patients, found an improvement of dysphagia in the 55.3% patients treated with SEPS and in the 21.8% of the patients treated with FCSEMS, however these data must be accepted with extreme caution because in 6 of the 8 studies was used the SEPS[41].
The FCSEMSs are effective also in the treatment of benign fistulas, perforations and anastomotic leakage.
Van Heel et al[42] treated 33 patients with esophageal perforation (19 iatrogenic type, 10 Boerhaave’s syndrome and 4 other pathologies), the closure of the perforation was obtained in 32 (97%) patients, recurrence occurred in 37% of cases, which required further stenting (3 patients were treated surgically), and the stents were removed within 6 wk of the placement without major complications[42].
A systematic review, that included 25 studies with a total of 267 patients, showed that the closure of the perforation was successful in 85% of patients, surgery was necessary in 13% and that patients treated with SEPS required a greater number of endoscopic reinterventions compared to patients treated with covered metal stents (26% vs 13%, P < 0.001)[43].
A particular problem is posed by the hypopharyngeal stenosis resulting from surgery and radiotherapy for cancer ear nose and throat[44,45]. Fibrosis caused by radiotherapy are interested in full thickness bowel and the remodeling of the stenosis is virtually impossible. Expansions of periodic increase fibrosis and therefore the risk of perforation. Generally, the appearance of a fistula requires, when possible, the surgery, with a considerable rate of mortality and morbidity. In these patients has proved useful the use of Niti-S Conio Stent (Taewoong Medical, Seoul, South Korea). The preliminary results are encouraging, but it is necessary to include a larger number of patients to assess the efficacy[46].
The biodegradable implant (Hella® stent - Ella®-CS, Hradec Kralove, Czech Republic) was introduced in the clinical setting in 2008.
One study that included 21 patients with refractory strictures reported a significant improvement in dysphagia at a mean 53 wk of follow-up, 45% of the had not dysphagia at the end of the study, the migration rate was 9.5%, 3 patients complained of retrosternal pain after the release of the stent and one patient presented a slight bleeding after the procedure[47].
Van Boeckel et al[48] compared the outcomes of SEPS (20 patients) and biodegradable stent (18 patients) in refractory strictures. In the group treated with SEPS, 6 (30%) patients were completely free of dysphagia with a median follow-up of 385 d while 10 (50%) had a recurrence of dysphagia, 1 severe bleeding and 1 perforation occurred. In the group treated with biodegradable stent, 6 (33%) patients were free of dysphagia with a median follow-up of 166 d, recurrence was observed in 12 (67%) patients and 2 severe bleeding and 2 cases of severe retrosternal pain occurred. The rate of endoscopic re-intervention was lower in the SEPS compared to the biodegradable stent grosup (15 vs 21)[48].
Esophageal stents in malignant diseases are mainly placed in presence of unresectable carcinoma of the esophagus, with a short life expectancy, and suffer from marked esophageal stenosis or fistula[49]. Other malignant conditions in which patients are eligible for stent placement are extrinsic esophageal compression or fistula formation as a result of pulmonary cancer, mediastinal cancer or metastatic disease. The main advantages of stent therapy are successful insertion of the device in almost all cases with rapid (24-48 h) improvement of dysphagia. Disadvantages of the stent therapy are the re-occurrence of dysphagia in up to one-third of patients, and other stent related complications, including hemorrhage, pain and fistula[50]. Although most stents are placed in the distal or mid esophagus, insertion in the cervical esophagus is most rarely and it is considered equally effective with dedicated stent[51].
Both SEMS and SEPS are most used in esophageal malignant diseases. Actually to prevent tumor ingrowth PC or FC SEMS were used[51]. Although a FC-SEMS prevents tissue ingrowth over the full length of the stent, it presents a considerable migration risk[52].
In the last 12 years, only five RCTs comparing different types of stent in patients with malignant esophageal strictures were published[53-56].
The first study randomized 100 patients to treatment with one of three SEMS: the PC-Ultraflex® stent (Boston Scientific, United States), the PC-Flamingo Wallstent® (Boston Scientific) and the FC-SEMS Gianturco Z-stent® (Wilson-Cook, Denmark). The three stents were equally effective in improving dysphagia scores without a significant difference in major complication rate.
The second trial randomized 53 patients with a distal esophageal tumor to a PC-Flamingo Wallstent® (Boston Scientific) or the more flexible PC-Ultraflex® stent (Boston Scientific). Clinical outcome was satisfactory in both groups without significant differences in improvement of dysphagia scores and complication rates.
A third study randomized 101 patients to a Polyflex® (Boston Scientific) or Ultraflex® stent (Boston Scientific), showing similar effectiveness in palliation of dysphagia. However, complications, especially late migration, occurred significantly more often after placement of a Polyflex® stent.
The fourth randomized study with 125 patients evaluated the Ultraflex® stent (Boston Scientific), the FC-double-layered Niti-S stent® (Taewong Medical, South Korea), and the Polyflex stent® (Boston Scientific). The Ultraflex® and Niti-S® stent were equally effective with equal overall complication rates, but recurrent dysphagia generally occurs more frequently with the Ultraflex® stent (52% vs 31%), mainly caused by a higher rate of food obstruction. The Polyflex® SEPS was associated with high failure of stent placement (17%) and increased migration risk. Because of a wider diameter of the Polyflex® delivery system, insertion is technically more difficult and dilation had to be performed more frequently. Furthermore, SEPS conform less easily to a stricture, making them more susceptible to slipping.
Observational series had initially demonstrated effectiveness of SEPS in malignant esophageal obstruction; however, the randomized studies revealed an unacceptable high complication rate[57,58].
A recently trial included 80 patients with dysphagia caused by malignant stenosis. Patients were randomized into two groups: PC-Evolution® stent (Cook Medical, Ireland) and Ultraflex® stent (Boston Scientific). The Evolution® stent was related with a significantly lower rate of stent dysfunction (8% vs 40%) and major complications (8% vs 25%). These data could not be confirmed in another single arm study, which included 44 patients with malignant dysphagia. In this study, the Evolution stent dysfunction rate was much higher (25%), mainly caused by tumor in- or overgrowth[59].
Stent innovations include anti-reflux and anti-migration features. The anti-reflux features were particularly developed for stents bridging the lower esophageal sphincter. This was generally done by attaching a valve to the distal end of the stent, inhibiting backflow from gastric contents into the esophagus. Theoretically, this should prevent reflux symptoms, esophagitis, and possibly aspiration. Although some studies have indicated that anti-reflux stents reduced gastro-esophageal reflux, a recent meta-analysis did not identify a significant difference in adverse events, symptoms and quality of life reflux-related[60]. Therefore, the use of anti-reflux stents has largely been abandoned. Antimigration features include uncovering of distinct areas of the metal mesh and a wider diameter of the stent flares, as well as addition of struts or rings to the outer side of the stent serving as anchoring devices. Both the Alimaxx-E® (Alveolus, United States) equipped with outer antimigration struts and the SX-ELLA® Esophageal HV stent (Ella-CS, Hradec Kralove, Czech Republic), with an anti-migration ring fall in the latter category. Several studies, however, have shown that, in spite of these design modifications, these stents frequently dislocate[61,62]. In addition, the SX-Ella stent seems to be associated with a major number of adverse events, such as hemorrhage, fistula formation, and severe pain, which likely relate to excessive pressure of the anti-migration ring.
The Niti-S stent has a dog-bone shape to prevent migration. Two design of the stent are present in commerce: a fully-covered self expandable metal stent and a double-layered covering with an FC inner layer made of polyurethane and an outer uncovered nitinol mesh to facilitate the attachment of the SEMS to the wall. Several studies have reported good clinical efficacy and acceptable migration rates (up to 12%) with both types Niti-S® stents[63,64]. In one study, the double-layered version was associated with a significantly lower combined recurrent dysphagia and complication rate than the single layer version (12% vs 58%). However, the high complication rate of the single-layered Niti-S® stent used in that study was not confirmed in a recent large single arm study[65]. The FC-Wallflex® stent (Boston Scientific) is characterized by two migration-resistant features: distinct shouldering at both sides and internal covering. This stent has so far only been evaluated in one study for the treatment of neoplastic stenosis. Although the migration risk was low (9%), major complications were commonly seen (30%), which might be associated to the relatively high Wallflex® RF[66].
In summary, the available studies suggest that no major differences in efficacy and safety exist between different stents. However, there is still insufficient evidence to recommend one type of SEMS in the treatment of malignant dysphagia. Specific features reduce migration rates of FC-SEMS; however, they can also induce traumatic injury and lead to major adverse events.
Fistulas usually result from infiltration of esophageal cancer to the respiratory tract or pleural cavity. Additionally, lung and mediastinal cancers can penetrate to the esophagus, also creating fistulas. Multiple series have reported on the use of covered SEMS to seal off fistulas, with closure rates ranging between 73% and 100%[67-69]. At the same time, it is also crucial that pleural and mediastinal fluid collections are drained aggressively. Both PC and FC-SEMS can be used as long as the covering completely seals the fistula. Unfortunately, randomized studies to recommend a specific type of SEMS are lacking. The largest non-comparative series to date reports on 61 patients with esophago-respiratory fistulas treated with covered SEMS. Ten patients also required a trachea-bronchial stent to seal the fistula. Complete fistula healing was reached in the 80% of the cases (49 subjects); the re-intervention was effective in the majority of 17 patients in whom the fistula had re-opened. Based on these data, and in the absence of effective alternative treatments, SEMS is considered the treatment of choice in malignant fistulas[70].
Nowadays, neoadjuvant chemoradiotherapy improves long-term survival after esophageal surgery[71]. Stent insertion before neoadjuvant therapy is an interesting new concept in the management of resectable esophageal malignancy. It could be useful as a bridge to surgery during the neoadjuvant chemotherapy, improving nutritional status by ensuring oral solid intake without the need for nasoenteral or percutaneous feeding tubes. Because esophagectomy is scheduled shortly after termination of neoadjuvant therapy, late stent-related complications can be averted. This approach has been evaluated in several studies, using different types of stents and various neoadjuvant regimes[72-74]. Stents were either extracted prior to esophagectomy or removed during surgery. They appear effective in improving dysphagia and maintaining nutrition. However, complications, although rare, may occur. These include esophageal perforation requiring urgent surgery, and stent migration. The latter has in case series been reported to result in small bowel perforation or obstruction. Furthermore, in one study, the number of patients proceeding to curative resection was surprisingly low due to progression or discovery of metastatic disease[75]. These findings indicate that adjunctive studies will clarify the use of the stents meanwhile the patient underwent neoadjuvant chemotherapy before implementing such use in regular practice. These studies should also clarify concerns about the possible spreading of viable tumor cells in the circulation after stent placement.
Recurrent dysphagia remains a problem after stent insertion and occurs in almost one-third of patients. Endoscopic reintervention is successful in most cases[76]. In cases of tumor over- or ingrowth, insertion of a second stent is effective to restore luminal patency. This can also be considered in cases of stent migration. Conio et al[77] in 2010 described the possibility to treat the dysphagia because of the over- or ingrowth by placement of a SEPS. They evaluated 13 patients, previously treated with metal stent developing dysphagia because of tissue in/overgrowth, underwent self-expandable plastic stent (SEPS). Before SEPS placement, the dysphagia score ranged from 3 and 4. After 1 wk from the stent placement the dysphagia score was 0% in 100% of the cases. All of the patients were free of dysphagia till their death. Mean survival after self-expandable plastic stent placement was of 4 mo[77].
However, either endoscopic repositioning or exchanging for a new stent is preferable. Obstruction due to impacted food can easily be managed by endoscopic stent clearance.
Another rare late complication is spontaneous stent fracture with collapse. The stent-in-stent technique seems safe and effective in these situations and can also be used to facilitate removal of the fractured SEMS[78].
Esophago-respiratory fistulas are mostly seen several months after stent placement. Due to the RF and resulting pressure necrosis, which is most extreme at the level of the flares, it is usually seen next to the proximal or distal margin of the stent. In these cases, placement of an additional covered-SEMS is an effective method.
Another complication is the development of retrosternal pain after stent insertion. Didden et al[79] found a 60% rate of moderate to severe pain in a prospective assessment of 50 patients after esophageal SEMS insertion for malignant stenosis.
Pain lasted for an average of 10 d and 91% of patients required analgesics, with good effect in all patients without the need for stent removal in any of them.
Complications of bariatric surgery: The sleeve gastrectomy (SG), described for the first time by Gagner et al[80] in 2003 is currently a well standardized therapeutic option for the surgical treatment of different degrees of obesity[81,82]. The described complications of the SG include bleeding of the suture line and the stenosis, while the dehiscence of the suture line is the most serious event associated with a high morbidity rate and for whose management have been proposed different therapeutic approaches[83,84].
The re-intervention is often required even if burdened by a high rate of morbidity and mortality.
In recent years some endoscopic methods such as the use of covered-SEMS, have been mostly used for the treatment of anastomotic leakage with the aim of obtaining a non-minimally invasive surgical repair of the fistula[85,86].
The dehiscence of the suture line of the SG could be present in 0.5%-7% of the cases, even if could be underestimated; a detailed review of the American Society for Bariatric and Metabolic Surgery shows an overall rate of complications after SG variable between 0% and 24% with a percentage of dehiscence of 16%-20% of the cases[87]. The esophago-gastric junction and the proximal portion of the stomach near the corner of His are the points where most of you will be dehiscence[88,89].
The use of FC-SEMS in the treatment of dehiscence of the suture line of the SG was proposed by several authors in recent years[90].
The stent constitute a physical barrier between the fistula and the content intraluminal favoring the healing and the closure of the wall defect at the same time allowing the nutrition per os. The results of this method are reported in the literature as never variables, even if it is mostly case reports or small case series, so at present there are no extensive data statistically reliable.
Two stents are currently marketed for the closure of fistulas post-SG: the Beta-stent® (Niti-S - TaeWoong), and the Hanaro® stent (M.I. Tech). There are no data about, it is recommended the extraction of the stent between 6 and 8 wk. Currently there are no data comparing the two stents. The migration of the stent is the most common complication, reported in 30% of cases in some papers[87,91] and up to 42%-50% of cases in others[90,92]. The two ends of the stents slightly flared and high profile allow a good anchor. The body of the stents is longer than any of the esophageal stent allowing the opening of the proximal bell at the level of distal esophagus and the distal to the level of the duodenal bulb, by eliminating the pressure gradient, favoring the closure of the wall defect. The large diameter ensures excellent fit of the prosthesis to the wall of the gastric tube.
Gastric outlet obstruction (GOO) is generally secondary to bilio-pancreatic and others. More rarely is due to gastric neoplasia[93]. Gastrojejunostomy (GJ) was the only therapeutic chance till the advent of the SEMS and is characterized by and higher mortality and morbidity, delayed symptoms resolution and longer hospitalization stay when compared to endoscopic stent placement[94,95].
In the last 20 years we observed an emerging role of self-expandable metal stent for palliation of GOO, substituting the GJ. A meta-analysis evaluating nine studies and 307 endoscopic and surgical intervention for palliation of malignant GOO evidenced better clinical success, minor morbidity and mortality, lower time-related procedure and hospital stay for endoscopic stent placement[96]. The rate of endoscopic clinical success was 84%-93%, with a technical success of 93%-97%[97,98].
The correct evaluation of the patients undergoing endoscopic stenting or surgical GJ plays a key role in the management of the malignant GOO. The GJ, in the opinion of some authors, is suggested in patients with a life expectancy more than 6 mo[99] despite a prospective randomized trial suggests GJ when the life expectancy is > 2 mo, and endoscopic SEMS when < 2 mo[100].
During the choice of the stent the endoscopist has to consider the site and the morphology of the stricture. The mean time for endoscopic duodenal SEMS placement is 17.5 min and the use of duodenoscope could be useful because offer a better view of the duodenal stenosis, moreover, the scope elevator allows also the orientation of the device used, maintaining correctly in place the wire during devices exchange. The use of the duodenoscope is also suggested from some authors in presence of a challenging situation: a concomitant biliary obstruction[101,102].
The concomitant bilio-duodenal strictures are classified in three types: type I: involving duodenal bulb/upper duodenal genu in absence of involvement of papillary area; type II: involving the medium and distal portion of the duodenum and the papillary area; and type III: involving the distal portion of the descending duodenum in absence of involvement of the papillary area[103].
In the type II, when a duodenal SEMS is placed, a particular condition is created, the “jailed papilla”. ERCP with biliary drainage through the metal mesh of the duodenal SEMS is possible fenestrating the SEMS with argon plasma coagulation (APC)[104]. In case of ERCP failure, percutaneous trans-hepatic biliary drainage is needed.
Actually the reported clinical success rate of duodenal stenting for GOO is 84%-93%, with a technical success of 93%-97%[98,99,101].
Tissue over- and ingrowth, food impaction and stent dislocation are the possible adverse events after SEMS placement, requiring endoscopic intervention in 20%-25% of the patients. Stent migration is more frequent for the covered than the uncovered SEMS[102].
Other complications of enteral SEMS are, bowel perforation and bleeding (< 1%), sometimes due to the uncovered ends of the SEMS[105,106]. The mesh pressure on the epithelium induces tissue regeneration, resulting in the ingrowth of the tissue, conditioning stent failure[107,108]. Then, the placement of a covered-SEMS is preferable in non-surgical patients, or patients with an high risk of mortality and morbidity, with a life expectance > 2-3 mo. Covered stent are usually placed inside of an uncovered stent, in presence of tissue ingrowth or for tumor recurrence and if a leak is present[109,110]. The disadvantage of the covered SEMS is the tendency to migration, even more rare for uncovered SEMS. The migration of a stent might be due to an inadequate stent diameter or after chemotherapy, if a reduction of the neoplastic mass is obtained[111].
Colo-rectal benign strictures are likely to endoscopic treatment: anastomotic strictures, post-ischemic, Crohn’s disease strictures and post-actinic stenosis[112]. Among these, the most frequent is the anastomotic stenosis. It appears on 22%-30% of patients undergoing colorectal surgery and is the most benign colonic pathology treated endoscopically, especially with pneumatic (balloon) or mechanical (Savary) dilation.
Stenting in non neoplastic colorectal stricture is proving to be a viable therapeutic alternative with the intent to bring down the number of endoscopic sessions required to achieve the resolution of the stenosis itself. The data published so far on the use of stents in this setting are still limited and often conflicting.
In the benign stenosis the stents are used with the aim of solving the occlusion or sub-occlusion bowel, which is sometimes an emergency surgical. On the use of stents in benign colorectal diseases are still a few data and with time follow-up is limited, lacking in the literature randomized studies. The results on the efficacy and safety of stents in benign colo-rectal obstruction is controversial because of the high numbers of adverse events, especially considering the high migration rate[113].
Published studies have demonstrated that colonic stenting in the benign disease has a technical success variable from 85% to 100% with a complication rate of around 30%. The most serious complications observed, although rare, are leaks, bleeding and perforation but the most frequent adverse event is SEMS dislocation[114].
Furthermore, from the “case series” published on colo-rectal inflammatory diseases treatable with SEMS, diverticular stricture are those associated with the higher rate of complications. In fact, as noted by the study of Keränen et al[115] the endoscopic stenting in diverticular stenosis is burdened by a considerable risk of adverse events (as leaks, abscesses and perforations) with the need for surgical management in 70% of patients treated with stent[115]. Therefore, the use of stents in diverticular stricture is actually not recommended. The most frequent stricture treated by insertion of stent is than the anastomotic one.
Published data on the use of self-expandable plastic stent in non-neoplastic colonical and rectal diseases consists of case reports and series only[114,116].
Dai et al[114] described a series of 14 patients with benign colon and rectal diseases in which SEPS was implanted, anastomotic leak healing in 67% of the patients (4/6) and colonical disobstruction was obtained in the 50% of the patients (7/14). In 2 of 7 patients (28.5%) re-intervention was performed because stricture recurrence at 37 mo[114].
Actually, the biggest series on the use of the FC-SEMS was published in 2013 by the French Society of Digestive Endoscopy (SFED). The study includes 43 patients with bowel obstruction because of anastomotic, post-ischemic or post-radiotherapy stenosis. Stent placement was successful in the 100% of the patients. Clinical success was 81%. Stent migration was in 63% of the cases. The median left in place of the stent was of 21 d. Statistical analysis evidenced that FC-SEMS with a diameter less than 20 mm have a major risk of migration. Recurrence of occlusion was observed in 53% of the cases (23 patients). No predictive factors for occlusive or sub-occlusive symptoms recurrence were individuated at multivariate analysis[117].
Although the use of this stent is limited to benign esophageal strictures, its application on colonic benign stenosis are reported.
Recently was reported its successful use for the treatment of a sigmoid stricture due to Crohn disease[118], however the majority of the published studies on the use this stent in colo-rectal benign strictures is referred to anastomosis.
Pérez Roldán et al[119] treated with the biodegradable stent 7 patients with postsurgical colorectal stricture and 3 with rectocutaneous fistula. In 9 patients the biodegradable stents were correctly placed; one early migration was observed. In one patients stent placement was not possible because of the distance to the anal orifice (30 cm) and the deformed anatomy site. Leak healing was obtained in 100% of the cases, despite recurrence was observed in one. Symptoms relief was observed in the 83.3% (6/7) of the occluded or sub-occluded patients; in the other case, the stent migrated 72 h after the placement[119].
Repici et al[120] studied 11 patients with anastomotic strictures within 20 cm from the anus, refractory to 3 sessions of endoscopic dilation. They obtained 100% of technical success. In the first 14 d after endoscopic stent placement Authors observed 4 dislocations, with subsequent stricture recurrence. Of the 7 cases with completely meshes biodegradation, 5 had no more symptoms and benign stenosis resolution. In 2 patients surgery was needed. The described clinical success was of 45%[120].
The endoscopic colo-rectal stenting is indicated for bowel obstruction caused by neoplastic stenosis of the colon-rectum determining a bowel obstruction.
Endoscopic stent placement is also indicated for decompression before of elective surgery (bridge to surgery) in patients affected by colo-rectal neoplasia to avoid emergent surgery and as palliation in presence of patients unfit for surgery candidates because of advanced disease or their poor clinical conditions.
The very low stenosis, which are less than 5 cm from the anus are a contraindication to the stenting. In the case of very low stenosis the use of the stent is invariably associated with the appearance of tenesmus, anal pain and incontinence, making intolerable the presence of the stent in the distal rectum.
More than 20% of patients with acute colo-rectal neoplastic occlusion present metastases and 2/3 of them are unfit for surgery[121,122].
Then, the SEMS placement, especially in patients not suitable for surgery, allows a re-canalization of the bowel patency, avoiding surgery.
In patients with advanced colo-rectal neoplasia causing bowel obstruction surgical intervention with stoma creation is generally performed, with negative implications for patient quality of life[123]. The endoscopic stenting by use of SEMS is nowadays accepted in the palliative therapy of the colo-rectal cancer, becoming a valid alternative to surgical stoma.
Different studies evaluated the role of the SEMS in the palliation of colo-rectal cancer. Three randomized studies are present in literature comparing endoscopic stenting with surgery in patients unfit for surgery affected by colo-rectal neoplasia, causing bowel obstruction.
In these 3 RCTs studies the technical and clinical success was of 92% and 92% respectively, with a morbidity rate of 30% (11/37) in the patients underwent endoscopic stenting and 17% (6/36) in the patients underwent surgery, and a mortality of 8% (3/37) only in the stent group[124-126]. Two of the three Authors of the RCTs suggest superior efficacy and safety of the SEMS group if compared to surgery for palliation of colorectal cancer obstruction, differently to the reported data by the Dutch Stent-in I multicenter RCT. However, in palliated patients with a longer lifespan, SEMS placement in comparison to a colostomy, presents an improvement of the life quality, and with a reduction in cost and length of hospital stay[127,128]. Stents used were the WallFlex (Boston Scientific). The study was closed before the total patients enrollment for the high recorded numbers of perforations related to the SEMS placement, with 3 consequently deaths in 10 patients of the group undergone stenting. Authors had not a clear reason for justifying the high rate of perforations. They supposed a doubtful safety of the WallFlex.
Moreover, no supporting results have been showed by other studies in which WallFlex SEMS was tested as palliative treatment in referral centres. This studies show as the experience of the endoscopist could be an explanation for the high rate of adverse events reported by the Dutch group[129-131].
Bridge to surgery has to be seriously considered in presence of patients with acute obstruction and fit for surgery. SEMS placement provides to bowel patency restoration allowing colonical preparation for surgery and an eventual pre or intra-operative endoscopy for the research of synchronous neoplastic and non-neoplastic diseases. The curative intent for these patients is a single-step intervention with primary anastomosis, especially when a laparoscopic approach is possible.
However, the role of SEMS as bridge to surgery, has been widely debated, because several RCTs studies have shown conflicting and mixed results.
In the 6 RCTs in which endoscopic stenting was evaluated as bridge to surgery (171 patients) compared to emergency surgical resection (169 patients), the technical and clinical success of stenting was 79% and 77% respectively, with a morbidity rate of 33% in the SEMS group and 53% surgical one with a comparable mortality rate (7% vs 8%)[132-137].
Notably evident is the difference in results between RCTs studies carried out in single centers vs those carried out in multicenters, particularly with respect to the stent placement outcomes and for the elevated number of stent-related perforations.
An elevated number of stent-related perforations were reported only in the studies specifically designed as multicenter trials and these studies were stopped prematurely.
In these trials, Pirlet et al[133] reported 3 stent-related perforations in 35 patients randomized to the stenting strategy and van Hooft et al[134] reported 6 stent-related perforations in 47 patients in the stenting group. The elevated number of perforations in these studies remains unexplained.
The worst results in SEMS placement outcomes come from RCTs which are specifically designed as multicenter trials involving low-volume centers. In the of Pirlet et al[133], of the nine participating centers, two of them enrolled 3 patients and one only 1 patient; in the study of van Hooft et al[134] 21 on 25 endoscopic centers were not referral.
The problem is that in planning RCTs regarding colonic stent placement, the need for involving multiple centers caused the inclusion of endoscopists with limited specific experience and low performance in placing stents. Therefore, this reality could result in confounding data on the real efficacy of the stenting strategy.
Huang et al[138] published a recent systematic review and meta-analysis evaluating safety and efficacy of colo-rectal stent placement as bridge to surgery compared to emergency surgery and considered for inclusion the 6 RCTs studies in english language and also another study in chinese language[138].
The technical success of colo-rectal stenting was of 76.9%, in absence of significant statistically difference in the postoperative mortality (10.7% vs 12.4%). The study evidenced lower morbidity (33.1% vs 53.9%, P = 0.03), higher rate of successful primary anastomosis (67.2% vs 55.1%, P < 0.01) and lower rate of definitive stoma (9% vs 27.4%, P < 0.01) for the group undergone stent placement[139].
None oncologic adverse events were recorded in the bridge to surgery group, but a major rate of lymphatic invasion was found[140-142]. No significant difference in survival were founded over 5 years (60% vs 58%)[143].
Colon stenting procedure does carry some risks, and complications are usually divided into early (within 30 d), including perforation, misplacement, and bleeding, and late, which include migration, reocclusion, tenesmus and delayed perforation.
The most common adverse event described in literature after stent placement as the migration (11%) SEMS obstruction caused by in and overgrowth tissue (12%) and bowel perforation (4.5%), as showed from a systematic review involving 88 published studies[144].
Stent obstruction is generally due to fecal impaction after tissue in or overgrowth, determining the long-term outcomes of the metal stent. The rate of SEMS obstruction by tissue in or overgrowth increases with the time because of the natural tendency of the neoplastic tissue to advance; then, SEMS occlusion is more frequent in patients in which the SEMS is placed for palliation. Literature data evidenced a 16% of SEMS occlusion when the treatment is made with palliative intent[145].
The endoscopic SEMS placement inside a stent is actually the best treatment to solve the stent obstruction due to the tissue in or overgrowth[146].
The migration of a SEMS could be asymptomatic or may cause occlusive or sub-occlusuve symptoms. More rarely is the bleeding. Tenesmus may be present when the SEMS reaches the rectum. Removal of a migrated stents from the rectal ampulla is not a challenging situation and can be also performed manually. Risk factors related to migration are the covering of the stent and the diameter < 24 mm. Some Authors stated that chemotherapy could be also related to the migration because of tumor reduction[147-149].
When the patient becoming symptomatic, the migration of the stent could be treated with the placement of a second one.
Bowel perforation is typically regarded as the only serious complication and is generally procedure or stent related. Most of the perforation occurred within 7 d after stent placement and may be caused by the SEMS delivery insertion into the stricture before the stent deployment, pneumatic dilatation of the stenosis or incorrect advancing of the wire. More rarely the perforation is due to the decubitus of the flared ends of the SEMS on the colonic wall. Over inflation with air can cause a perforation in a yet dilated colon far away from the site of obstruction, usually in the cecum[150-152].
Datye et al[152] reviewed the factors involved into the bowel perforation after stent placement, collecting the data from 82 published articles with 2287 patients. They showed a mortality rate related to perforation of 16.2% for patients who had stent-related perforation. The majority of adverse events (> 80%) were recorded within 1 mo from SEMS deployment, and 50% within 24 h from the procedure. Concomitant chemotherapy, steroids, and radiotherapy were significantly associated with perforation[153].
Bevacizumab therapy is considered now a considerable risk factor for post-stenting bowel perforation. The antiangiogenic effect could impair the colonic wall promoting the perforation at the site of maximal stent exercised pressure. Moreover, this perforation risk might be not dependent from the SEMS placement because is nowadays well known that spontaneous bowel perforation can occur during the addition of bevacizumab to chemotherapy.
P- Reviewer: Lorenzo-Zuniga V, Maccioni F, Nakazawa T S- Editor: Ji FF L- Editor: A E- Editor: W u HL
| 1. | Knyrim K, Wagner HJ, Bethge N, Keymling M, Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med. 1993;329:1302-1307. [PubMed] |
| 2. | Siersema PD, Hop WC, Dees J, Tilanus HW, van Blankenstein M. Coated self-expanding metal stents versus latex prostheses for esophagogastric cancer with special reference to prior radiation and chemotherapy: a controlled, prospective study. Gastrointest Endosc. 1998;47:113-120. [PubMed] |
| 3. | Sharma P, Kozarek R. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol. 2010;105:258-373; quiz 274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Schembre DB. Recent advances in the use of stents for esophageal disease. Gastrointest Endosc Clin N Am. 2010;20:103-121, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch Intern Med. 1985;145:1443-1446. [PubMed] |
| 6. | Isomoto H, Yamaguchi N, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2011;11:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Dua KS. Stents for palliating malignant dysphagia and fistula: is the paradigm shifting? Gastrointest Endosc. 2007;65:77-81. [PubMed] |
| 8. | Pereira-Lima JC, Ramires RP, Zamin I, Cassal AP, Marroni CA, Mattos AA. Endoscopic dilation of benign esophageal strictures: report on 1043 procedures. Am J Gastroenterol. 1999;94:1497-1501. [PubMed] |
| 9. | Lew RJ, Kochman ML. A review of endoscopic methods of esophageal dilation. J Clin Gastroenterol. 2002;35:117-126. [PubMed] |
| 10. | Spechler SJ. American gastroenterological association medical position statement on treatment of patients with dysphagia caused by benign disorders of the distal esophagus. Gastroenterology. 1999;117:229-233. [PubMed] |
| 11. | Kochman ML, McClave SA, Boyce HW. The refractory and the recurrent esophageal stricture: a definition. Gastrointest Endosc. 2005;62:474-475. [PubMed] |
| 12. | Ramage JI, Rumalla A, Baron TH, Pochron NL, Zinsmeister AR, Murray JA, Norton ID, Diehl N, Romero Y. A prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection therapy for recalcitrant esophageal peptic strictures. Am J Gastroenterol. 2005;100:2419-2425. [PubMed] |
| 13. | Hordijk ML, Siersema PD, Tilanus HW, Kuipers EJ. Electrocautery therapy for refractory anastomotic strictures of the esophagus. Gastrointest Endosc. 2006;63:157-163. [PubMed] |
| 14. | London, UK: Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, 2009. . |
| 15. | Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg. 1995;169:634-640. [PubMed] |
| 16. | Griffin SM, Lamb PJ, Dresner SM, Richardson DL, Hayes N. Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg. 2001;88:1346-1351. [PubMed] |
| 17. | Pross M, Manger T, Reinheckel T, Mirow L, Kunz D, Lippert H. Endoscopic treatment of clinically symptomatic leaks of thoracic esophageal anastomoses. Gastrointest Endosc. 2000;51:73-76. [PubMed] |
| 18. | Qadeer MA, Dumot JA, Vargo JJ, Lopez AR, Rice TW. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc. 2007;66:605-611. [PubMed] |
| 19. | Hünerbein M, Stroszczynski C, Moesta KT, Schlag PM. Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surg. 2004;240:801-807. [PubMed] |
| 20. | Schubert D, Scheidbach H, Kuhn R, Wex C, Weiss G, Eder F, Lippert H, Pross M. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc. 2005;61:891-896. [PubMed] |
| 21. | Gelbmann CM, Ratiu NL, Rath HC, Rogler G, Lock G, Schölmerich J, Kullmann F. Use of self-expandable plastic stents for the treatment of esophageal perforations and symptomatic anastomotic leaks. Endoscopy. 2004;36:695-699. [PubMed] |
| 22. | Langer FB, Wenzl E, Prager G, Salat A, Miholic J, Mang T, Zacherl J. Management of postoperative esophageal leaks with the Polyflex self-expanding covered plastic stent. Ann Thorac Surg. 2005;79:398-403; discussion 404. [PubMed] |
| 23. | Griffin SM, Lamb PJ, Shenfine J, Richardson DL, Karat D, Hayes N. Spontaneous rupture of the oesophagus. Br J Surg. 2008;95:1115-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Ivey TD, Simonowitz DA, Dillard DH, Miller DW. Boerhaave syndrome. Successful conservative management in three patients with late presentation. Am J Surg. 1981;141:531-533. [PubMed] |
| 25. | de Schipper JP, Pull ter Gunne AF, Oostvogel HJ, van Laarhoven CJ. Spontaneous rupture of the oesophagus: Boerhaave’s syndrome in 2008. Literature review and treatment algorithm. Dig Surg. 2009;26:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Petruzziello L, Tringali A, Riccioni ME, Mutignani M, Margaritora S, Cesario A, Costamagna G. Successful early treatment of Boerhaave’s syndrome by endoscopic placement of a temporary self-expandable plastic stent without fluoroscopy. Gastrointest Endosc. 2003;58:608-612. [PubMed] |
| 27. | Ghassemi KF, Rodriguez HJ, Vesga L, Stewart L, McQuaid KR, Shah JN. Endoscopic treatment of Boerhaave syndrome using a removable self-expandable plastic stent. J Clin Gastroenterol. 2007;41:863-864. [PubMed] |
| 28. | García-Cano J. Esophageal insertion of polyflex stents without fluoroscopy in peptic strictures. Endoscopy. 2007;39 Suppl 1:E133. [PubMed] |
| 29. | Lazaraki G, Katsinelos P, Nakos A, Chatzimavroudis G, Pilpilidis I, Paikos D, Tzilves D, Katsos I. Malignant esophageal dysphagia palliation using insertion of a covered Ultraflex stent without fluoroscopy: a prospective observational study. Surg Endosc. 2011;25:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Verschuur EM, Repici A, Kuipers EJ, Steyerberg EW, Siersema PD. New design esophageal stents for the palliation of dysphagia from esophageal or gastric cardia cancer: a randomized trial. Am J Gastroenterol. 2008;103:304-312. [PubMed] |
| 31. | Dai YY, Gretschel S, Dudeck O, Rau B, Schlag PM, Hünerbein M. Treatment of oesophageal anastomotic leaks by temporary stenting with self-expanding plastic stents. Br J Surg. 2009;96:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Verschuur EM, Steyerberg EW, Kuipers EJ, Siersema PD. Effect of stent size on complications and recurrent dysphagia in patients with esophageal or gastric cardia cancer. Gastrointest Endosc. 2007;65:592-601. [PubMed] |
| 33. | Repici A, Conio M, De Angelis C, Battaglia E, Musso A, Pellicano R, Goss M, Venezia G, Rizzetto M, Saracco G. Temporary placement of an expandable polyester silicone-covered stent for treatment of refractory benign esophageal strictures. Gastrointest Endosc. 2004;60:513-519. [PubMed] |
| 34. | Dua KS, Vleggaar FP, Santharam R, Siersema PD. Removable self-expanding plastic esophageal stent as a continuous, non-permanent dilator in treating refractory benign esophageal strictures: a prospective two-center study. Am J Gastroenterol. 2008;103:2988-2994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Repici A, Hassan C, Sharma P, Conio M, Siersema P. Systematic review: the role of self-expanding plastic stents for benign oesophageal strictures. Aliment Pharmacol Ther. 2010;31:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Sandha GS, Marcon NE. Expandable metal stents for benign esophageal obstruction. Gastrointest Endosc Clin N Am. 1999;9:437-446. [PubMed] |
| 37. | Hirdes MM, Siersema PD, Houben MH, Weusten BL, Vleggaar FP. Stent-in-stent technique for removal of embedded esophageal self-expanding metal stents. Am J Gastroenterol. 2011;106:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Eloubeidi MA, Lopes TL. Novel removable internally fully covered self-expanding metal esophageal stent: feasibility, technique of removal, and tissue response in humans. Am J Gastroenterol. 2009;104:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Bakken JC, Wong Kee Song LM, de Groen PC, Baron TH. Use of a fully covered self-expandable metal stent for the treatment of benign esophageal diseases. Gastrointest Endosc. 2010;72:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Senousy BE, Gupte AR, Draganov PV, Forsmark CE, Wagh MS. Fully covered Alimaxx esophageal metal stents in the endoscopic treatment of benign esophageal diseases. Dig Dis Sci. 2010;55:3399-3403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Thomas T, Abrams KR, Subramanian V, Mannath J, Ragunath K. Esophageal stents for benign refractory strictures: a meta-analysis. Endoscopy. 2011;43:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | van Heel NC, Haringsma J, Spaander MC, Bruno MJ, Kuipers EJ. Short-term esophageal stenting in the management of benign perforations. Am J Gastroenterol. 2010;105:1515-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther. 2011;33:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 44. | Nguyen NP, Moltz CC, Frank C, Vos P, Smith HJ, Karlsson U, Dutta S, Midyett FA, Barloon J, Sallah S. Dysphagia following chemoradiation for locally advanced head and neck cancer. Ann Oncol. 2004;15:383-388. [PubMed] |
| 45. | Kronenberger MB, Meyers AD. Dysphagia following head and neck cancer surgery. Dysphagia. 1994;9:236-244. [PubMed] |
| 46. | Conio M, Blanchi S, Filiberti R, Repici A, Barbieri M, Bilardi C, Siersema PD. A modified self-expanding Niti-S stent for the management of benign hypopharyngeal strictures. Gastrointest Endosc. 2007;65:714-720. [PubMed] |
| 47. | Repici A, Vleggaar FP, Hassan C, van Boeckel PG, Romeo F, Pagano N, Malesci A, Siersema PD. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc. 2010;72:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 48. | van Boeckel PG, Vleggaar FP, Siersema PD. A comparison of temporary self-expanding plastic and biodegradable stents for refractory benign esophageal strictures. Clin Gastroenterol Hepatol. 2011;9:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Homs MY, Steyerberg EW, Eijkenboom WM, Tilanus HW, Stalpers LJ, Bartelsman JF, van Lanschot JJ, Wijrdeman HK, Mulder CJ, Reinders JG. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet. 2004;364:1497-1504. [PubMed] |
| 50. | Homs MY, Steyerberg EW, Kuipers EJ, van der Gaast A, Haringsma J, van Blankenstein M, Siersema PD. Causes and treatment of recurrent dysphagia after self-expanding metal stent placement for palliation of esophageal carcinoma. Endoscopy. 2004;36:880-886. [PubMed] |
| 51. | Vakil N, Morris AI, Marcon N, Segalin A, Peracchia A, Bethge N, Zuccaro G, Bosco JJ, Jones WF. A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. Am J Gastroenterol. 2001;96:1791-1796. [PubMed] |
| 52. | Uitdehaag MJ, van Hooft JE, Verschuur EM, Repici A, Steyerberg EW, Fockens P, Kuipers EJ, Siersema PD. A fully-covered stent (Alimaxx-E) for the palliation of malignant dysphagia: a prospective follow-up study. Gastrointest Endosc. 2009;70:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Siersema PD, Hop WC, van Blankenstein M, van Tilburg AJ, Bac DJ, Homs MY, Kuipers EJ. A comparison of 3 types of covered metal stents for the palliation of patients with dysphagia caused by esophagogastric carcinoma: a prospective, randomized study. Gastrointest Endosc. 2001;54:145-153. [PubMed] |
| 54. | Sabharwal T, Hamady MS, Chui S, Atkinson S, Mason R, Adam A. A randomised prospective comparison of the Flamingo Wallstent and Ultraflex stent for palliation of dysphagia associated with lower third oesophageal carcinoma. Gut. 2003;52:922-926. [PubMed] |
| 55. | Conio M, Repici A, Battaglia G, De Pretis G, Ghezzo L, Bittinger M, Messmann H, Demarquay JF, Blanchi S, Togni M. A randomized prospective comparison of self-expandable plastic stents and partially covered self-expandable metal stents in the palliation of malignant esophageal dysphagia. Am J Gastroenterol. 2007;102:2667-2677. [PubMed] |
| 56. | van Heel NC, Haringsma J, Boot H, Cats A, Vanhoutvin SA, Kuipers EJ. Comparison of 2 expandable stents for malignant esophageal disease: a randomized controlled trial. Gastrointest Endosc. 2012;76:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Conigliaro R, Battaglia G, Repici A, De Pretis G, Ghezzo L, Bittinger M, Messmann H, Demarquay JF, Togni M, Blanchi S. Polyflex stents for malignant oesophageal and oesophagogastric stricture: a prospective, multicentric study. Eur J Gastroenterol Hepatol. 2007;19:195-203. [PubMed] |
| 58. | Szegedi L, Gál I, Kósa I, Kiss GG. Palliative treatment of esophageal carcinoma with self-expanding plastic stents: a report on 69 cases. Eur J Gastroenterol Hepatol. 2006;18:1197-1201. [PubMed] |
| 59. | van Boeckel PG, Repici A, Vleggaar FP, Solito B, Rando G, Cortelezzi C, Rossi M, Pagano N, Malesci A, Siersema PD. A new metal stent with a controlled-release system for palliation of malignant dysphagia: a prospective, multicenter study. Gastrointest Endosc. 2010;71:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Sreedharan A, Harris K, Crellin A, Forman D, Everett SM. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev. 2009;CD005048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Uitdehaag MJ, Siersema PD, Spaander MC, Vleggaar FP, Verschuur EM, Steyerberg EW, Kuipers EJ. A new fully covered stent with antimigration properties for the palliation of malignant dysphagia: a prospective cohort study. Gastrointest Endosc. 2010;71:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Talreja JP, Eloubeidi MA, Sauer BG, Al-Awabdy BS, Lopes T, Kahaleh M, Shami VM. Fully covered removable nitinol self-expandable metal stents (SEMS) in malignant strictures of the esophagus: a multicenter analysis. Surg Endosc. 2012;26:1664-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Choi SJ, Kim JH, Choi JW, Lim SG, Shin SJ, Lee KM, Lee KJ. Fully covered, retrievable self-expanding metal stents (Niti-S) in palliation of malignant dysphagia: long-term results of a prospective study. Scand J Gastroenterol. 2011;46:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Verschuur EM, Homs MY, Steyerberg EW, Haringsma J, Wahab PJ, Kuipers EJ, Siersema PD. A new esophageal stent design (Niti-S stent) for the prevention of migration: a prospective study in 42 patients. Gastrointest Endosc. 2006;63:134-140. [PubMed] |
| 65. | Kim ES, Jeon SW, Park SY, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH. Comparison of double-layered and covered Niti-S stents for palliation of malignant dysphagia. J Gastroenterol Hepatol. 2009;24:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Hirdes MM, Siersema PD, Vleggaar FP. A new fully covered metal stent for the treatment of benign and malignant dysphagia: a prospective follow-up study. Gastrointest Endosc. 2012;75:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Bethge N, Sommer A, Vakil N. Treatment of esophageal fistulas with a new polyurethane-covered, self-expanding mesh stent: a prospective study. Am J Gastroenterol. 1995;90:2143-2146. [PubMed] |
| 68. | Do YS, Song HY, Lee BH, Byun HS, Kim KH, Chin SY, Park JH. Esophagorespiratory fistula associated with esophageal cancer: treatment with a Gianturco stent tube. Radiology. 1993;187:673-677. [PubMed] |
| 69. | Dumonceau JM, Cremer M, Lalmand B, Devière J. Esophageal fistula sealing: choice of stent, practical management, and cost. Gastrointest Endosc. 1999;49:70-78. [PubMed] |
| 70. | Shin JH, Song HY, Ko GY, Lim JO, Yoon HK, Sung KB. Esophagorespiratory fistula: long-term results of palliative treatment with covered expandable metallic stents in 61 patients. Radiology. 2004;232:252-259. [PubMed] |
| 71. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4082] [Article Influence: 314.0] [Reference Citation Analysis (0)] |
| 72. | Adler DG, Fang J, Wong R, Wills J, Hilden K. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest Endosc. 2009;70:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 73. | Langer FB, Schoppmann SF, Prager G, Tomaselli F, Pluschnig U, Hejna M, Schmid R, Zacherl J. Temporary placement of self-expanding oesophageal stents as bridging for neo-adjuvant therapy. Ann Surg Oncol. 2010;17:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 74. | Pellen MG, Sabri S, Razack A, Gilani SQ, Jain PK. Safety and efficacy of self-expanding removable metal esophageal stents during neoadjuvant chemotherapy for resectable esophageal cancer. Dis Esophagus. 2012;25:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 75. | Siddiqui AA, Sarkar A, Beltz S, Lewis J, Loren D, Kowalski T, Fang J, Hilden K, Adler DG. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc. 2012;76:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Homann N, Noftz MR, Klingenberg-Noftz RD, Ludwig D. Delayed complications after placement of self-expanding stents in malignant esophageal obstruction: treatment strategies and survival rate. Dig Dis Sci. 2008;53:334-340. [PubMed] |
| 77. | Conio M, Blanchi S, Filiberti R, De Ceglie A. Self-expanding plastic stent to palliate symptomatic tissue in/overgrowth after self-expanding metal stent placement for esophageal cancer. Dis Esophagus. 2010;23:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Didden P, Kuipers EJ, Bruno MJ, Spaander MC. Endoscopic removal of a broken self-expandable metal stent using the stent-in-stent technique. Endoscopy. 2012;44 Suppl 2 UCTN:E232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Didden P, Spaander MC, Bruno MJ, Kuipers EJ. Esophageal stents in malignant and benign disorders. Curr Gastroenterol Rep. 2013;15:319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Gagner M, Rogula T. Laparoscopic reoperative sleeve gastrectomy for poor weight loss after biliopancreatic diversion with duodenal switch. Obes Surg. 2003;13:649-654. [PubMed] |
| 81. | Baltasar A, Serra C, Pérez N, Bou R, Bengochea M, Ferri L. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes Surg. 2005;15:1124-1128. [PubMed] |
| 82. | Tucker ON, Szomstein S, Rosenthal RJ. Indications for sleeve gastrectomy as a primary procedure for weight loss in the morbidly obese. J Gastrointest Surg. 2008;12:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 83. | Frezza EE, Reddy S, Gee LL, Wachtel MS. Complications after sleeve gastrectomy for morbid obesity. Obes Surg. 2009;19:684-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 84. | Casella G, Soricelli E, Rizzello M, Trentino P, Fiocca F, Fantini A, Salvatori FM, Basso N. Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 85. | Nguyen NT, Nguyen XM, Dholakia C. The use of endoscopic stent in management of leaks after sleeve gastrectomy. Obes Surg. 2010;20:1289-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Blackmon SH, Santora R, Schwarz P, Barroso A, Dunkin BJ. Utility of removable esophageal covered self-expanding metal stents for leak and fistula management. Ann Thorac Surg. 2010;89:931-936; discussion 937-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 87. | Clinical Issues Committee of the American Society for Metabolic and Bariatric Surgery. Updated position statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis. 2010;6:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 88. | de Aretxabala X, Leon J, Wiedmaier G, Turu I, Ovalle C, Maluenda F, Gonzalez C, Humphrey J, Hurtado M, Benavides C. Gastric leak after sleeve gastrectomy: analysis of its management. Obes Surg. 2011;21:1232-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 89. | Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26:1509-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 439] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 90. | Márquez MF, Ayza MF, Lozano RB, Morales Mdel M, Díez JM, Poujoulet RB. Gastric leak after laparoscopic sleeve gastrectomy. Obes Surg. 2010;20:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 91. | Serra C, Baltasar A, Andreo L, Pérez N, Bou R, Bengochea M, Chisbert JJ. Treatment of gastric leaks with coated self-expanding stents after sleeve gastrectomy. Obes Surg. 2007;17:866-872. [PubMed] |
| 92. | Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, Miedema BW, Scott JS. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935-938; discussion 938-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 94. | Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7:18. [PubMed] |
| 95. | Siddiqui A, Spechler SJ, Huerta S. Surgical bypass versus endoscopic stenting for malignant gastroduodenal obstruction: a decision analysis. Dig Dis Sci. 2007;52:276-281. [PubMed] |
| 96. | Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol. 2007;42:283-290. [PubMed] |
| 97. | Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543-550. [PubMed] |
| 98. | van Hooft JE, Uitdehaag MJ, Bruno MJ, Timmer R, Siersema PD, Dijkgraaf MG, Fockens P. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc. 2009;69:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 99. | van Heek NT, van Geenen RC, Busch OR, Gouma DJ. Palliative treatment in “peri”-pancreatic carcinoma: stenting or surgical therapy? Acta Gastroenterol Belg. 2002;65:171-175. [PubMed] |
| 100. | Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, Kuipers EJ, Siersema PD. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 361] [Article Influence: 24.1] [Reference Citation Analysis (2)] |
| 101. | Laasch HU, Martin DF, Maetani I. Enteral stents in the gastric outlet and duodenum. Endoscopy. 2005;37:74-81. [PubMed] |
| 102. | Maetani I, Isayama H, Mizumoto Y. Palliation in patients with malignant gastric outlet obstruction with a newly designed enteral stent: a multicenter study. Gastrointest Endosc. 2007;66:355-360. [PubMed] |
| 103. | Mutignani M, Tringali A, Shah SG, Perri V, Familiari P, Iacopini F, Spada C, Costamagna G. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy. 2007;39:440-447. [PubMed] |
| 104. | Topazian M, Baron TH. Endoscopic fenestration of duodenal stents using argon plasma to facilitate ERCP. Gastrointest Endosc. 2009;69:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 105. | Thumbe VK, Houghton AD, Smith MS. Duodenal perforation by a Wallstent. Endoscopy. 2000;32:495-497. [PubMed] |
| 106. | Stoeckel D, Pelton A, Duerig T. Self-expanding nitinol stents: material and design considerations. Eur Radiol. 2004;14:292-301. [PubMed] |
| 107. | Bethge N, Sommer A, Gross U, von Kleist D, Vakil N. Human tissue responses to metal stents implanted in vivo for the palliation of malignant stenoses. Gastrointest Endosc. 1996;43:596-602. [PubMed] |
| 108. | Vakil N, Gross U, Bethge N. Human tissue responses to metal stents. Gastrointest Endosc Clin N Am. 1999;9:359-365. [PubMed] |
| 109. | Kim GH, Kang DH, Lee DH, Heo J, Song GA, Cho M, Yang US. Which types of stent, uncovered or covered, should be used in gastric outlet obstructions? Scand J Gastroenterol. 2004;39:1010-1014. [PubMed] |
| 110. | Song HY, Yang DH, Kuh JH, Choi KC. Obstructing cancer of the gastric antrum: palliative treatment with covered metallic stents. Radiology. 1993;187:357-358. [PubMed] |
| 111. | Tringali A, Mutignani M, Spera G, Costamagna G. Duodenal stent migration. Gastrointest Endosc. 2003;58:759. [PubMed] |
| 112. | Hayden GE, Sprouse KL. Bowel obstruction and hernia. Emerg Med Clin North Am. 2011;29:319-345, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Small AJ, Young-Fadok TM, Baron TH. Expandable metal stent placement for benign colorectal obstruction: outcomes for 23 cases. Surg Endosc. 2008;22:454-462. [PubMed] |
| 114. | Dai Y, Chopra SS, Wysocki WM, Hünerbein M. Treatment of benign colorectal strictures by temporary stenting with self-expanding stents. Int J Colorectal Dis. 2010;25:1475-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 115. | Keränen I, Lepistö A, Udd M, Halttunen J, Kylänpää L. Outcome of patients after endoluminal stent placement for benign colorectal obstruction. Scand J Gastroenterol. 2010;45:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Geiger TM, Miedema BW, Tsereteli Z, Sporn E, Thaler K. Stent placement for benign colonic stenosis: case report, review of the literature, and animal pilot data. Int J Colorectal Dis. 2008;23:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Vanbiervliet G, Bichard P, Demarquay JF, Ben-Soussan E, Lecleire S, Barange K, Canard JM, Lamouliatte H, Fontas E, Barthet M. Fully covered self-expanding metal stents for benign colonic strictures. Endoscopy. 2013;45:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 118. | Rejchrt S, Kopacova M, Brozik J, Bures J. Biodegradable stents for the treatment of benign stenoses of the small and large intestines. Endoscopy. 2011;43:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 119. | Pérez Roldán F, González Carro P, Villafáñez García MC, Aoufi Rabih S, Legaz Huidobro ML, Sánchez-Manjavacas Múñoz N, Roncero García-Escribano O, Ynfante Ferrús M, Bernardos Martín E, Ruiz Carrillo F. Usefulness of biodegradable polydioxanone stents in the treatment of postsurgical colorectal strictures and fistulas. Endoscopy. 2012;44:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 120. | Repici A, Pagano N, Rando G, Carlino A, Vitetta E, Ferrara E, Strangio G, Zullo A, Hassan C. A retrospective analysis of early and late outcome of biodegradable stent placement in the management of refractory anastomotic colorectal strictures. Surg Endosc. 2013;27:2487-2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Kim JS, Hur H, Min BS, Sohn SK, Cho CH, Kim NK. Oncologic outcomes of self-expanding metallic stent insertion as a bridge to surgery in the management of left-sided colon cancer obstruction: comparison with nonobstructing elective surgery. World J Surg. 2009;33:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 122. | Trompetas V. Emergency management of malignant acute left-sided colonic obstruction. Ann R Coll Surg Engl. 2008;90:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 123. | Liu L, Herrinton LJ, Hornbrook MC, Wendel CS, Grant M, Krouse RS. Early and late complications among long-term colorectal cancer survivors with ostomy or anastomosis. Dis Colon Rectum. 2010;53:200-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 124. | Fiori E, Lamazza A, De Cesare A, Bononi M, Volpino P, Schillaci A, Cavallaro A, Cangemi V. Palliative management of malignant rectosigmoidal obstruction. Colostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004;24:265-268. [PubMed] |
| 125. | Xinopoulos D, Dimitroulopoulos D, Theodosopoulos T, Tsamakidis K, Bitsakou G, Plataniotis G, Gontikakis M, Kontis M, Paraskevas I, Vassilobpoulos P. Stenting or stoma creation for patients with inoperable malignant colonic obstructions? Results of a study and cost-effectiveness analysis. Surg Endosc. 2004;18:421-426. [PubMed] |
| 126. | van Hooft JE, Fockens P, Marinelli AW, Timmer R, van Berkel AM, Bossuyt PM, Bemelman WA. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008;40:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 127. | Nagula S, Ishill N, Nash C, Markowitz AJ, Schattner MA, Temple L, Weiser MR, Thaler HT, Zauber A, Gerdes H. Quality of life and symptom control after stent placement or surgical palliation of malignant colorectal obstruction. J Am Coll Surg. 2010;210:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 128. | Varadarajulu S, Roy A, Lopes T, Drelichman ER, Kim M. Endoscopic stenting versus surgical colostomy for the management of malignant colonic obstruction: comparison of hospital costs and clinical outcomes. Surg Endosc. 2011;25:2203-2209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 129. | Repici A, De Caro G, Luigiano C, Fabbri C, Pagano N, Preatoni P, Danese S, Fuccio L, Consolo P, Malesci A. WallFlex colonic stent placement for management of malignant colonic obstruction: a prospective study at two centers. Gastrointest Endosc. 2008;67:77-84. [PubMed] |
| 130. | Luigiano C, Ferrara F, Fabbri C, Ghersi S, Bassi M, Billi P, Polifemo AM, Landi P, Cennamo V, Consolo P. Through-the-scope large diameter self-expanding metal stent placement as a safe and effective technique for palliation of malignant colorectal obstruction: a single center experience with a long-term follow-up. Scand J Gastroenterol. 2011;46:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | Meisner S, González-Huix F, Vandervoort JG, Repici A, Xinopoulos D, Grund KE, Goldberg P, Registry Group TW. Self-Expanding Metal Stenting for Palliation of Patients with Malignant Colonic Obstruction: Effectiveness and Efficacy on 255 Patients with 12-Month’s Follow-up. Gastroenterol Res Pract. 2012;2012:296347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 132. | Cheung HY, Chung CC, Tsang WW, Wong JC, Yau KK, Li MK. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: a randomized controlled trial. Arch Surg. 2009;144:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 133. | Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc. 2011;25:1814-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 134. | van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011;12:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 135. | Alcántara M, Serra-Aracil X, Falcó J, Mora L, Bombardó J, Navarro S. Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg. 2011;35:1904-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 136. | Ho KS, Quah HM, Lim JF, Tang CL, Eu KW. Endoscopic stenting and elective surgery versus emergency surgery for left-sided malignant colonic obstruction: a prospective randomized trial. Int J Colorectal Dis. 2012;27:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 137. | Ghazal AH, El-Shazly WG, Bessa SS, El-Riwini MT, Hussein AM. Colonic endolumenal stenting devices and elective surgery versus emergency subtotal/total colectomy in the management of malignant obstructed left colon carcinoma. J Gastrointest Surg. 2013;17:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 138. | Huang X, Lv B, Zhang S, Meng L. Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction: a meta-analysis. J Gastrointest Surg. 2014;18:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 139. | Cui J, Zhang JL, Wang S, Sun ZQ, Jiang XL. [A preliminary study of stenting followed by laparoscopic surgery for obstructing left-sided colon cancer]. Zhonghua Wei Chang Waike Zazhi. 2011;14:40-43. [PubMed] |
| 140. | Saida Y, Sumiyama Y, Nagao J, Uramatsu M. Long-term prognosis of preoperative “bridge to surgery” expandable metallic stent insertion for obstructive colorectal cancer: comparison with emergency operation. Dis Colon Rectum. 2003;46:S44-S49. [PubMed] |
| 141. | Kavanagh DO, Nolan B, Judge C, Hyland JM, Mulcahy HE, O’Connell PR, Winter DC, Doherty GA. A comparative study of short- and medium-term outcomes comparing emergent surgery and stenting as a bridge to surgery in patients with acute malignant colonic obstruction. Dis Colon Rectum. 2013;56:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 142. | Kim HJ, Choi GS, Park JS, Park SY, Jun SH. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis. 2013;28:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 143. | Knight AL, Trompetas V, Saunders MP, Anderson HJ. Does stenting of left-sided colorectal cancer as a “bridge to surgery” adversely affect oncological outcomes? A comparison with non-obstructing elective left-sided colonic resections. Int J Colorectal Dis. 2012;27:1509-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 144. | Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ. Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg. 2007;246:24-30. [PubMed] |
| 145. | Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096-1102. [PubMed] |
| 146. | Suh JP, Kim SW, Cho YK, Park JM, Lee IS, Choi MG, Chung IS, Kim HJ, Kang WK, Oh ST. Effectiveness of stent placement for palliative treatment in malignant colorectal obstruction and predictive factors for stent occlusion. Surg Endosc. 2010;24:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 147. | Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX. Comparison of efficacy between uncovered and covered self-expanding metallic stents in malignant large bowel obstruction: a systematic review and meta-analysis. Colorectal Dis. 2012;14:e367-e374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 148. | Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010;71:560-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 149. | Fernández-Esparrach G, Bordas JM, Giráldez MD, Ginès A, Pellisé M, Sendino O, Martínez-Pallí G, Castells A, Llach J. Severe complications limit long-term clinical success of self-expanding metal stents in patients with obstructive colorectal cancer. Am J Gastroenterol. 2010;105:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 150. | Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057. [PubMed] |
| 151. | Baron TH. Colonic stenting: a palliative measure only or a bridge to surgery? Endoscopy. 2010;42:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 152. | Datye A, Hersh J. Colonic perforation after stent placement for malignant colorectal obstruction--causes and contributing factors. Minim Invasive Ther Allied Technol. 2011;20:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 153. | Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |