Published online Oct 10, 2015. doi: 10.4253/wjge.v7.i14.1150
Peer-review started: July 4, 2015
First decision: July 29, 2015
Revised: August 5, 2015
Accepted: September 7, 2015
Article in press: September 8, 2015
Published online: October 10, 2015
Processing time: 110 Days and 14.5 Hours
Laparoscopic wedge resection is a useful procedure for treating patients with submucosal tumor (SMT) including gastrointestinal stromal tumor (GIST) of the stomach. However, resection of intragastric-type SMTs can be problematic due to the difficulty in accurately judging the location of endoluminal tumor growth, and often excessive amounts of healthy mucosa are removed; thus, full-thickness local excision using laparoscopic and endoscopic cooperative surgery (LECS) is a promising procedure for these cases. Our experience with LECS has confirmed this procedure to be a safe, feasible, and minimally invasive treatment method for gastric GISTs less than 5 cm in diameter, with outcomes similar to conventional laparoscopic wedge resection. The important advantage of LECS is the reduction in the resected area of the gastric wall compared to that in conventional laparoscopic wedge resection using a linear stapler. Early gastric cancer fits the criteria for endoscopic resection; however, if performing endoscopic submucosal dissection is difficult, the LECS procedure might be a good alternative. In the future, LECS is also likely to be indicated for duodenal tumors, as well as gastric tumors. Furthermore, developments in endoscopic and laparoscopic technology have generated various modified LECS techniques, leading to even less invasive surgery.
Core tip: Resection of intragastric-type submucosal tumor can be problematic due to the difficulty in accurately judging the location of endoluminal tumor growth, and often excessive amounts of healthy mucosa are removed; thus, full-thickness local excision using laparoscopic and endoscopic cooperative surgery (LECS) is a promising procedure for these cases. The important advantage of LECS is the reduction in the resected area of the gastric wall compared to that in conventional laparoscopic wedge resection using a linear stapler. Developments in endoscopic and laparoscopic technology have generated various modified LECS techniques, leading to even less invasive surgery.
- Citation: Namikawa T, Hanazaki K. Laparoscopic endoscopic cooperative surgery as a minimally invasive treatment for gastric submucosal tumor. World J Gastrointest Endosc 2015; 7(14): 1150-1156
- URL: https://www.wjgnet.com/1948-5190/full/v7/i14/1150.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i14.1150
Gastrointestinal stromal tumors (GISTs) are common mesenchymal tumors among submucosal tumors (SMTs) of the gastrointestinal tract. Complete resection of GISTs is recommended because of their malignant potential, such as risk of tumor recurrence and progression to metastatic disease[1,2]. Lymphadenectomy is not recommended as lymph node involvement is rare. Laparoscopic surgery for GISTs is safe and effective and provides a minimally invasive approach for tumors less than 5 cm in diameter[3-5].
Extragastric-type SMTs can be treated relatively easily using a conventional laparoscopic wedge resection with adequate margins. However, resection of intragastric-type SMTs can be more problematic due to the difficulty of accurately judging the tumor’s location under laparoscopic examination. This results in the necessity of removing relatively large sections of healthy stomach to remove the tumor, which sometimes leads to postoperative deformity of the stomach[6].
Laparoscopic endoscopic cooperative surgery (LECS) was first reported by Hiki et al[7,8] in 2008, and is a minimally invasive surgical technique designed to resect SMTs originating in the gastrointestinal tract. In recent years we have performed LECS in patients presenting with gastric SMT with mainly intraluminal growth. In the present study, we reviewed a number of clinical reports describing LECS, including our own series, to evaluate the procedure and its clinical outcomes.
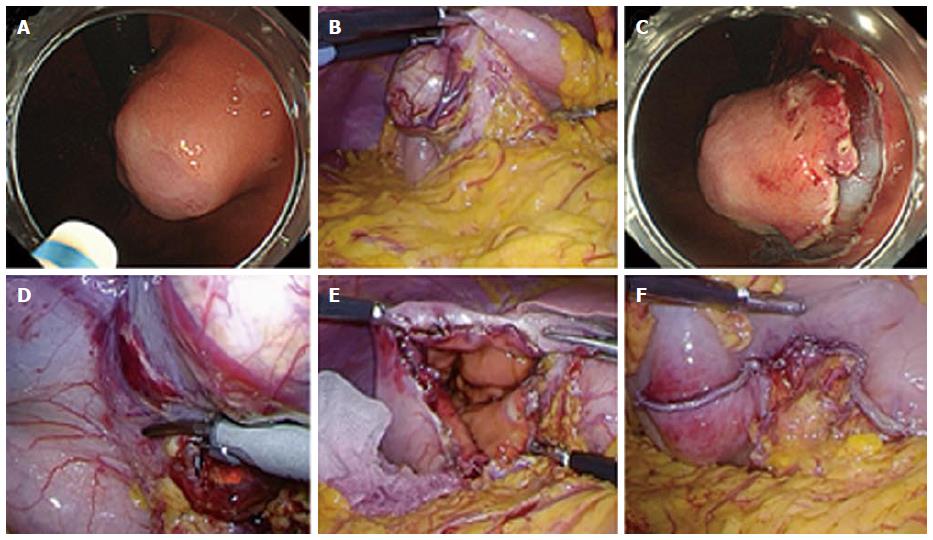
LECS requires the participation of experienced laparoscopic surgeons and experienced endoscopists[7,8]. After inducing general anesthesia, a port for a camera is inserted through the umbilicus using an open technique, and a pneumoperitoneum is established by insufflation of carbon dioxide to 8-10 mmHg abdominal pressure. Four additional ports, two 5-mm ports and two 12-mm ports, are inserted into the upper left and right and lower left and right quadrants, respectively. The proximal jejunum near the ligament of Treitz is clamped using detachable forceps to avoid air inflating the distal intestine during endoscopic manipulation. When confirmation of the tumor location from the serosal side is difficult, it is confirmed by an intraluminal approach using endoscopy (Figure 1A). Blood vessels in the excision area around the tumor are then prepared using ultrasonically activated laparoscopic coagulating shears (Figure 1B).
The location of the tumor is first confirmed endoscopically, followed by submucosal dissection using intraluminal endoscopy to determine an appropriate resection line (Figure 1C). Endoscopic submucosal dissection (ESD) is widely accepted as the standard treatment for early gastric cancer without lymph node metastasis and enables a clinician to resect a target lesion en bloc[9]. This technique is applied to LECS using various endoscopic devices such as a needle knife to mark the mucosal resection line and an insulation-tipped diathermic electrosurgical (IT) knife to dissect the submucosal layer. After circumferential dissection of the mucosal to submucosal layers, a full-thickness incision into the serosal layer around the lesion is made using the needle knife to connect the endoscopic and laparoscopic approaches.
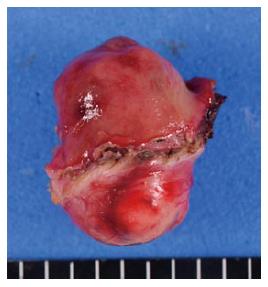
Subsequently, an ultrasonic coagulation incision device is inserted into the artificial perforation under laparoscopic view, and the seromuscular layer is dissected along the incision line made by ESD (Figure 1D). After circumferential full-thickness resection (Figure 1E), the resected specimen is put into a plastic bag, which is then removed through the umbilical incision. The edges of the incision line are then lifted up by an assistant using forceps, and the incision line is closed using laparoscopic stapling devices (Figure 1F). After completing the full-thickness closure, the endoscope can be inserted into the stomach to confirm that there is no air leakage, despite insufflation of the stomach. Gross examination of the resected specimen reveal that the resection margin of healthy gastric wall is limited to the minimum necessary (Figure 2).
Table 1 details the clinicopathological results of 19 patients who underwent laparoscopic resection for SMT in the stomach at the Kochi Medical School Hospital. Conventional laparoscopic resection of the tumor was undertaken in 11 patients, and LECS was performed in 8 patients. Basically, the indications of laparoscopic surgery including LECS for gastric SMT include the tumors less than 5 cm detected on esophagogastroduodenoscopy, computed tomography or upper gastrointestinal barium study. We performed LECS for gastric SMT when the main tumor location was intragastric type. Until the advent of LECS, we performed conventional laparoscopic resection for the gastric SMT less than 5 cm in diameter even if the main tumor location was intragastric type.
| Patient | Age | Gender | Tumor location | Main location of tumor | Operation method | Operating time (min) | Estimated blood loss (mL) | Tumor size (cm) | Histology |
| 1 | 78 | M | M, Less | Extragastric | Conventional laparoscopic approach | 145 | 20 | 4.0 × 3.5 | GIST (low risk) |
| 2 | 60 | F | U, Gre | Intragastric | Conventional laparoscopic approach | 175 | 20 | 1.7 × 1.7 | GIST (very low risk) |
| 3 | 66 | F | U, Post | Intragastric | Conventional laparoscopic approach | 85 | 30 | 5.5 × 4.5 | GIST (high risk) |
| 4 | 42 | M | L, Gre | Mixed | Conventional laparoscopic approach | 155 | 5 | 3.0 × 2.7 | Schwannoma |
| 5 | 86 | F | M, Less | Extragastric | Conventional laparoscopic approach | 190 | 40 | 3.5 × 2.5 | GIST (low risk) |
| 6 | 35 | F | U, Gre | Extragastric | Conventional laparoscopic approach | 115 | 5 | 3.8 × 3.0 | GIST (intermediate risk) |
| 7 | 84 | F | U, Gre | Intragastric | Conventional laparoscopic approach | 235 | 130 | 4.0 × 3.3 | GIST (intermediate risk) |
| 8 | 78 | F | M, Less | Extragastric | Conventional laparoscopic approach | 145 | 5 | 4.5 × 4.0 | GIST (low risk) |
| 9 | 61 | F | U, Post | Intragastric | LECS | 130 | 5 | 4.4 × 2.2 | GIST (low risk) |
| 10 | 64 | F | U, Post | Intragastric | LECS | 250 | 5 | 3.1 × 3.0 | GIST (low risk) |
| 11 | 43 | F | L, Less | Intragastric | LECS | 155 | 5 | 2.0 × 1.7 | GIST (very low risk) |
| 12 | 72 | M | M, Less | Extragastric | Conventional laparoscopic approach | 165 | 70 | 5.0 × 3.5 | GIST (low risk) |
| 13 | 77 | M | U, Gre | Mixed | LECS | 230 | 5 | 3.0 × 1.5 | GIST (low risk) |
| 14 | 63 | F | L, Gre | Intragastric | LECS | 202 | 0 | 3.0 × 2.0 | Schwannoma |
| 15 | 73 | M | U, Ant | Intragastric | Conventional laparoscopic approach | 226 | 0 | 3.0 × 2.0 | GIST (low risk) |
| 16 | 36 | F | M, Less | Mixed | LECS | 214 | 0 | 4.0 × 2.5 | GIST (low risk) |
| 17 | 82 | F | L, Gre | Mixed | LECS | 212 | 10 | 2.8 × 2.0 × 1.8 | GIST (low risk) |
| 18 | 81 | F | U, Post | Extragastric | Conventional laparoscopic approach | 130 | 0 | 4.5 × 3.0 | GIST (low risk) |
| 19 | 81 | F | M, Less | Intragastric | LECS | 221 | 0 | 3.2 × 3.0 | GIST (low risk) |
The median age of patients was 72 years (range, 35-86 years), and there was a female predominance, with a male-to-female ratio of 5:14. The tumor was located in the upper third of the stomach in 9 patients, in the middle third in 6 patients, and in the lower third in 4 patients. The tumor circumference included the lesser curvature in 7 patients, greater curvature in 7 patients, posterior wall in 4 patients, and anterior wall in 1 patient. The median tumor diameter was 3.5 cm (range, 1.7-5.5 cm), the median operating time was 175 min (range, 85-250 min), and the median estimated blood loss was 5 mL (range, 0-130 mL). The main location of the tumor was intragastric in 9 patients, extragastric in 6, and mixed (both intragastric and extragastric) in 4. The histological diagnosis was GIST in 17 patients, and schwannoma in 2 patients. Of the patients with GISTs, 2 were classified as very low risk, 12 as low risk, 2 as intermediate risk, and 1 patient as high risk, according to the modified Fletcher classification[10,11]. There were no remarkable postoperative complications including mortality, leakage or surgical site infection.
Table 2 compares the clinicopathological characteristics of the 19 patients in our current series who underwent either conventional laparoscopic surgery (n = 11) or LECS (n = 8) for gastric SMT. The clinicopathological characteristics included the tumor location, major axis of the tumor, estimated blood loss, and operating time. The median estimated blood loss in patients who underwent LECS tended to be smaller than in those who underwent conventional laparoscopic surgery (5 mL vs 20 mL, respectively; P = 0.06). The median operating time of patients who underwent LECS also tended to be longer than those who underwent conventional laparoscopic surgery (213 min vs 155 min, respectively; P = 0.05). There were no significant differences in the median age, gender, tumor location, or median tumor size in patients who underwent conventional laparoscopic surgery compared to LECS.
| LECS(n = 8) | Conventional laparoscopic approach (n = 11) | P value | |
| Age, median (range), yr | 64 (36-82) | 73 (35-86) | 0.51 |
| Gender | 0.52 | ||
| Male | 1 | 4 | |
| Female | 7 | 7 | |
| Tumor location | 0.7 | ||
| Upper third | 3 | 6 | |
| Middle third | 2 | 4 | |
| Lower third | 3 | 1 | |
| Tumor size, median (range), cm | 3.1 (2.0-4.4) | 4.0 (1.7-5.5) | 0.12 |
| Estimated blood loss, median (range), mL | 5 (0-10) | 20 (0-130) | 0.06 |
| Operating time, median (range), min | 213 (130-250) | 155 (85-235) | 0.05 |
Conventional surgical operations may be excessively invasive for the treatment of gastrointestinal SMT. Successful laparoscopic wedge resection has been reported for 2-5 cm gastric GISTs, and confirmed by studies examining long-term surgical outcomes[3,12,13]. However, it is sometimes difficult to determine the appropriate resection line from the outside of the stomach when tumors are located intragastrically. There have been several reports on the use of LECS for SMT of the stomach including GISTs, leiomyomas, and schwannomas[6,7,14-19]. Table 3 summarizes the clinical variables of patients from a number of studies who underwent LECS, including our own series.
| Ref. | Year | Number of patients | Median tumor size (cm) | Median operating time (min) | Median estimated blood loss (mL) | Conversion to open surgery |
| Hiki et al[7] | 2008 | 7 | 4.6 | 169 | 7 | 0 |
| Kawahira et al[14] | 2012 | 16 | 2.8 | 172 | 5 | 0 |
| Tsujimoto et al[6] | 2012 | 20 | 3.8 | 157 | 3.5 | 0 |
| Dong et al[15] | 2013 | 6 | 3.5 | 83.3 | NA | 0 |
| Qiu et al[16] | 2013 | 69 | 2.8 | 81.6 | 29.8 | 0 |
| Hoteya et al[17] | 2014 | 25 | NA | 156.3 | NA | 0 |
| Waseda et al[18] | 2014 | 27 | 3.6 | 167.5 | 5 | 0 |
| Mori et al[19] | 2014 | 12 | 3.9 | 146.3 | NA | 0 |
| Our case | 2015 | 8 | 3.1 | 213 | 5 | 0 |
Kawahira et al[14] reported that the median ratio of the longest diameter of the tumor divided by the longest diameter of the surgical specimen was significantly greater in LECS compared to laparoscopic wedge resection (0.86 vs 0.69, respectively; P = 0.02). This means that LECS results in the resection of a smaller area of healthy gastric wall, which is an important advantage of LECS over conventional laparoscopic wedge resection using linear staplers. Therefore, LECS has good prospects for the treatment of GISTs, applying the modern concept of minimally invasive surgery, regardless of the location of the tumor even if it is adjacent to the esophagogastric junction or pyloric ring[16].
In our series, there was a tendency for the estimated blood loss to be lower in the LECS group than in the conventional laparoscopic resection group, with the difference just failing to reach significance. A major reason for this lower blood loss is that in the case of intragastric-type SMT, division of the extended gastric vessels or the omentum in the excision area around the tumor is needed to maintain a safe margin from the tumor. There was also a tendency for longer operation times in the LECS group than in the conventional laparoscopic resection group, although this difference was not significant. This longer operation time for LECS is not surprising since time is required for both the ESD and laparoscopic procedures. Previous studies have not shown any difference in perioperative outcomes between LECS and the conventional approach, and a larger sample size would be useful to clarify this issue.
Developments in endoscopic and laparoscopic technology have yielded various modified LECS techniques aimed at further minimizing invasiveness. Examples of these improved techniques include the laparoscopy-assisted endoscopic full-thickness resection, a full-thickness resection method using the non-exposure technique (CLEAN-NET), and non-exposed endoscopic wall-inversion surgery (NEWS)[20-22]. Because LECS has an inherent risk of peritoneal infection due to the necessity for gastric perforation, CLEAN-NET and NEWS have been developed to prevent the risk of cancer cells seeding during open gastrectomy. These procedures might thus have potential minimally invasive resections of gastric tumors, even those in an ulcerated state[22].
Single-port LECS, which is a single-incision laparoscopic surgery combined with an endoscopic approach, might provide an alternative to gastric wedge resection with minimal transformation of the stomach[23]. It may contribute to reduced pain, faster recovery, and improved cosmesis for patients. However, careful selection of patients based on the tumor location and growth morphology is needed to clearly identify the risks and benefits of this new approach, also taking into account the need for needlescopic instruments.
Initially, the indication criteria for LECS was limited to SMT of the stomach measuring up to 5 cm in diameter without ulceration of the mucosa[7]. In the future, it is likely that LECS will also be indicated for tumors of the duodenum[24,25]. Although ESD is widely accepted as the standard treatment for gastric lesions including early gastric cancer, the duodenal wall is generally thinner than that of the stomach and ESD for duodenal tumors is associated with an increased risk of perforation[25]. Furthermore, maneuvering a flexible endoscope is technically difficult in the tiny duodenal lumen. In these cases, LECS might be a useful therapeutic modality not only for avoiding perforation of the duodenal wall by ESD, but also for achieving a more precise incision line[24].
Moreover, Nunobe et al[26] reported the successful use of LECS for a large spreading mucosal cancer in the stomach that would have been difficult to treat with ESD because of the high likelihood of complications and the long surgical time required for ESD. Thus, early gastric cancer that fits the criteria for endoscopic resection, but presents difficulties for ESD, is likely to be a candidate for the LECS procedure[26].
In conclusion, recent advances in endoscopic and laparoscopic techniques have facilitated several variations of endoscopic procedures derived from ESD, and created a fusion of endoscopy and laparoscopy technologies suitable for upper gastrointestinal SMTs. LECS is a useful and safe procedure for SMT that avoids excessive resection of healthy gastric wall. Further investigations, including a prospective randomized controlled trial and a study exploring long-term consequences, are needed to verify the usefulness of the LECS for gastrointestinal SMT.
The authors demonstrated the patients with gastric submucosal tumor treated by laparoscopic surgery.
The subjects were the gastric submucosal tumors including intragastric and extragastric type.
Histopathological diagnosis of submucosal tumors includes gastrointestinal stromal tumor and schwannoma.
Laboratory findings were within the normal range.
The gastric submucosal tumors were diagnosed by computed tomography and esophagogastroduodenoscopy.
Pathological findings of gastrointestinal stromal tumor were characterized by interlacing bundles of elongated cells with c-KIT expression.
The patients were treated by conventional laparoscopic wedge resection or laparoscopic endoscopic cooperative surgery.
The authors summarized the clinical variables of patients from a number of studies who underwent laparoscopic endoscopic cooperative surgery, including their own series.
Laparoscopic surgery for submucosal tumors effective and provides a minimally invasive approach for tumors less than 5 cm in diameter.
Laparoscopic endoscopic cooperative surgery is a useful and safe procedure for submucosal tumor that avoids excessive resection of healthy gastric wall.
Further investigations, including a prospective randomized controlled trial and a study exploring long-term consequences, are needed to verify the usefulness of the laparoscopic endoscopic cooperative surgery for gastrointestinal submucosal tumors.
P- Reviewer: Tomizawa M S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3203] [Cited by in RCA: 3110] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 2. | Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1206] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 3. | Kakeji Y, Nakanoko T, Yoshida R, Eto K, Kumashiro R, Ikeda K, Egashira A, Saeki H, Oki E, Morita M. Laparoscopic resection for gastrointestinal stromal tumors in the stomach. Surg Today. 2012;42:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Catena F, Di Battista M, Fusaroli P, Ansaloni L, Di Scioscio V, Santini D, Pantaleo M, Biasco G, Caletti G, Pinna A. Laparoscopic treatment of gastric GIST: report of 21 cases and literature’s review. J Gastrointest Surg. 2008;12:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Lee CM, Kim HH. Minimally invasive surgery for submucosal (subepithelial) tumors of the stomach. World J Gastroenterol. 2014;20:13035-13043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. 2012;36:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (2)] |
| 8. | Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. 2015;27:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 9. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1149] [Article Influence: 47.9] [Reference Citation Analysis (4)] |
| 10. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 865] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 11. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. [PubMed] |
| 12. | Hwang SH, Park do J, Kim YH, Lee KH, Lee HS, Kim HH, Lee HJ, Yang HK, Lee KU. Laparoscopic surgery for submucosal tumors located at the esophagogastric junction and the prepylorus. Surg Endosc. 2009;23:1980-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Honda M, Hiki N, Nunobe S, Ohashi M, Kiyokawa T, Sano T, Yamaguchi T. Long-term and surgical outcomes of laparoscopic surgery for gastric gastrointestinal stromal tumors. Surg Endosc. 2014;28:2317-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Kawahira H, Hayashi H, Natsume T, Akai T, Uesato M, Horibe D, Mori M, Hanari N, Aoyama H, Nabeya Y. Surgical advantages of gastric SMTs by laparoscopy and endoscopy cooperative surgery. Hepatogastroenterology. 2012;59:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Dong HY, Wang YL, Li J, Pang QP, Li GD, Jia XY. New-style laparoscopic and endoscopic cooperative surgery for gastric stromal tumors. World J Gastroenterol. 2013;19:2550-2554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Qiu WQ, Zhuang J, Wang M, Liu H, Shen ZY, Xue HB, Shen L, Ge ZZ, Cao H. Minimally invasive treatment of laparoscopic and endoscopic cooperative surgery for patients with gastric gastrointestinal stromal tumors. J Dig Dis. 2013;14:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Hoteya S, Haruta S, Shinohara H, Yamada A, Furuhata T, Yamashita S, Kikuchi D, Mitani T, Ogawa O, Matsui A. Feasibility and safety of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors, including esophagogastric junction tumors. Dig Endosc. 2014;26:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Waseda Y, Doyama H, Inaki N, Nakanishi H, Yoshida N, Tsuji S, Takemura K, Yamada S, Okada T. Does laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors preserve residual gastric motility? Results of a retrospective single-center study. PLoS One. 2014;9:e101337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Mori H, Kobara H, Tsushimi T, Fujihara S, Nishiyama N, Matsunaga T, Ayaki M, Yachida T, Tani J, Miyoshi H. Reduction effect of bacterial counts by preoperative saline lavage of the stomach in performing laparoscopic and endoscopic cooperative surgery. World J Gastroenterol. 2014;20:15763-15770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Abe N, Takeuchi H, Yanagida O, Masaki T, Mori T, Sugiyama M, Atomi Y. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc. 2009;23:1908-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Suzuki M, Kudo SE. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am. 2012;21:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Mitsui T, Niimi K, Yamashita H, Goto O, Aikou S, Hatao F, Wada I, Shimizu N, Fujishiro M, Koike K. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer. 2014;17:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Obuchi T, Sasaki A, Baba S, Nitta H, Otsuka K, Wakabayashi G. Single-port laparoscopic and endoscopic cooperative surgery for a gastric gastrointestinal stromal tumor: report of a case. Surg Today. 2015;45:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Irino T, Nunobe S, Hiki N, Yamamoto Y, Hirasawa T, Ohashi M, Fujisaki J, Sano T, Yamaguchi T. Laparoscopic-endoscopic cooperative surgery for duodenal tumors: a unique procedure that helps ensure the safety of endoscopic submucosal dissection. Endoscopy. 2015;47:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, Inoue Y, Umegaki E, Uchiyama K. Laparoscopic and endoscopic cooperative surgery for duodenal tumor resection. Endoscopy. 2014;46 Suppl 1 UCTN:E26-E27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Nunobe S, Hiki N, Gotoda T, Murao T, Haruma K, Matsumoto H, Hirai T, Tanimura S, Sano T, Yamaguchi T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |