Published online Oct 10, 2015. doi: 10.4253/wjge.v7.i14.1142
Peer-review started: April 21, 2015
First decision: June 3, 2015
Revised: June 21, 2015
Accepted: September 7, 2015
Article in press: September 8, 2015
Published online: October 10, 2015
Processing time: 182 Days and 21.8 Hours
AIM: To compare the usefulness of endoscopic ultrasonography-guided fine-needle aspiration biopsy (EUS-FNAB) without cytology and mucosal cutting biopsy (MCB) in the histological diagnosis of gastric submucosal tumor (SMT).
METHODS: We prospectively compared the diagnostic yield, feasibility, and safety of EUS-FNAB and those of MCB based on endoscopic submucosal dissection. The cases of 20 consecutive patients with gastric SMT ≥ 1 cm in diameter. who underwent both EUS-FNAB and MCB were investigated.
RESULTS: The histological diagnoses were gastrointestinal stromal tumors (n = 7), leiomyoma (n = 6), schwannoma (n = 2), aberrant pancreas (n = 2), and one case each of glomus tumor, metastatic hepatocellular carcinoma, and no-diagnosis. The tumors’ mean size was 23.6 mm. Histological diagnosis was made in 65.0% of the EUS-FNABs and 60.0% of the MCBs, a nonsignificant difference. There were no significant differences in the diagnostic yield concerning the tumor location or tumor size between the two methods. However, diagnostic specimens were significantly more frequently obtained in lesions with intraluminal growth than in those with extraluminal growth by the MCB method (P = 0.01). All four SMTs with extraluminal growth were diagnosed only by EUS-FNAB (P = 0.03). No complications were found in either method.
CONCLUSION: MCB may be chosen as an alternative diagnostic modality in tumors showing the intraluminal growth pattern regardless of tumor size, whereas EUS-FNAB should be performed for SMTs with extraluminal growth.
Core tip: We prospectively compared the diagnostic yield and the safety between endoscopic ultrasonography-guided fine-needle aspiration biopsy (EUS-FNAB) without cytology and mucosal cutting biopsy (MCB) based on endoscopic submucosal dissection. Although no significant difference in histological diagnosis was found between EUS-FNAB and MCB, diagnostic specimens were significantly more frequently obtained in the lesions with intraluminal growth compared to those with extraluminal growth by the MCB method. All submucosal tumors (SMTs) with extraluminal growth were diagnosed only by EUS-FNAB. No complications were found in either method. Therefore, MCB may be chosen as an alternative diagnostic modality in tumors showing intraluminal growth, whereas EUS-FNAB should be performed for SMTs with extraluminal growth.
- Citation: Ikehara H, Li Z, Watari J, Taki M, Ogawa T, Yamasaki T, Kondo T, Toyoshima F, Kono T, Tozawa K, Ohda Y, Tomita T, Oshima T, Fukui H, Matsuda I, Hirota S, Miwa H. Histological diagnosis of gastric submucosal tumors: A pilot study of endoscopic ultrasonography-guided fine-needle aspiration biopsy vs mucosal cutting biopsy. World J Gastrointest Endosc 2015; 7(14): 1142-1149
- URL: https://www.wjgnet.com/1948-5190/full/v7/i14/1142.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i14.1142
Gastric submucosal tumors (SMTs) including gastrointestinal stromal tumors (GISTs), leiomyomas, schwannomas, aberrant pancreas and more are frequently identified during routine upper endoscopies. Although endoscopic ultrasonography (EUS) is a useful modality for diagnosing gastric SMTs[1], it is not always possible to differentiate a GIST from a leiomyoma or schwannoma by EUS, especially when the tumor originated from the muscularis propria layer. GISTs are rare neoplasms that account for only 0.1%-3% of all gastrointestinal (GI) malignancies[2-4], whereas they represent approximately 80% of GI mesenchymal tumors[5]. As GISTs are potentially malignant, histological diagnosis by an EUS- fine-needle aspiration biopsy (FNAB) is recommended[6,7]. It is thus very important to discriminate these lesions from benign SMTs originating from the muscularis propria, such as leiomyomas and schwannomas. However, it may be difficult to arrive at the correct histological diagnosis with only a standard endoscopic biopsy, because the surface of an SMT is covered with normal epithelium.
EUS-FNAB is a reliable, useful and suitable method for the histological evaluation of SMTs[8-10]. Although EUS-FNAB is used widely, only a limited number of cases are subjected to this method, even in hospitals specializing in gastroenterology. In addition, EUS-FNAB systems including an echoendoscope and its observing system are very expensive and require not only experienced pathologists but also cytology technicians capable of handling and processing biopsy specimens[7]. The successful diagnostic rate for SMT by an EUS-FNAB combined with cytology has been reported to be relatively high (83%), but the success rate for histology is not satisfactory (50%)[9,11-13]. An alternative modality for the histological diagnosis of SMTs is thus needed.
Endoscopic submucosal dissection (ESD) was developed in Japan in the 2000s[14] and has since been widely adopted for the treatment of superficial gastric neoplasms. By applying this method, Lee et al[15] described cases in which an ESD-associated technique rather than EUS-FNAB was useful for the tissue sampling of SMTs. The applications of several similar methods for the histological diagnosis of gastrointestinal (GI) SMTs were also reported: mucosal cutting biopsy (MCB), a mucosal incision-assisted biopsy technique, and an “unroofing” biopsy based on endoscopic mucosal resection (EMR)[16-20].
A comparison of the histological diagnostic yield of SMTs between EUS-FNAB without its combination with cytology and MCB has not been published, to our knowledge. The aim of the present study was to prospectively compare the diagnostic yield of gastric SMTs and the feasibility, safety and complications between EUS-FNAB and MCB by performing both diagnostic modalities simultaneously for the same SMT patients.
Between May 2012 and February 2015 in our department, both EUS-FNAB and MCB were prospectively performed for 20 consecutive patients with gastric SMTs ≥ 1 cm in diameter which were diagnosed by EUS (UM2000, UM-2R and 3R; Olympus Optical Corp., Tokyo) prior to the EUS-FNAB and MCB procedures. If the EUS finding of SMT showed mainly inward or outward growth from the gastric wall, the lesion was judged as intraluminal or extraluminal growth, respectively. Since hyperechoic lesions on EUS that originate from the submucosal layer are generally diagnosed as lipoma, these lesions were excluded from the study. All patients were admitted on the day of EUS-FNAB and MCB, and were usually discharged the day after the procedures. Thus the hospital stay for the patients without any clinical complications was generally 1 d, based on the clinical protocol at our hospital.
Written informed consent was obtained from all patients prior to the study, and the study design was approved by the Ethics Committee of Hyogo College of Medicine (No. 1710).
Operator skill may affect the diagnostic yield and the complications of these procedures. In Japan, endoscopists receive board certification from the Japan Gastroenterological Endoscopy (JGES) after 5 years of training in a JGES-approved educational institution of endoscopy and after passing an examination administered by the JGES. Accordingly, the EUS-FNAB and MCB procedures in the present study were performed by expert endoscopists with board certification from the JGES. The same endoscopist performed the EUS-FNAB and MCB in a given patient.
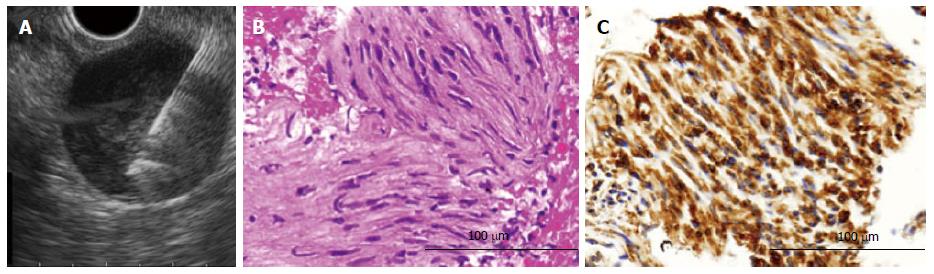
The EUS-FNAB procedure was performed first with the patient under conscious sedation by midazolam with or without pethidine. The EUS-FNAB procedure was performed by expert endoscopists. Fundamentally, a convex linear-array echoendoscope (GF-UCT260; Olympus Optical) connected to an observing system (UM-ME1; Olympus Optical) was used in this procedure. A 22-gauge needle (EchoTip ProCore High Definition Ultrasound Biopsy Needle; Cook Japan, Tokyo) was used to obtain specimens for the histological analysis. After properly targeting the mass, the endoscopist punctured the lesion with the needle. Thereafter, the inner needle was pulled out, and the endoscopist moved the needle back and forth 20 times while applying suction using the connected 10-mL syringe. The EUS-FNAB was performed by making 1 to 4 passes, at the discretion of the endoscopist. That is, when the endoscopist judged that grossly visible material was obtained, the procedure was stopped.
The obtained material was immediately and directly exposed to 10% formalin, and then processed as a tissue block for histopathological evaluation using hematoxylin-eosin and immunohistochemistry (IHC) staining. Cytology was not performed as an on-site cytologist was not available in this procedure, and a cell block for confirmatory IHC was also not prepared.
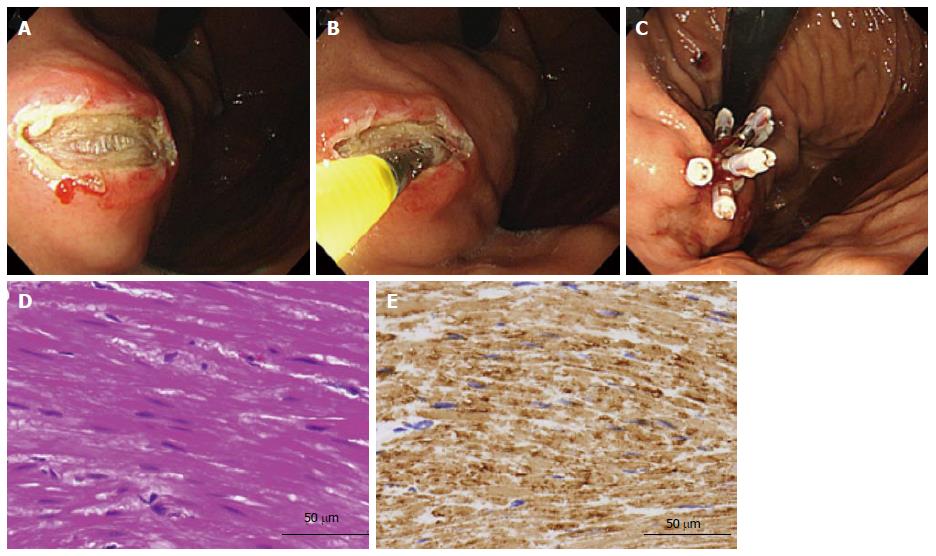
Immediately following the EUS-FNAB in each patient, an MCB was performed. The MCB technique was as follows: first, saline was injected into the submucosa and then mucosal cutting was performed using a needle-knife (KD-1L-1; Olympus Optical). Under direct vision of the SMT, several biopsy specimens were taken using conventional biopsy forceps (Radial Jaw™ 4: Boston Scientific, Natick, MA). One to six biopsy samples were taken at the discretion of the operators. As in the EUS-FNAB procedure, when the endoscopist judged that grossly visible material was obtained, the procedure was stopped. Thereafter, the mucosal incision was closed with hemoclips (EZ Clip™; Olympus Optical) to prevent post-procedure bleeding (Figure 1) and to reduce the risk of ulceration that may cause peritoneal dissemination.
The patient’s oral intake was allowed starting the morning after the day of the procedure, and then the patient was discharged. A proton pump inhibitor was administered for 2 wk after the procedure.
IHC staining of the samples obtained by both methods was performed using the following antibodies: c-kit, CD34, S100 protein, and desmin. Patients diagnosed with a GIST were offered surgical resection.
We evaluated the diagnostic yield and post-procedure bleeding and other complications between the EUS-FNAB and MCB methods, and we tried to determine the causes of nondiagnostic cases.
The data were assessed by Welch’s t test between two groups, and the chi-square test or Fisher’s exact test was used to examine differences between two proportions. Statistical significance was defined as a P value < 0.05. Statistical analyses were performed with GraphPad Prism5 software (GraphPad Software, La Jolla, CA).
Table 1 provides the characteristics of the 20 patients and a summary of the targeted SMTs. All patients underwent EUS prior to the EUS-FNAB and MCB procedures and were diagnosed as having a gastric SMT originating from the submucosal (third layer) or the muscularis propria layer (fourth layer). The mean age of the patients was 61.8 ± 12.5 years (range 39-77 years), and women accounted for 50.0% of the patients. The tumors had a mean size of 23.6 mm (range 10-57). Among the 20 cases, four showed extraluminal growth on EUS. The histological diagnoses were GIST (n = 7), leiomyoma (n = 6), schwannoma (n = 2), aberrant pancreas (n = 2), glomus tumor (n = 1), metastatic hepatocellular carcinoma (HCC, n = 1), and nondiagnostic (n = 1).
| Age, mean ± SD (yr) | 61.8 ± 12.5 |
| Male: Female | 10:10 |
| Tumor location (upper/middle/lower) | 11/8/1 |
| Tumor size ± SD (range) (mm) | 23.6 ± 11.5 (10-57) |
| ≤ 20 mm : > 20 mm | 7:13 |
| Growth pattern | |
| Intraluminal | 16 |
| Extraluminal | 4 |
| Histological diagnosis | |
| Gastrointestinal stromal tumor | 7 |
| Leiomyoma | 6 |
| Schwannoma | 2 |
| Aberrant pancreas | 2 |
| Glomus tumor | 1 |
| Metastatic hepatocellular carcinoma | 1 |
| Not diagnosed | 1 |
Of the three cases that could not be diagnosed by either method, two were treated surgically and diagnosed histologically as a schwannoma and a glomus tumor, respectively, and the third case is being followed closely without treatment. The tumors were located at the lesser curvature of the middle corpus in the schwannoma, at the greater curvature of the fornix for the glomus tumor, and at the greater curvature of the antrum in the nondiagnostic case. All seven GIST cases diagnosed by EUS-FNAB or MCB were surgically resected and confirmed histologically as GISTs.
The median values 3.0 [interquartile range (IQR): 2.0, 4.0] for the EUS-FNAB samples and 3.0 (IQR: 1.5, 4.5) for the MCB samples were obtained per patient. All 15 cases of GIST, leiomyoma and schwannoma were diagnosed by IHC. The rates of histological definitive diagnosis were 65.0% (13 of 20) by EUS-FNAB and 60.0% (12 of 20) by MCB, a nonsignificant difference. The concordance rate of the histological diagnosis between the two methods was 100%. There were also no significant differences in the diagnostic yield regarding tumor location or tumor size between the EUS-FNAB and MCB methods (Table 2). However, diagnostic specimens were significantly more frequently obtained in lesions with intraluminal growth than in those with extraluminal growth in the MCB method (P = 0.01). All four of the SMTs that showed extraluminal growth, including three GISTs and the single HCC, were correctly diagnosed only by EUS-FNAB (Figure 2), and not by MCB (P = 0.03). Seventeen of the SMTs (85.0%) were histologically diagnosed by both methods.
| EUS-FNAB | P value | MCB | P value | |||||||
| Diagnosed (%)(n = 13) | Not diagnosed (%)(n = 7) | Diagnosed (%)(n = 12) | Not diagnosed (%)(n = 8) | |||||||
| Histological diagnosis | 13 (65.0) | 12 (60.0) | > 0.99 | |||||||
| Location 1 | ||||||||||
| Upper | 7 | (63.6) | 4 | (36.4) | 0.33 | 8 | (72.7) | 3 | (27.3) | 0.28 |
| Middle | 6 | (75.0) | 2 | (25.0) | 4 | (50.0) | 4 | (50.0) | ||
| Lower | 0 | (0) | 1 | (100) | 0 | (0) | 1 | (100) | ||
| Location 2 | ||||||||||
| Lesser curvature | 4 | (66.7) | 2 | (33.3) | 0.81 | 3 | (50.0) | 3 | (50.0) | 0.27 |
| Greater curvature | 2 | (50.0) | 2 | (50.0) | 1 | (25.0) | 3 | (75.0) | ||
| Anterior wall | 4 | (80.0) | 1 | (20.0) | 4 | (80.0) | 1 | (20.0) | ||
| Posterior wall | 3 | (60.0) | 2 | (40.0) | 4 | (80.0) | 1 | (20.0) | ||
| Tumor size | ||||||||||
| ≤ 20 mm | 7 | (58.3)a | 5 | (41.7) | 0.641 | 9 | (75.0)a | 3 | (25.0) | 0.171 |
| > 20 mm | 6 | (75.0)c | 2 | (25.0) | 3 | (37.5)c | 5 | (62.5) | ||
| Growth pattern | ||||||||||
| Intraluminal | 9 | (56.3)e | 7 | (43.8) | 0.101 | 12 | (75.0)e | 4 | (25.0) | 0.011 |
| Extraluminal | 4 | (100)b | 0 | (0) | 0 | (0)b | 4 | (100) | ||
| Median number of samples to the diagnosis (IQR) | 1.0 | (1.0, 1.0) | 1.0 | (1.0, 1.75) | ||||||
| Median number of samples (IQR) | 3.0 | (2.5, 3.5) | 3.0 | (3.0, 3.0) | 0.93 | 5.0 | (3.0, 6.0) | 2.5 | (1.0, 5.75) | 0.17 |
Two cases showed mild bleeding during the MCB procedure, but both were successfully managed by hemoclips. The mean number of hemoclips for closing the incised mucosa was 3.4 (range: 1-6 clips). No post-procedural hemorrhage, fever, or peritonitis was seen following either procedure.
To date, there are many reports on the methods of tissue acquisition from SMTs: EMR, MCB and EUS-FNAB. Histological diagnosis by a standard biopsy or EMR may be confined to SMTs that arise from the muscularis mucosa or submucosa, which corresponds to second- or third-layer lesions on EUS. In contrast, it may be impossible to make a histologic diagnosis of the lesions located in the muscularis propria by these methods. Therefore, EUS-FNAB was suggested to play an important role in histological diagnoses such as gastric SMTs, although the results can be quite variable[10]. However, although the use of EUS-FNAB is quite prevalent, only a limited number of patients undergo this procedure - even in hospitals specializing in gastroenterology - because an expensive dedicated endoscopic system is needed to conduct an EUS-FNAB. For example, the price of the needle for EUS-FNAB is approximately $300 United States dollars (USD), and the total prices of devices such as the needle knife, injection needle and EZ Clip™ for MCB are also approximately $100 USD, and thus the cost may be significantly cheaper in the MCB method than in the EUS-FNAB method. Additionally, the needle knife used in the MCB method is reusable. The EUS-FNAB system comprised of an echoendoscope and its observing system is very expensive, over $100000 USD. Therefore, MCB may be the less expensive procedure compared to EUS-FNAB.
Generally, the accuracy rates of EUS-FNAB in the histological diagnosis of gastric SMTs vary from approximately 60% to 80%[9,11]. It was noted that the sensitivity of EUS-FNAB for the diagnosis of GIST, especially in cases of small GISTs, is relatively low compared to that for other types of SMTs[21,22], indicating that the diagnostic yield may be influenced by the lesion’s size[11,21-24], because technical expertise is required to diagnose smaller lesions by EUS-FNAB. Several studies showed that the diagnostic rate increased with the increase of tumor size[22-24], but another study did not observe this association[11]. A recent report by Sekine et al[25] showed that cytological or histological specimens obtained by EUS-FNAB met the diagnostic criteria of GIST in 81.3% of the cases, even among small GISTs (< 20 mm). In their procedure, the samples obtained by EUS-FNAB were examined immediately with a rapid staining method to verify the adequacy of the specimen and to provide a presumptive diagnosis. It was reported that when an on-site cytopathologist immediately reviewed the adequacy of the samples, the sensitivity of EUS-FNAB was > 90%[23,26]. It was also suggested that the sensitivity of EUS-FNAB drops by 10%-15% in the absence of an on-site pathologist to evaluate the cellular adequacy of the samples[27]. However, this diagnostic procedure for cytology during an EUS-FNAB may be troublesome for not only endoscopists but also cytology technicians in daily medical practice. For this reason, an easy and useful diagnostic tool for SMT regardless of the tumor size is needed.
In the present study, the histological diagnosis rate of MCB was significantly higher than that of EUS-FNAB in the lesions with intraluminal growth (P = 0.01). In addition, all four extraluminal-growth tumors could be histologically diagnosed only by EUS-FNAB. The diagnostic capability of MCB was thus increased from 60% to 75% when the four cases with extraluminal growth were excluded. Additionally, when we examined the histological diagnosis for both methods together, the accurate histological diagnosis increased to 85%. In previous studies, the accurate histological diagnosis of MCB ranged from 85% to 100%[16-18], which is relatively higher than that of our study. One of the reasons for the differences in diagnostic yield may be that the numbers of cases in those studies were relatively small, and they were retrospective studies. To date, there are two studies that compared the diagnostic yield between the jumbo biopsy “unroofing” technique and EUS-FNAB[28,29]. The results of both studies indicated a lower diagnostic yield than ours. In those studies, the diagnostic procedures (i.e., jumbo biopsy vs EUS-FNAB) were not performed during the same session in the same patients, as was done in our study. In addition, both studies[28,29] included many cases of lipoma (16.6%-22.1%), which is considered to be easily diagnosed by the jumbo biopsy unroofing technique or only by EUS.
The diagnostic yield of the EUS-FNAB method in the present study was relatively lower compared to previous reports[26,30]. One of the reasons might be an effect of the difference in the FNA needle size used for the EUS-FNABs. The larger-bore 19-gauge needle may actually show a higher diagnostic yield compared to the 22-gauge needle used in the present study[24,31], but the exact difference in diagnostic yield between 19- and 22-gauge FNA needles remains unclear[24,30]. We did not adequately assess procedural factors such as the needle gauge and the number of needle passes in the present study. More passes or the use of a larger-bore needle would provide more tissue. However, Sepe et al[23] reported that the number of passes did not significantly affect the diagnostic capability of EUS-FNAB. In their study, as is standard practice, this decision regarding the number of passes was made at the discretion of the individual endosonographer and was based on a real-time assessment of presumed tissue adequacy, as in our study, and our finding is in agreement with their result[23].
No major complications were caused by either the EUS-FNAB or MCB method in the present study, although mild bleeding occurred in two cases during the MCB; both were successfully managed by hemoclips. Perforation did not occur in any of the 20 patients during MCB, but extra care should be taken to prevent perforation in cases with extraluminal growth[17]. A laparoscopic and endoscopic cooperative surgery (LECS) is now being performed for the treatment of gastrointestinal SMTs[32,33]. However, the MCB method is unlikely to preclude LECS for the treatment of SMTs.
The present study had some potential limitations. First, the sample size of this study was small and drawn from a single institution. When the diagnostic yield is assumed to be approximately 70% for the EUS-FNAB without cytology method and approximately 90% for the MCB method, 62 patients with SMT are needed in each group in order to have a power of 80% to detect a difference at the level significance of α = 0.05 (two-sided).
Second, there is the issue of EUS-FNAB- and MCB-related dissemination as a late complication, but this has not been reported to date. It is important to close the mucosal incisions appropriately with endoclips after tissue sampling to prevent post-procedure complications in MCB[17]. Third, if the diagnostic yield of the combination of EUS-FNAB and MCB is assessed, the histological diagnosis by the two methods should be compared to that of a surgically resected whole specimen as a “golden standard.”
In conclusion, although EUS-FNAB is the widely used gold standard for the histological and cytological diagnoses of gastric SMTs, MCB may be chosen as an alternative diagnostic modality in tumors showing the intraluminal growth pattern. A randomized controlled trial to compare the capability of MCB with that of EUS-FNAB is needed.
As gastric submucosal tumors (SMTs) comprise both benign and malignant lesions, histological diagnosis is needed. Endoscopic ultrasonography-guided fine-needle aspiration biopsy (EUS-FNAB) is a useful method for the histological evaluation of SMTs. However, EUS-FNAB systems are very expensive and require experienced pathologists and cytology technicians, and thus this procedure may be unavailable in hospitals not specializing in gastroenterology.
Although the diagnostic yields of EUS-FNAB and mucosal cutting biopsy (MCB) have been reported, there are no studies comparing the diagnostic capabilities of EUS-FNAB and MCB based on endoscopic submucosal resection in the same patients. The authors prospectively compared the diagnostic yield, feasibility, and safety of these two methods.
In this prospective study, no significant difference in histological diagnosis was found between EUS-FNAB and MCB regardless of tumor location and tumor size. However, diagnostic specimens were significantly more frequently obtained in the lesions with intraluminal growth compared to those with extraluminal growth by the MCB method. All SMTs with extraluminal growth were diagnosed only by EUS-FNAB, not by MCB. No complications were produced by either method.
MCB may be chosen as an alternative diagnostic modality in tumors showing an intraluminal growth pattern regardless of tumor size, whereas EUS-FNAB should be performed for SMTs with extraluminal growth.
EUS-FNAB: This method is a needle biopsy procedure considered to be a reliable and accurate method for the evaluation of SMTs in the gastrointestinal tract; gastrointestinal stromal tumor (GIST): GISTs are the most common mesenchymal neoplasms of the gastrointestinal tract.
The authors present an interesting result regarding the efficacy of MCB in the histological diagnosis of SMTs. This procedure will be accepted widely even in hospitals not specializing in gastroenterology.
P- Reviewer: Amornyotin S, Pauli E, Yan SL S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 2. | Crosby JA, Catton CN, Davis A, Couture J, O’Sullivan B, Kandel R, Swallow CJ. Malignant gastrointestinal stromal tumors of the small intestine: a review of 50 cases from a prospective database. Ann Surg Oncol. 2011;8:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1682] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 4. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. [PubMed] |
| 5. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1178] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 6. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 866] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 7. | Japan Society of Clinical Oncology, Japanese Gastric Cancer Association, Japanese Study Group on GIST. GIST Therapeutic Guidelines. Tokyo: Kanehara Co., Ltd 2008; . |
| 8. | Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Matsui M, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Preliminary results of fine needle aspiration biopsy histology in upper gastrointestinal submucosal tumors. Endoscopy. 1998;30:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Philipper M, Hollerbach S, Gabbert HE, Heikaus S, Böcking A, Pomjanski N, Neuhaus H, Frieling T, Schumacher B. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Mekky MA, Yamao K, Sawaki A, Mizuno N, Hara K, Nafeh MA, Osman AM, Koshikawa T, Yatabe Y, Bhatia V. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 736] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 13. | Wiech T, Walch A, Werner M. Histopathological classification of nonneoplastic and neoplastic gastrointestinal submucosal lesions. Endoscopy. 2005;37:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Lee HL, Kwon OW, Lee KN, Jun DW, Eun CS, Lee OY, Jeon YC, Han DS, Yoon BC, Choi HS. Endoscopic histologic diagnosis of gastric GI submucosal tumors via the endoscopic submucosal dissection technique. Gastrointest Endosc. 2011;74:693-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Kataoka M, Kawai T, Yagi K, Sugimoto H, Yamamoto K, Hayama Y, Nonaka M, Aoki T, Fukuzawa M, Fukuzawa M. Mucosal cutting biopsy technique for histological diagnosis of suspected gastrointestinal stromal tumors of the stomach. Dig Endosc. 2013;25:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Ihara E, Matsuzaka H, Honda K, Hata Y, Sumida Y, Akiho H, Misawa T, Toyoshima S, Chijiiwa Y, Nakamura K. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J Gastrointest Endosc. 2013;5:191-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Kobara H, Mori H, Fujihara S, Nishiyama N, Kobayashi M, Kamata H, Masaki T. Bloc biopsy by using submucosal endoscopy with a mucosal flap method for gastric subepithelial tumor tissue sampling (with video). Gastrointest Endosc. 2013;77:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Lee CK, Chung IK, Lee SH, Lee SH, Lee TH, Park SH, Kim HS, Kim SJ, Cho HD. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010;71:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | de la Serna-Higuera C, Pérez-Miranda M, Díez-Redondo P, Gil-Simón P, Herranz T, Pérez-Martín E, Ochoa C, Caro-Patón A. EUS-guided single-incision needle-knife biopsy: description and results of a new method for tissue sampling of subepithelial GI tumors (with video). Gastrointest Endosc. 2011;74:672-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, Heresbach D, Pujol B, Fernández-Esparrach G, Vazquez-Sequeiros E. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 22. | Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077-2082. [PubMed] |
| 23. | Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Watson RR, Binmoeller KF, Hamerski CM, Shergill AK, Shaw RE, Jaffee IM, Stewart L, Shah JN. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig Dis Sci. 2011;56:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Sekine M, Imaoka H, Mizuno N, Hara K, Hijioka S, Niwa Y, Tajika M, Tanaka T, Ishihara M, Ito S. Clinical course of gastrointestinal stromal tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Dig Endosc. 2015;27:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration of intramural and extraintestinal mass lesions: diagnostic accuracy, complication assessment, and impact on management. Endoscopy. 2005;37:984-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 299] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 28. | Komanduri S, Keefer L, Jakate S. Diagnostic yield of a novel jumbo biopsy “unroofing” technique for tissue acquisition of gastric submucosal masses. Endoscopy. 2011;43:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 29. | Buscaglia JM, Nagula S, Jayaraman V, Robbins DH, Vadada D, Gross SA, DiMaio CJ, Pais S, Patel K, Sejpal DV. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012;75:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Eckardt AJ, Adler A, Gomes EM, Jenssen C, Siebert C, Gottschalk U, Koch M, Röcken C, Rösch T. Endosonographic large-bore biopsy of gastric subepithelial tumors: a prospective multicenter study. Eur J Gastroenterol Hepatol. 2012;24:1135-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Larghi A, Verna EC, Ricci R, Seerden TC, Galasso D, Carnuccio A, Uchida N, Rindi G, Costamagna G. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: a prospective study. Gastrointest Endosc. 2011;74:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (2)] |
| 33. | Kang WM, Yu JC, Ma ZQ, Zhao ZR, Meng QB, Ye X. Laparoscopic-endoscopic cooperative surgery for gastric submucosal tumors. World J Gastroenterol. 2013;19:5720-5726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |