Published online Oct 10, 2015. doi: 10.4253/wjge.v7.i14.1114
Peer-review started: May 29, 2015
First decision: July 6, 2015
Revised: August 27, 2015
Accepted: September 7, 2015
Article in press: September 8, 2015
Published online: October 10, 2015
Processing time: 144 Days and 10.3 Hours
Endoscopic submucosal dissection (ESD) is very useful in en bloc resection of large superficial colorectal tumors but is a technically difficult procedure because the colonic wall is thin and endoscopic maneuverability is poor because of colonic flexure and extensibility. A high risk of perforation has been reported in colorectal ESD. To prevent complications such as perforation and unexpected bleeding, it is crucial to ensure good visualization of the submucosal layer by creating a mucosal flap, which is an exfoliated mucosa for inserting the tip of the endoscope under it. The creation of a mucosal flap is often technically difficult; however, various types of equipment, appropriate strategy, and novel procedures including our clip-flap method, appear to facilitate mucosal flap creation, improving the safety and success rate of ESD. Favorable treatment outcomes with colorectal ESD have already been reported in many advanced institutions, and appropriate understanding of techniques and development of training systems are required for world-wide standardization of colorectal ESD. Here, we describe recent technical advances for safe and successful colorectal ESD.
Core tip: Endoscopic submucosal dissection (ESD) is useful for en bloc resection of large colorectal tumors but is a technically difficult procedure. Good visualization of the submucosal layer is crucial for safely and successfully performing colorectal ESD because poor visualization of the operative field may result in perforation or unexpected bleeding. Creating a mucosal flap solves these problems; however, it is the process that requires the most skill in this procedure. To facilitate the mucosal flap creation, we developed the clip-flap method, which is simple and very effective for colorectal ESD. We described recent advances in colorectal ESD techniques and devices.
- Citation: Yamamoto K, Michida T, Nishida T, Hayashi S, Naito M, Ito T. Colorectal endoscopic submucosal dissection: Recent technical advances for safe and successful procedures. World J Gastrointest Endosc 2015; 7(14): 1114-1128
- URL: https://www.wjgnet.com/1948-5190/full/v7/i14/1114.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i14.1114
Endoscopic submucosal dissection (ESD) was recently developed for en bloc resection of early stage gastrointestinal neoplasms with negligible risk of lymph node metastasis[1-5]. Higher rates of en bloc resection of large colorectal tumors have been reported with colorectal ESD than with endoscopic mucosal resection (EMR); however, colorectal ESD confers an increased risk of perforation[6-10]. A high degree of technical skill and the development of specific strategies for colorectal ESD are required because of the anatomical characteristics of the colon, namely being a long and winding tube with a thinner wall than other regions of the gastrointestinal tract[4,6]. To prevent complications such as perforation and uncontrollable bleeding, it is crucial to maintain good visualization of the submucosal layer to be dissected[4,11,12]. Therefore, the mucosal flap creation is the key procedure[12], although this process is technically challenging. To facilitate the mucosal flap creation, we recently developed the clip-flap method in which an endoclip is initially substituted for the mucosal flap[13-15]. Several types of endoknives were developed and properly utilizing them according to the requirements is also important. In this review, recent advances in techniques using various devices in colorectal ESD will be described.
Before performing colorectal ESD, the determination of the indications for ESD by preoperative examination is highly important. EMR using a snare remains the main treatment for superficial colorectal tumors. However, EMR is not adequate for en bloc resection of flat lesions larger than 20 mm in diameter because incomplete removal and local recurrence are occasionally observed[16,17]. The indications for ESD are therefore considered for a tumor when using EMR for en bloc resection is difficult. The guidelines on the indications for colorectal ESD were published in Japanese and Spanish academic societies of gastrointestinal endoscopy[18,19]. Basically, the indications for ESD are colorectal tumors for which endoscopic en bloc resection is required but en bloc resection with EMR is difficult to apply. The primary objective lesions are large colorectal tumors, such as the laterally spreading tumor granular type (LST-G) with a large nodule or the laterally spreading tumor non-granular type (LST-NG)[20,21], which are suspected to be intramucosal or with slightly invaded submucosal cancers > 20 mm in diameter in the preoperative examinations. Large protruding lesions are also indications for colorectal ESD[18,19]. However, an abundance of caution is required to treat large protruding lesions because even experienced endoscopists sometimes cannot avoid discontinuation of submucosal dissection due to severe submucosal fibrosis and retracted muscle[22]. Even if the size of the tumor is less than 20 mm, mucosal lesions with submucosal fibrosis, which cannot be resected with EMR, can be the indications for ESD.
In contrast, the technical simplicity of EMR can permit its utilization for colorectal tumors > 20 mm in diameter when the preoperative diagnosis is adenoma or mucosal cancer in adenoma[18-20], although piecemeal mucosal resection includes the problem of a high local recurrence rate[20]. Magnifying chromoendoscopy for pit pattern observation[23] and magnifying image-enhanced endoscopy (narrow band imaging[24,25] or blue laser imaging[26], etc.) are useful for preoperative differential diagnosis of adenoma, intramucosal cancer, and submucosal invasive cancers. It is better to avoid preoperative biopsy if the endoscopic treatment is planned to be performed because biopsy often causes submucosal fibrosis, complicating further endoscopic treatment[18]. In addition, the endoscope maneuverability should be analyzed before performing ESD because poor endoscope maneuverability may cause incomplete resection or complications[27,28].
Bowel preparation is required for adequate visualization of the operative field and as prophylaxis against bacterial peritonitis in case of perforation. Patients are restricted to a low-fiber diet on the day before colorectal ESD and are instructed to orally consume 10 mL picosulfate after the last meal on the day before the procedure. Two-four liters of an electrolyte solution is orally administrated before the procedure[11,29].
In contrast, no food or drink is allowed on the day of the procedure or the following day. Provided that there are no signs or symptoms of complications, patients will begin drinking water on day 1 and have light meals (rice porridge) on day 2. Meals are upgraded to normal food with alcohol excluded from day 2 until day 3-5 or the date of hospital discharge[30-32].
Light or conscious sedation is appropriate for colorectal ESD because deep sedation makes alteration of the patient’s position difficult and often leads to severe respiratory fluctuations[11]. At our institution, midazolam (2 mg) and pethidine (17.5-35 mg) is initially intravenously administered. Light sedation is maintained with additional administration of midazolam or pethidine during the procedure. In cases where a long procedure duration is expected, the use of dexmedetomidine may be useful in maintaining good sedation levels[33,34]. Use of a carbon dioxide (CO2) insufflation system (UCR; Olympus Co., Tokyo, Japan) is extremely helpful for reducing the patient’s discomfort and risk of peritonitis in case of perforation[35-37]. Excessive air present during the procedure decreases the endoscope maneuverability, but carbon dioxide can be quickly absorbed[35-37]. Yoshida et al[38] reported that CO2 insufflation during colorectal ESD was safe even for patients with obstructive ventilator disturbance.
Scopolamine butylbromide (10 mg) is administrated to all patients except those contraindicated because of reduced bowel movement immediately prior to the procedure. Additional doses may be administered during the procedure. Administration of intravenous glucagon[39] or intraluminal peppermint oil[40] may be useful for patients who are contraindicated for scopolamine.
The patient’s position is critical in performing successful colorectal ESD. In principle, the lesion should be moved upward as far as possible against the force of gravity prior to ESD and followed by a postural change to take advantage of the counter-traction of gravity[4,11,41,42]. The direction of gravity can be understood by the pooling of water or indigo carmine dye[11]. However, the intestinal lumen may become narrower or broader on alteration of the patient’s position due to the movement of air. ESD becomes particularly challenging in narrowed lumen. Therefore, it is recommended that ESD should be commenced after each position (supine, prone, left lateral decubitus position, and right lateral decubitus position) has been adequately assessed as far as possible. In case of large lesions, changing the patient’s position during the procedure is often required to ensure optimal operative field[4].
ESD is generally performed using a single-channel colonoscope. At our institution, PCF-H290I or CF-H290I (Olympus), which have a water-jet function, are currently predominantly used because the water-jet function is convenient for hemostasis during ESD. Moreover, a gastroendoscope (GIF-HQ290, GIF-Q260J; Olympus) may be used for lesions in the rectum or distal sigmoid colon because the shorter endoscope can be easily operated in such locations[11,43]. In addition, a gastroendoscope can be used to approach lesions from the oral side in retroflexion more easily than with a conventional colonoscope.
Endoscope maneuverability is crucial to precisely perform ESD. ESD is challenging in cases of poor endoscope maneuverability, although experts can overcome these difficulties in most cases. Straightening of the endoscope is important for maintaining good endoscope maneuverability. Single-balloon[44] (OBCU; Olympus) or double-balloon endoscopy systems[45,46] (PB-20; Fujifilm Co., Tokyo, Japan) may be useful in cases of extremely poor maneuverability.
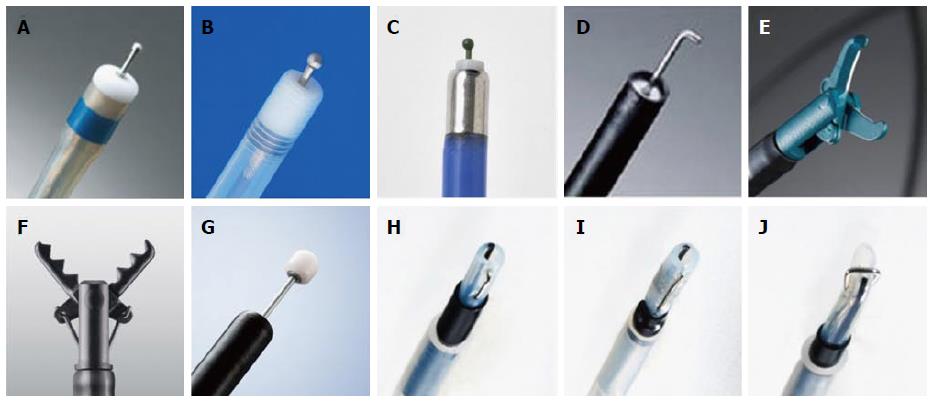
The use of distal attachments is essential in safely performing colorectal ESD. The cutting area can be broadened and visualized with the use of distal attachments during the procedure. The shapes of distal attachments for colorectal ESD are mainly divided into straight types (D-201; Olympus, Figure 1A) and tapered types. Straight distal attachments allow larger working spaces for the operation of endoknives or forceps; however, the submucosal layer must be cut more deeply to insert the attachment under the exfoliated mucosa compared with tapered distal attachments. At our institution, a distal attachment (F-050/020, M-02/03/01; Top Corp., Tokyo, Japan, Figure 1B and C), which is slightly tapered, is attached to the tip of the endoscope. Small-caliber tip transparent hoods (ST-hood; Fujifilm) (Figure 1D) are useful for accessing narrow cutting areas[4,47]. Furthermore, this distal attachment is used for the tunnel[41,48] or pocket-creation method[49].
Various types of endoknives are used for colorectal ESD. Short-needle knives are the most widely used type of endoknife for colorectal ESD. The DualKnife[50] (Olympus, Figure 2A) is a short-needle endoknife that has a small disk at the tip of a short needle. The FlushKnife BT[51] /FlushKnife (Fujifilm, Figure 2B), Jet B-knife (Zeon Medical, Tokyo, Japan, Figure 2C)[52], and Splash needle (Pentax Medical, Tokyo, Japan) are all short-needle knives with a water-jet function that enable submucosal injection without requiring the injection needle to be changed. The HookKnife[53] (Olympus, Figure 2D) has a hook on the tip that enables hooking and cutting of submucosal tissue. The HookKnife is particularly useful when the tangential approach is difficult or submucosal fibrosis is present because the submucosal tissue can be easily hooked and cut with this endoknife. The SBknife Jr[54-56] (Sumitomo Bakelite, Tokyo, Japan, Figure 2E) and Clutch Cutter[57] (Fujifilm) (Figure 2F) are scissor-type endoknives that have a rotation function. Scissor-type endoknives can be easily operated in the manner of forceps even by inexperienced operators. In addition, it can be efficiently operated even in cases when the tangential approach is difficult or endoscope maneuverability is extremely poor, because the submucosal tissue can be dissected simply by grasping, lifting, and applying an electrical current. The ITknife-nano (Olympus, Figure 2G) is an endoknife with an insulator on the tip of the blade that was developed for colorectal or esophageal ESD. Its use may allow increased dissection speeds[21] because it has a long blade between the insulated-tip and the sheath. The Mucosectom[58] (PENTAX, Figure 2H and I) and Swanblade (PENTAX, Figure 2J) have blades on an insulated rod that has a rotation function. These endoknives were developed for the safe and rapid dissection of the submucosal layer. At our institution, the FlushKnife BT (DK2618JB15/20) is predominantly used. According to the specific situation, other endoknives may be used in conjunction.
A high-frequency generator with an automated control system is required for ESD. At our institution, the VIO 300D (Erbe Elektromedizin GmbH, Tubingen, Germany) is predominantly used. ICC-200 (Erbe) or ESG-100 (Olympus) are also used for colorectal ESD. The settings on each instrument when using short-needle knives (FlushKnife BT, DualKnife) and hemostatic forceps (FD-410LR, FD-411QR; Olympus) are shown in Table 1[11,50,59].
| Device | Mucosal incision | Submucosal dissection | Hemostasis |
| FlushKnifeBT with VIO 300D (at our institution) | Endocut I, effect 2, duration 3, interval 3 | Forced coag, effect 2, 40-50 W Swift coag, effect 2, 40-50 W | Forced coag, effect 2, 40-50 W Swift coag, effect 2, 40-50 W |
| with ICC 200 (at our institution) | Endocut, effect 2-3, 80-120 W | Forced coag, 40-50 W Endocut, effect 2-3, 80-120 W | Forced coag, 40-50 W |
| DualKnife with VIO 300D[49] | Dry cut, effect 2, 30 W | Swift coag, effect 4, 30 W | Swift coag, effect 4, 30 W |
| with ESG-100[59] | Pulse-cut-slow, 50 W | Forced coag, effect 2 | Forced coag, effect 2 |
| Hemostatic forceps | |||
| FD-410LR with VIO 300D[11] | Soft coag, effect 5, 50 W | ||
| with ICC 200 (at our institution) | Soft coag, 80 W | ||
| with ESG-100[59] | Soft coag, 80 W | ||
| FD-411QR with VIO 300D (at our institution) | Soft coag, effect 6, 80-100 W |
ESD is usually initiated either from the anal side of the lesion in a forward direction or from the oral side in retroflexion[11,43]. There are benefits and limitations to both methods. Dissection from the anal side can be performed in almost all cases; however, endoscope maneuverability is somewhat unstable, and the treatment of the mucosa just beyond a haustrum or a colonic flexure is occasionally challenging. Dissection from the oral side in retroflexion requires adequate space with a broad lumen; however, endoscope maneuverability is comparatively stable using this method[11,60]. The method selection by endoscopists largely depends on the institutions’ established procedures and lesion location. At our institution, dissection from the anal side is predominantly performed and dissection from the oral side in retroflexion is occasionally performed in cases where approaching from anal side is difficult.
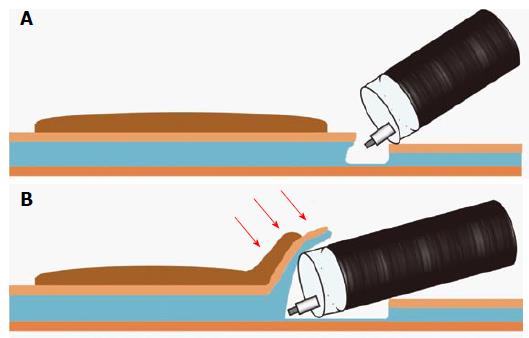
In either case, it is important to start dissecting the submucosa immediately proximal to the tip of the endoscope to avoid complications, such as perforation and unexpected bleeding, caused by blind procedures. Therefore, insertion of a distal attachment under the exfoliated mucosa of the lesion side is a crucial step in safely and effectively dissecting the submucosal layer. The lesional exfoliated mucosa is called the mucosal flap (Figure 3B)[12,61]. Formation of the mucosal flap facilitates safe and sequential dissection.
While approaching from the anal side, a solution is injected in the submucosal layer of the anal side of the lesion and then the lesion tends to be more tangentially approached and more easily dissected (Figure 4B). Saline, 0.4% sodium hyaluronate solution (MucoUp; Johnson and Johnson, Tokyo, Japan) (Sigmavisc; Hyaltech Ltd., Livingston, United Kingdom), or 10% glycerin with a small amount of indigo carmine dye and 0.001% epinephrine are usually used as the injected solution[11,41,47,62]. Sodium hyaluronate solution is the most long-acting agent that can be locally injected for colorectal ESD[63]. Suvenyl (2% hyaluronate, Chugai, Tokyo, Japan) or Artz (1% hyaluronate, Seikagaku Corp. Tokyo, Japan) may be used after coordinating their concentrations[4,47,63].
Following submucosal injection, the mucosa adjacent to the lesion is incised with an adequate margin before incision of the submucosal layer. A complete or partial circumferential mucosal incision is initially made according to the institutions’ established procedures or characteristics of the lesion. A partial circumferential mucosal incision has recently been introduced at an increasing number of institutions because initial complete circumferential mucosal incision can make insertion of the distal attachment under the exfoliated mucosa difficult because of the loss of mucosal tension caused by extensive mucosal incision[4,11,30]. At our institution, a partial circumferential mucosal incision from the anal side is usually made (Figure 4C) because it allows widening of the gap between lesional and non-lesional mucosa and greater ease of insertion of the distal attachment under the exfoliated mucosa. In partially circumferential mucosal incision, a complete circumferential mucosal incision is made after the creation of the mucosal flap.
At our institution, mucosal incision with the FlushKnife BT is performed with the endocut I mode. Deeper cut of the submucosal layer is performed with the forced coagulation or swift coagulation mode.
Insertion of the distal attachment under the exfoliated mucosa is critical in allowing dissection of the submucosal layer while maintaining a good operative field. However, adequate visualization of the submucosal area at the beginning of the dissection is difficult because it is commonly hidden under the exfoliated mucosa. Poor visualization of the submucosal layer to be dissected may cause perforation and unexpected bleeding. To enhance visualization and ensure safe dissection of the submucosal layer, a mucosal flap must be created. Insertion of the distal attachment under the mucosal flap elevates the mucosal flap and provides counter-traction to the submucosal layer that allows easier dissection (Figure 4J). Therefore, creation of the mucosal flap is the most important step of the ESD procedure[12]; however, this process requires the most technical skill. The presence of submucosal fibrosis or vasculature often hinders smooth dissection and vertical approaches make creation of the mucosal flap more challenging. Changing the type of endoknife (Figure 5A-C) or using a tapered-type distal attachment may have utility in cases where the creation of the mucosal flap proves difficult.
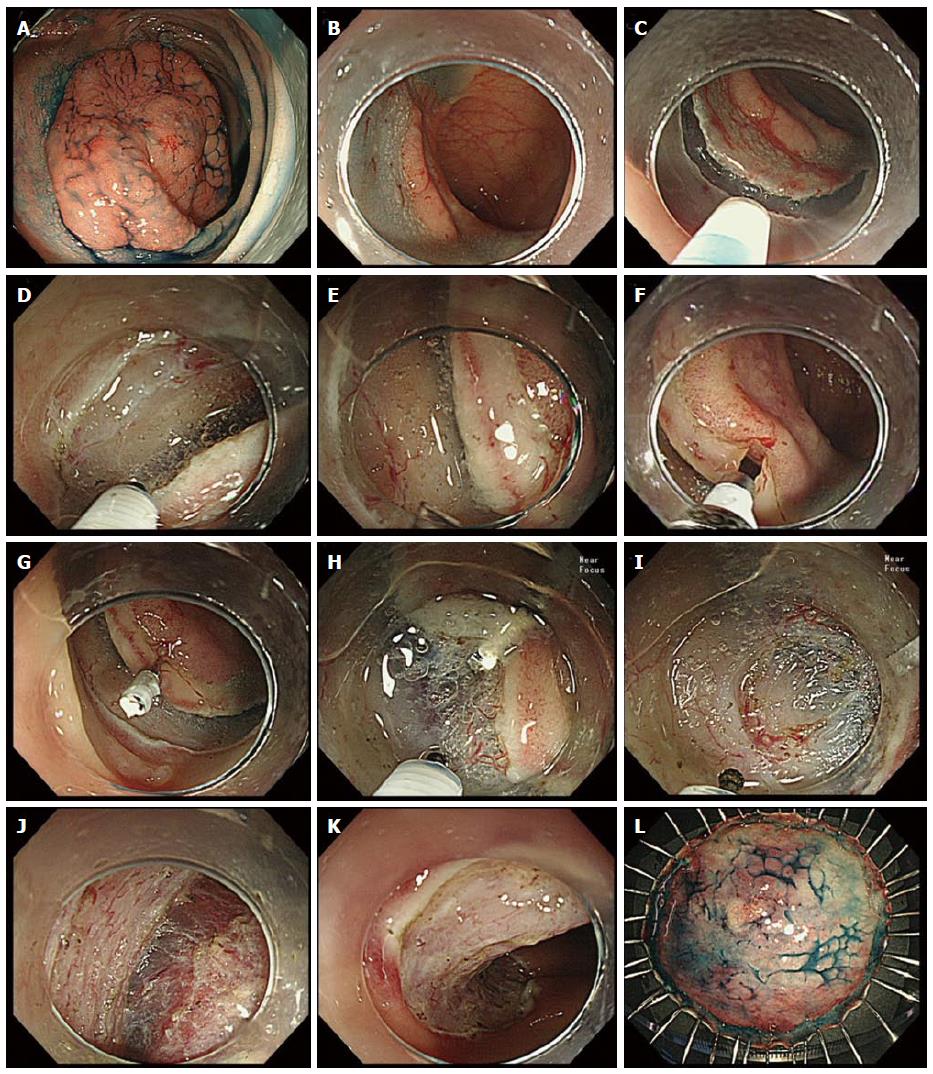
To facilitate the mucosal flap creation, we developed the clip-flap method, in which an endoclip is substituted for the mucosal flap until the flap is completed[13-15]. The basic procedure is as follows.
After submucosal injection, the mucosa adjacent to the lesion on the anal side is incised with an adequate margin and then the submucosal layer is cut deeply (Figure 4A-E). The edge of the exfoliated mucosa is clipped with an endoclip (EZ Clip, HX-610-135; Olympus; Figure 4F and G) while lifting the exfoliated mucosa with the prongs of the endoclip, so that the deep layer of the submucosa is not grasped by the endoclip. The distal attachment is inserted under the endoclip, and then the endoclip is lifted with the distal attachment. Consequently, the exfoliated mucosa is pulled up by the endoclip, allowing clear visualization and effective dissection of the submucosal layer by counter-traction using the endoclip (Figure 4H and I). In addition, the distal attachment can be easily inserted under the endoclip when the tail end of the endoclip is directed toward the intestinal lumen (Figure 4G) by using gravity after a postural change or temporarily lifting the endoclip with the endoknife.
Other than a single endoclip (Figure 4G and H), a cross pattern of endoclips created by attaching one endoclip to another endoclip is used to provide good counter-traction according to the situations[14]. We use the EZ clip in the clip-flap method because it can be easily rotated, and it has a joint between the metal prongs and sheath, most of which is made of plastic. The joint may be utilized as a step difference with which to hook it to the distal attachment. A long endoclip may be inappropriate because it can be a hindrance in a narrow lumen.
In our experience, the clip-flap method was effective in most cases, even in the presence of submucosal fibrosis or with a vertical approach, but can be difficult to use in some situations. When lesions are located within a very narrow lumen, such as in the anal tube, just beyond the colonic flexure, or when endoscope maneuverability is extremely poor, attaching the endoclip to the exfoliated mucosa and inserting the distal attachment under the endoclip may be difficult[14].
The clip-flap method is very simple and requires no special equipment other than common rotatable endoclips. Furthermore, various types of distal attachments, including a tapered type, can be used in the clip-flap method.
The endoscopists may apply the clip-flap method or change the endoknife or distal attachment according to the situation, when inserting the distal attachment under the exfoliated mucosa is difficult.
Following mucosal flap formation, adequate visualization of the submucosal layer to be dissected is ensured by lifting the mucosal flap with the distal attachment. Many vessels are present in the submucosal layer. Bleeding worsens the translucency of submucosal layer and makes dissection of the submucosal layer much more challenging after bleeding. Thick vessels are pre-coagulated with hemostatic forceps using the soft coagulation mode and cut after precoagulation with an endoknife[12]. Fat tissue is occasionally observed in the submucosal layer, and the translucent layer to be dissected is found below submucosal fat tissue. The deep submucosal layer should be dissected to determine the presence or absence of massive malignant submucosal invasion[12].
At our institution, submucosal dissection is predominantly performed with the FlushKnife BT using forced or swift coagulation mode. Forced coagulation mode is superior to swift coagulation mode for hemostasis but inferior for incision. Therefore, we initially use forced coagulation mode and change to swift coagulation mode in cases where the submucosal tissue cannot be easily incised with forced coagulation mode because of submergence, fat rich tissue, fibrosis, or burnt tissue. Endocut I mode can also be used for incision of burnt tissue or tissue with severe fibrosis.
Submucosal fibrosis is an important factor that has a large impact on the technical difficulty of dissection[10,27,64-66]. Submucosal fibrosis complicates dissection by losing the translucency of the submucosal layer or narrowing the space between the mucosa and muscle. Furthermore, the presence of submucosal fibrosis is often preoperatively unexpected. Endoscopists must dissect the submucosal layer more carefully in cases of submucosal fibrosis because submucosal fibrosis increases perforation risk. Additional submucosal injection of solution widens the gap between the exfoliated mucosa and muscle layer and enhances the safety of submucosal dissection. A short needle knife with a water-jet function, such as FlushKnife BT, is very useful in these situations because it enables repeated submucosal injection without changing the injection needle[12,51,67,68]. A HookKnife or scissor-type endoknife, which enable the endoscopists to resect the submucosal tissue while pulling up on it, may also be useful in those situations[55].
Single- and multi-center studies of colorectal ESD have reported en bloc resection rates of 61%-99.3%, perforation rates of 0%-20.4%, and bleeding rates ranging from 0% to 11.9% (Table 2)[8-10,12,14,29,52,54,56,62,64,65,69-88]. Numerous studies regarding colorectal ESD were reported in Japan where colorectal ESD was initially developed; furthermore, the reports from some other Asian countries and Western countries are continuously increasing. Direct comparison of treatment outcomes is difficult because the technical difficulty of ESD is greatly affected by tumor location, tumor size, the presence of submucosal fibrosis, and endoscope maneuverability. In addition, in some studies, treatment outcomes do not include data of earlier stage of colorectal ESD. However, recent single- and multi-center studies have reported improved treatment outcomes compared with previous studies[6,61,72,86,89]. Nakajima et al[86] recently reported a comparatively high en bloc resection rate (94.5%) and low perforation rate (2.0%) of colorectal ESD in a Japanese large multi-center prospective study. The development of various devices and improvement of the endoscopist’s skill appear to have contributed to recent improvements in treatment outcomes[21,52,55,90,91]. Probst et al[62] reported low perforation rate (1.9%) and permissible en bloc resection rate (81.6%) of colorectal ESD in a European single-center study. Furthermore, higher en bloc resection rate (96.2%) in their late stage was reported compared with that in their early (60.0%) and middle stage (88.0%). These data reveal that colorectal ESD may be widely spread even in European countries where ESD experience is low.
| Ref. | Year | Country | Studydesign | No. ofcases | Tumorsize(mm) | En blocresectionrate (%) | Completeen blocresectionrate (%) | Perforation(%) | Bleeding (%) |
| Fujishiro et al[69] | 2007 | Japan | S, R | 200 | 29.9 | 91.5 | 70.5 | 6 | 1 |
| Tamegai et al[70] | 2007 | Japan | S, R | 71 | 32.7 | 98.6 | 95.6 | 1.4 | |
| Hurlstome et al[29] | 2007 | United Kingdom | S, R | 42 | 78.6 | 73.8 | 2.4 | 11.9 | |
| Taku et al[8] | 2007 | Japan | M, R | 43 | 14 | ||||
| Zhou et al[9] | 2009 | China | S, R | 74 | 32.6 | 93.2 | 89.2 | 8.1 | 1.3 |
| Iizuka et al[71] | 2009 | Japan | S, R | 38 | 39 | 61 | 58 | 8 | |
| Isomoto et al[64] | 2009 | Japan | S, R | 292 | 26.8 | 90.1 | 79.8 | 8.2 | 0.7 |
| Hotta et al[72] | 2010 | Japan | S, R | 120 | 35 | 93.3 | 85 | 7.5 | |
| Niimi et al[73] | 2010 | Japan | S, R | 310 | 28.9 | 90.3 | 74.5 | 4.8 | 1.6 |
| Matsumoto et al[65] | 2010 | Japan | S, R | 203 | 32.4 | 85.7 | 6.9 | ||
| Yoshida et al[74] | 2010 | Japan | S, R | 250 | 29.1 | 86.8 | 81.2 | 6 | 2.4 |
| Tanaka et al[75] | 2010 | Japan | M, R | 8303 | 83.8 | 4.8 | 1.6 | ||
| Oka et al[76] | 2010 | Japan | M, R | 688 | 3.3 | 1.7 | |||
| Saito et al[77] | 2010 | Japan | M, P | 1111 | 35 | 88 | 4.9 | 1.5 | |
| Kim et al[10] | 2011 | South Korea | S, R | 108 | 27.6 | 78.7 | 20.4 | ||
| Shono et al[78] | 2011 | Japan | S, R | 137 | 29.2 | 89.1 | 85.4 | 3.6 | |
| Uraoka et al[79] | 2011 | Japan | S, R | 202 | 39.9 | 91.6 | 2.5 | 0.5 | |
| Takeuchi et al[80] | 2012 | Japan | S, R | 348 | 30 | 91.1 | 2.3 | 4.6 | |
| Probst et al[62] | 2012 | Germany | S, R | 82 | 45.5 | 81.6 | 69.7 | 1.9 | 7.9 |
| Toyonaga et al[12] | 2012 | Japan | S, R | 1143 | 99.3 | 1.4 | 1.2 | ||
| Homma et al[54] | 2012 | Japan | M, R | 102 | 32.4 | 100 | 1 | ||
| Tseng et al[81] | 2013 | Taiwan | S, R | 92 | 37.2 | 90.2 | 89.1 | 12 | 0 |
| Thorlacius et al[82] | 2013 | Sweden | S, R | 29 | 26 | 72 | 69 | 6.9 | 3.4 |
| Hülagü et al[83] | 2013 | Turkey | S, R | 44 | 30 | 77.3 | 4.5 | 9.1 | |
| Hsu et al[84] | 2013 | Taiwan | S, R | 50 | 33 | 86 | 82 | 6 | 0 |
| Saito et al[52] | 2013 | Japan | S, R | 806 | 37 | 90 | 2.8 | 1.9 | |
| Lee et al[85] | 2013 | South Korea | S, R | 1000 | 24.1 | 97.5 | 5.3 | 0.4 | |
| Nakajima et al[86] | 2013 | Japan | M, P | 816 | 94.5 | 2 | 2.2 | ||
| Hori et al[87] | 2014 | Japan | S, P | 247 | 35 | 93.1 | 92.3 | 2 | 0.4 |
| Bialek et al[88] | 2014 | Poland | S, R | 37 | 37 | 86.5 | 81.1 | 0 | 5.7 |
| Nawata et al[56] | 2014 | Japan | S, R | 150 | 98.6 | 91.3 | 0 | 0 | |
| Yamamoto et al[14] | 2015 | Japan | S, R | 119 | 32.5 | 97.5 | 90.8 | 0.8 | 1.7 |
In contrast, some studies have compared the local recurrence rates after EMR and ESD for large colorectal tumors (Table 3)[92-96]. Those studies demonstrated that local recurrence rates after ESD were significantly lower than after EMR because of the high en bloc resection rates with ESD despite the larger tumor sizes compared with EMR[92,93,95,96]. Oka et al[96] reported that piecemeal resection was the most important risk factor for local recurrence regardless of EMR or ESD in a large multicenter prospective study. Most local recurrences of mucosal lesions may be addressed with additional endoscopic treatment; however, close follow-up colonoscopy is required to detect local recurrence after piecemeal resection[92-96], even with ESD.
| Ref. | Study design | Recurrence rate after EMR(En bloc resection with EMR)(Tumor size with EMR) | Recurrence rate after ESD(En bloc resection with ESD)(Tumor size with ESD) | P value |
| Saito et al[92] | S, R | 14.0%; 33/228 | 2%; 3/145 | P < 0.0001 |
| (33%; 74/228) | (84%; 122/145) | P < 0.0001 | ||
| (28 ± 8 mm) | (37 ± 14 mm) | P = 0.0006 | ||
| Tajika et al[93] | S, R | 15.4%; 16/104 | 1.2%; 1/85 | P = 0.002 |
| (48.1%; 50/104) | (83.5%; 71/85) | P < 0.001 | ||
| (25.5 ± 6.8 mm) | (31.6 ± 9.0 mm) | P < 0.001 | ||
| Terasaki et al[94] | S, R | 8.0%; 14/176 | 0%; 0/56 | |
| (39.3%; 70/178) | ||||
| Lee et al[95] | S, R | 25.7%; 29/113 | 0.8%; 2/257 | P < 0.001 |
| (42.9%; 60/140) | (92.7%; 291/314) | P < 0.001 | ||
| (21.7 ± 3.5 mm) | (28.9 ± 12.7 mm) | P < 0.001 | ||
| Oka et al[96] | M, P | 6.8%; 55/808 | 1.4%; 10/716 | P < 0.01 |
| (53.2%; 430/808) | (95.0%; 680/716) | |||
| (32.8 ± 15.7 mm) | (39.6 ± 18.6 mm) | P < 0.01 |
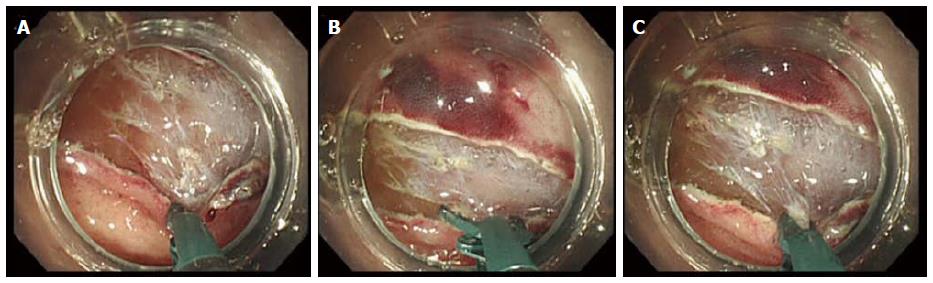
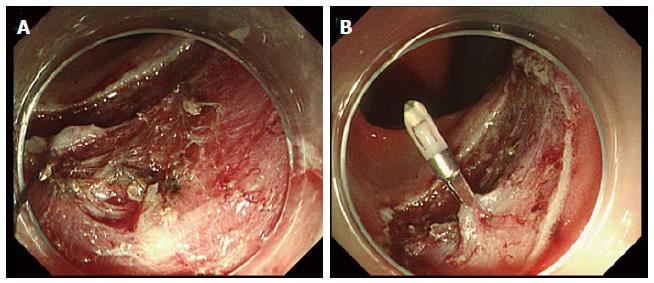
Perforation is a major complication of colorectal ESD; however, most cases of perforation can be conservatively treated by closure with endoclips (Figure 6A and B). However, endoscopists should give particular attention to the risks of perforation because open or laparoscopic surgery may be required for bacterial peritonitis, particularly with delayed perforations[77,85,97]. Larger lesional size, submucosal fibrosis, colonic location, and less experienced ESD operators have all been reported as risk factors for perforation during colorectal ESD[10,27,28,77,87].
Post-operative bleeding is less common with colorectal ESD than with gastric ESD and can conservatively managed with hemostatic forceps or endoscopic clipping in the majority of cases[77,85].
Abdominal pain or fever due to electrocoagulation syndrome after ESD is occasionally observed, particularly in the proximal colon, and when conservatively managed[98]. The occurrence of adverse events may cause an extension in hospital stay[31,32,98].
The safety and success rates of colorectal ESD have recently improved to favorable levels predominantly in advanced institutions in Japan, some Asian, and a few Western countries. However, colorectal ESD is still a technically difficult procedure for majority of endoscopists, and development of training systems is required for world-wide adoption of colorectal ESD[99,100]. ESD for rectal and smaller lesions, which is less technically difficult, is suitable for initial adoption of colorectal ESD. Substantial experience of gastric ESD, which is less technically challenging than colorectal ESD, is highly useful for performing colorectal ESD; however, it is difficult in Western countries because of the low morbidity rate of gastric cancer. EMR with circumferential mucosal incision may be option in cases where ESD cannot be successfully performed[101]. Before performing colorectal ESD, ESD training using animal models or observing the performance of procedure by ESD experts at other institutions have been shown to be extremely useful in improving operator skill[102-104].
In contrast, some cases are challenging even for experts in colorectal ESD, particularly because of the poor endoscope maneuverability or poor visualization of the operative field due to colonic flexure. Colonic flexure and extensibility commonly causes paradoxical movement of the endoscope. Therefore, double- or single-balloon endoscopy systems have recently been introduced for colorectal ESD at several institutions[44-46] because these endoscopy systems enable the endoscope to be straightened more easily than conventional endoscopy. Ohya et al[44] reported that a short-type single-balloon overtube through which a thin conventional endoscope can be introduced was useful for colorectal ESD, particularly for poor endoscope maneuverability in the proximal colon.
Sinker-assisted ESD[105], magnet anchor-guided ESD[106], clip with line-assisted ESD[107,108], clip with rubber- or spring-assisted ESD[109,110], clip-band ESD[111], a double-channel scope method[112,113], and a double endoscopic intraluminal procedure[114,115] have all been described as traction systems that facilitate ESD. Each system has a unique traction system that utilizes specialized equipment to provide counter-traction[107]. Because these traction systems are somewhat complicated or commercially unavailable, they are not widely used in colorectal ESD at present. The improvement of these traction systems or development of new tractions systems or devices[116] may facilitate improvements in the safety or efficacy of colorectal ESD in the future.
In this review, we have described the technical aspects and recent progresses in colorectal ESD. Maintaining good visualization of the operative field is the most important for safely and successfully performing colorectal ESD. Developments of various devices, novel procedures, and appropriate strategies have resulted in the recent improvement of the treatment outcome in colorectal ESD. Further development of training systems or devices will promote world-wide standardization of colorectal ESD.
We would like to thank Dr. Takashi Toyonaga, Dr. Yoshinori Morita, and CE Ken Yoshimura, all at Kobe University Hospital, Japan, for their technical advice.
P- Reviewer: Matsumoto K, Yoshida N S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1149] [Article Influence: 47.9] [Reference Citation Analysis (4)] |
| 2. | Oyama T, Kikuchi Y. Aggressive endoscopic mucosal resection in the upper GI tract-Hook knife EMR method. Minim Invasive Ther Allied Technol. 2002;11:291-295. |
| 3. | Yahagi N, Fujishiro M, Kakushima N, Kobayashi K, Hashimoto T, Oka M, Iguchi M, Enomoto S, Ichinose M, Niwa H. Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type). Dig Endosc. 2004;16:34-38. [RCA] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Yamamoto H, Yahagi N, Oyama T. Mucosectomy in the colon with endoscopic submucosal dissection. Endoscopy. 2005;37:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Rösch T, Sarbia M, Schumacher B, Deinert K, Frimberger E, Toermer T, Stolte M, Neuhaus H. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy. 2004;36:788-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 7. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc. 2007;66:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Taku K, Sano Y, Fu KI, Saito Y, Matsuda T, Uraoka T, Yoshino T, Yamaguchi Y, Fujita M, Hattori S. Iatrogenic perforation associated with therapeutic colonoscopy: a multicenter study in Japan. J Gastroenterol Hepatol. 2007;22:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Zhou PH, Yao LQ, Qin XY. Endoscopic submucosal dissection for colorectal epithelial neoplasm. Surg Endosc. 2009;23:1546-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Kim ES, Cho KB, Park KS, Lee KI, Jang BK, Chung WJ, Hwang JS. Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy. 2011;43:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Yahagi N. Endoscopic submucosal dissection in the colon. Colonoscopy: Principles and Practice, 2nd ed. Waye JD, Rex DK, Williams CB, editors. Oxford: Wiley-Blackwell 2009; 603-612. |
| 12. | Toyonaga T, Nishino E, Man-I M, East JE, Azuma T. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc. 2012;45:362-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Yamamoto K, Hayashi S, Nakabori T, Shibuya M, Ichiba M, Inada M. Endoscopic submucosal dissection using endoclips to assist in mucosal flap formation (novel technique: “clip flap method”). Endoscopy. 2012;44 Suppl 2 UCTN:E334-E335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Yamamoto K, Hayashi S, Saiki H, Indo N, Nakabori T, Yamamoto M, Shibuya M, Nishida T, Ichiba M, Inada M. Endoscopic submucosal dissection for large superficial colorectal tumors using the “clip-flap method”. Endoscopy. 2015;47:262-265. [PubMed] |
| 15. | Yamamoto K, Hayashi S, Nishida T, Saiki H, Naito M, Michida T, Ito T. Effective use of the “clip-flap” method for the endoscopic submucosal dissection of a difficult-to-approach superficial gastric tumor. Endoscopy. 2015;47 Suppl 1 UCTN:E318-E319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Walsh RM, Ackroyd FW, Shellito PC. Endoscopic resection of large sessile colorectal polyps. Gastrointest Endosc. 1992;38:303-309. [PubMed] |
| 17. | Hotta K, Fujii T, Saito Y, Matsuda T. Local recurrence after endoscopic resection of colorectal tumors. Int J Colorectal Dis. 2009;24:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 437] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 19. | Fernández-Esparrach G, Calderón A, de la Peña J, Díaz Tasende JB, Esteban JM, Gimeno-García AZ, Herreros de Tejada A, Martínez-Ares D, Nicolás-Pérez D, Nogales O. Endoscopic submucosal dissection. Endoscopy. 2014;46:361-370. [PubMed] |
| 20. | Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, Fujii T. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 21. | Hotta K, Yamaguchi Y, Saito Y, Takao T, Ono H. Current opinions for endoscopic submucosal dissection for colorectal tumors from our experiences: indications, technical aspects and complications. Dig Endosc. 2012;24 Suppl 1:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Toyonaga T, Tanaka S, Man-I M, East J, Ono W, Nishino E, Ishida T, Hoshi N, Morita Y, Azuma T. Clinical significance of the muscle-retracting sign during colorectal endoscopic submucosal dissection. Endosc Int Open. 2015;3:E246-E251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 708] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 24. | Hirata M, Tanaka S, Oka S, Kaneko I, Yoshida S, Yoshihara M, Chayama K. Evaluation of microvessels in colorectal tumors by narrow band imaging magnification. Gastrointest Endosc. 2007;66:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Ikematsu H, Matsuda T, Emura F, Saito Y, Uraoka T, Fu KI, Kaneko K, Ochiai A, Fujimori T, Sano Y. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol. 2010;10:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Yoshida N, Hisabe T, Inada Y, Kugai M, Yagi N, Hirai F, Yao K, Matsui T, Iwashita A, Kato M. The ability of a novel blue laser imaging system for the diagnosis of invasion depth of colorectal neoplasms. J Gastroenterol. 2014;49:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Hayashi N, Tanaka S, Nishiyama S, Terasaki M, Nakadoi K, Oka S, Yoshihara M, Chayama K. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc. 2014;79:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Lee EJ, Lee JB, Choi YS, Lee SH, Lee DH, Kim do S, Youk EG. Clinical risk factors for perforation during endoscopic submucosal dissection (ESD) for large-sized, nonpedunculated colorectal tumors. Surg Endosc. 2012;26:1587-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Hurlstone DP, Atkinson R, Sanders DS, Thomson M, Cross SS, Brown S. Achieving R0 resection in the colorectum using endoscopic submucosal dissection. Br J Surg. 2007;94:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 30. | Yoshida N, Naito Y, Sakai K, Sumida Y, Kanemasa K, Inoue K, Morimoto Y, Konishi H, Wakabayashi N, Kokura S. Outcome of endoscopic submucosal dissection for colorectal tumors in elderly people. Int J Colorectal Dis. 2010;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Aoki T, Nakajima T, Saito Y, Matsuda T, Sakamoto T, Itoi T, Khiyar Y, Moriyasu F. Assessment of the validity of the clinical pathway for colon endoscopic submucosal dissection. World J Gastroenterol. 2012;18:3721-3726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Tomiki Y, Kawai M, Takehara K, Tashiro Y, Munakata S, Kure K, Ishiyama S, Sugimoto K, Kamiyama H, Takahashi M. Clinical pathway to discharge 3 days after colorectal endoscopic submucosal dissection. Dig Endosc. 2015;27:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Takimoto K, Ueda T, Shimamoto F, Kojima Y, Fujinaga Y, Kashiwa A, Yamauchi H, Matsuyama K, Toyonaga T, Yoshikawa T. Sedation with dexmedetomidine hydrochloride during endoscopic submucosal dissection of gastric cancer. Dig Endosc. 2011;23:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Dere K, Sucullu I, Budak ET, Yeyen S, Filiz AI, Ozkan S, Dagli G. A comparison of dexmedetomidine versus midazolam for sedation, pain and hemodynamic control, during colonoscopy under conscious sedation. Eur J Anaesthesiol. 2010;27:648-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Bretthauer M, Thiis-Evensen E, Huppertz-Hauss G, Gisselsson L, Grotmol T, Skovlund E, Hoff G. NORCCAP (Norwegian colorectal cancer prevention): a randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut. 2002;50:604-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Uraoka T, Kato J, Kuriyama M, Hori K, Ishikawa S, Harada K, Takemoto K, Hiraoka S, Fujita H, Horii J. CO(2) insufflation for potentially difficult colonoscopies: efficacy when used by less experienced colonoscopists. World J Gastroenterol. 2009;15:5186-5192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Yoshida M, Imai K, Hotta K, Yamaguchi Y, Tanaka M, Kakushima N, Takizawa K, Matsubayashi H, Ono H. Carbon dioxide insufflation during colorectal endoscopic submucosal dissection for patients with obstructive ventilatory disturbance. Int J Colorectal Dis. 2014;29:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Tamai N, Matsuda K, Sumiyama K, Yoshida Y, Tajiri H. Glucagon facilitates colonoscopy and reduces patient discomfort: a randomized double-blind controlled trial with salivary amylase stress analysis. Eur J Gastroenterol Hepatol. 2013;25:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Asao T, Mochiki E, Suzuki H, Nakamura J, Hirayama I, Morinaga N, Shoji H, Shitara Y, Kuwano H. An easy method for the intraluminal administration of peppermint oil before colonoscopy and its effectiveness in reducing colonic spasm. Gastrointest Endosc. 2001;53:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Lee BI. Debates on colorectal endoscopic submucosal dissection - traction for effective dissection: gravity is enough. Clin Endosc. 2013;46:467-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Fujishiro M. Endoscopic submucosal dissection for colorectal neoplasms. World J Gastrointest Endosc. 2009;1:32-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 44. | Ohya T, Ohata K, Sumiyama K, Tsuji Y, Koba I, Matsuhashi N, Tajiri H. Balloon overtube-guided colorectal endoscopic submucosal dissection. World J Gastroenterol. 2009;15:6086-6090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Sunada K, Yamamoto H. Double-balloon endoscopy: past, present, and future. J Gastroenterol. 2009;44:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Katsuki S, Fujita T, Waga E, Takahashi K, Kitaoka K, Komatsu Y, Ohta H. More beneficial colonic endoscopic submucosal dissection (ESD) method for patients by employing double balloon endoscopes (DBE). Gastrointestinal Endoscopy. 2014;79 Suppl 5:AB271. [DOI] [Full Text] |
| 47. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 48. | Linghu E, Feng X, Wang X, Meng J, Du H, Wang H. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy. 2013;45:60-62. [PubMed] |
| 49. | Hayashi Y, Sunada K, Takahashi H, Shinhata H, Lefor AT, Tanaka A, Yamamoto H. Pocket-creation method of endoscopic submucosal dissection to achieve en bloc resection of giant colorectal subpedunculated neoplastic lesions. Endoscopy. 2014;46 Suppl 1 UCTN:E421-E422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Yahagi N, Uraoka T, Ida Y, Hosoe N, Nakamura R, Kitagawa Y, Ogata H, Hibi T. Endoscopic submucosal dissection using the Flex and the Dual knives. Techniques in gastrointestinal endoscopy. 2011;13:74-78. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Toyonaga T, Man-I M, Fujita T, Nishino E, Ono W, Morita Y, Sanuki T, Masuda A, Yoshida M, Kutsumi H. The performance of a novel ball-tipped Flush knife for endoscopic submucosal dissection: a case-control study. Aliment Pharmacol Ther. 2010;32:908-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Saito Y, Otake Y, Sakamoto T, Nakajima T, Yamada M, Haruyama S, So E, Abe S, Matsuda T. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver. 2013;7:263-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 459] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 54. | Homma K, Otaki Y, Sugawara M, Kobayashi M. Efficacy of novel SB knife Jr examined in a multicenter study on colorectal endoscopic submucosal dissection. Dig Endosc. 2012;24 Suppl 1:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Oka S, Tanaka S, Takata S, Kanao H, Chayama K. Usefulness and safety of SB knife Jr in endoscopic submucosal dissection for colorectal tumors. Dig Endosc. 2012;24 Suppl 1:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 56. | Nawata Y, Homma K, Suzuki Y. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for large superficial colorectal tumors. Dig Endosc. 2014;26:552-555. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Akahoshi K, Okamoto R, Akahane H, Motomura Y, Kubokawa M, Osoegawa T, Nakama N, Chaen T, Oya M, Nakamura K. Endoscopic submucosal dissection of early colorectal tumors using a grasping-type scissors forceps: a preliminary clinical study. Endoscopy. 2010;42:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Okamoto K, Kitamura S, Muguruma N, Takaoka T, Fujino Y, Kawahara Y, Okahisa T, Takayama T. Mucosectom2-short blade for safe and efficient endoscopic submucosal dissection of colorectal tumors. Endoscopy. 2013;45:928-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Chung HC, Hsu HY Chia WY, Yang YC, Wei WS, Maw SS. Short-term outcomes of endoscopic submucosal dissection for colorectal neoplasms in a single medical center. Advances in Digestive Medicine. 2015;2:54-60. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Sakamoto T, Takamaru H, Mori G, Yamada M, Kinjo Y, So E, Abe S, Otake Y, Nakajima T, Matsuda T. Endoscopic submucosal dissection for colorectal neoplasms. Ann Transl Med. 2014;2:26. [PubMed] |
| 61. | Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Knipschield MA, Marler RJ. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc. 2007;65:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 62. | Probst A, Golger D, Anthuber M, Märkl B, Messmann H. Endoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European center. Endoscopy. 2012;44:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 63. | Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Enomoto S, Kakushima N, Kobayashi K, Hashimoto T, Iguchi M. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy. 2004;36:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 199] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 64. | Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2009;41:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 65. | Matsumoto A, Tanaka S, Oba S, Kanao H, Oka S, Yoshihara M, Chayama K. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol. 2010;45:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 66. | Lee SP, Kim JH, Sung IK, Lee SY, Park HS, Shim CS, Han HS. Effect of submucosal fibrosis on endoscopic submucosal dissection of colorectal tumors: pathologic review of 173 cases. J Gastroenterol Hepatol. 2015;30:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 67. | Takeuchi Y, Uedo N, Ishihara R, Iishi H, Kizu T, Inoue T, Chatani R, Hanaoka N, Taniguchi T, Kawada N. Efficacy of an endo-knife with a water-jet function (FlushKnife) for endoscopic submucosal dissection of superficial colorectal neoplasms. Am J Gastroenterol. 2010;105:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Takeuchi Y, Shimokawa T, Ishihara R, Iishi H, Hanaoka N, Higashino K, Uedo N. An electrosurgical endoknife with a water-jet function (flushknife) proves its merits in colorectal endoscopic submucosal dissection especially for the cases which should be removed en bloc. Gastroenterol Res Pract. 2013;2013:530123. [PubMed] |
| 69. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678-683; quiz 645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 275] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 70. | Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, Liu Y, Uemura N, Saito K. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy. 2007;39:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 71. | Iizuka H, Okamura S, Onozato Y, Ishihara H, Kakizaki S, Mori M. Endoscopic submucosal dissection for colorectal tumors. Gastroenterol Clin Biol. 2009;33:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Hotta K, Oyama T, Shinohara T, Miyata Y, Takahashi A, Kitamura Y, Tomori A. Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig Endosc. 2010;22:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 73. | Niimi K, Fujishiro M, Kodashima S, Goto O, Ono S, Hirano K, Minatsuki C, Yamamichi N, Koike K. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2010;42:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 74. | Yoshida N, Naito Y, Kugai M, Inoue K, Wakabayashi N, Yagi N, Yanagisawa A, Yoshikawa T. Efficient hemostatic method for endoscopic submucosal dissection of colorectal tumors. World J Gastroenterol. 2010;16:4180-4186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 75. | Tanaka S, Tamegai Y, Tsuda S, Saito Y, Yahagi N, Yamano HO. Multicenter questionnaire survey on the current situation of colorectal endoscopic submucosal dissection in Japan. Dig Endosc. 2010;22 Suppl 1:S2-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Oka S, Tanaka S, Kanao H, Ishikawa H, Watanabe T, Igarashi M, Saito Y, Ikematsu H, Kobayashi K, Inoue Y. Current status in the occurrence of postoperative bleeding, perforation and residual/local recurrence during colonoscopic treatment in Japan. Dig Endosc. 2010;22:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 77. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 593] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 78. | Shono T, Ishikawa K, Ochiai Y, Nakao M, Togawa O, Nishimura M, Arai S, Nonaka K, Sasaki Y, Kita H. Feasibility of endoscopic submucosal dissection: a new technique for en bloc resection of a large superficial tumor in the colon and rectum. Int J Surg Oncol. 2011;2011:948293. [PubMed] |
| 79. | Uraoka T, Higashi R, Kato J, Kaji E, Suzuki H, Ishikawa S, Akita M, Hirakawa T, Saito S, Hori K. Colorectal endoscopic submucosal dissection for elderly patients at least 80 years of age. Surg Endosc. 2011;25:3000-3007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 80. | Takeuchi Y, Ohta T, Matsui F, Nagai K, Uedo N. Indication, strategy and outcomes of endoscopic submucosal dissection for colorectal neoplasm. Dig Endosc. 2012;24 Suppl 1:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Tseng MY, Lin JC, Huang TY, Shih YL, Chu HC, Chang WK, Hsieh TY, Chen PJ. Endoscopic submucosal dissection for early colorectal neoplasms: clinical experience in a tertiary medical center in taiwan. Gastroenterol Res Pract. 2013;2013:891565. [PubMed] |
| 82. | Thorlacius H, Uedo N, Toth E. Implementation of endoscopic submucosal dissection for early colorectal neoplasms in Sweden. Gastroenterol Res Pract. 2013;2013:758202. [PubMed] |
| 83. | Hülagü S, Şentürk Ö, Korkmaz U, Şirin G, Duman AE, Dindar G, Çelebi A, Koç DÖ, Bozkurt N, Yılmaz H. Endoscopic submucosal dissection for colorectal laterally spreading tumors. Turk J Gastroenterol. 2013;24:532-540. [PubMed] |
| 84. | Hsu WH, Sun MS, Lo HW, Tsai CY, Tsai YJ. Clinical practice of endoscopic submucosal dissection for early colorectal neoplasms by a colonoscopist with limited gastric experience. Gastroenterol Res Pract. 2013;2013:262171. [PubMed] |
| 85. | Lee EJ, Lee JB, Lee SH, Kim do S, Lee DH, Lee DS, Youk EG. Endoscopic submucosal dissection for colorectal tumors--1,000 colorectal ESD cases: one specialized institute’s experiences. Surg Endosc. 2013;27:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 86. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 87. | Hori K, Uraoka T, Harada K, Higashi R, Kawahara Y, Okada H, Ramberan H, Yahagi N, Yamamoto K. Predictive factors for technically difficult endoscopic submucosal dissection in the colorectum. Endoscopy. 2014;46:862-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 88. | Białek A, Pertkiewicz J, Karpińska K, Marlicz W, Bielicki D, Starzyńska T. Treatment of large colorectal neoplasms by endoscopic submucosal dissection: a European single-center study. Eur J Gastroenterol Hepatol. 2014;26:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Saito Y, Kawano H, Takeuchi Y, Ohata K, Oka S, Hotta K, Okamoto K, Homma K, Uraoka T, Hisabe T. Current status of colorectal endoscopic submucosal dissection in Japan and other Asian countries: progressing towards technical standardization. Dig Endosc. 2012;24 Suppl 1:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Tanaka S, Terasaki M, Kanao H, Oka S, Chayama K. Current status and future perspectives of endoscopic submucosal dissection for colorectal tumors. Dig Endosc. 2012;24 Suppl 1:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 91. | Yoshida N, Yagi N, Naito Y, Yoshikawa T. Safe procedure in endoscopic submucosal dissection for colorectal tumors focused on preventing complications. World J Gastroenterol. 2010;16:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 93. | Tajika M, Niwa Y, Bhatia V, Kondo S, Tanaka T, Mizuno N, Hara K, Hijioka S, Imaoka H, Ogura T. Comparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumors. Eur J Gastroenterol Hepatol. 2011;23:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 94. | Terasaki M, Tanaka S, Oka S, Nakadoi K, Takata S, Kanao H, Yoshida S, Chayama K. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol. 2012;27:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 95. | Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012;26:2220-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 96. | Oka S, Tanaka S, Saito Y, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol. 2015;110:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 97. | Hotta K, Shinohara T, Oyama T, Ishii E, Tomori A, Takahashi A, Miyata Y. Criteria for non-surgical treatment of perforation during colorectal endoscopic submucosal dissection. Digestion. 2012;85:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Yamashina T, Takeuchi Y, Uedo N, Hamada K, Aoi K, Yamasaki Y, Matsuura N, Kanesaka T, Akasaka T, Yamamoto S. Features of electrocoagulation syndrome after endoscopic submucosal dissection for colorectal neoplasm. J Gastroenterol Hepatol. 2015;Jul 22; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 99. | Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection in Japan and Western countries. Dig Endosc. 2012;24 Suppl 1:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Ohata K, Ito T, Chiba H, Tsuji Y, Matsuhashi N. Effective training system in colorectal endoscopic submucosal dissection. Dig Endosc. 2012;24 Suppl 1:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Toyonaga T, Man-I M, Morita Y, Sanuki T, Yoshida M, Kutsumi H, Inokuchi H, Azuma T. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S31-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 102. | Yoshida N, Yagi N, Inada Y, Kugai M, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Handa O. Possibility of ex vivo animal training model for colorectal endoscopic submucosal dissection. Int J Colorectal Dis. 2013;28:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Parra-Blanco A, Arnau MR, Nicolás-Pérez D, Gimeno-García AZ, González N, Díaz-Acosta JA, Jiménez A, Quintero E. Endoscopic submucosal dissection training with pig models in a Western country. World J Gastroenterol. 2010;16:2895-2900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 104. | Draganov PV, Chang M, Coman RM, Wagh MS, An Q, Gotoda T. Role of observation of live cases done by Japanese experts in the acquisition of ESD skills by a western endoscopist. World J Gastroenterol. 2014;20:4675-4680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | Saito Y, Emura F, Matsuda T, Uraoka T, Nakajima T, Ikematsu H, Gotoda T, Saito D, Fujii T. A new sinker-assisted endoscopic submucosal dissection for colorectal cancer. Gastrointest Endosc. 2005;62:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 106. | Gotoda T, Oda I, Tamakawa K, Ueda H, Kobayashi T, Kakizoe T. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos). Gastrointest Endosc. 2009;69:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 107. | Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc. 2012;45:375-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 108. | Okamoto K, Muguruma N, Kitamura S, Kimura T, Takayama T. Endoscopic submucosal dissection for large colorectal tumors using a cross-counter technique and a novel large-diameter balloon overtube. Dig Endosc. 2012;24 Suppl 1:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Mori H, Kawabe M, Nagahara A, Otaka M, Ogihara T. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc. 2009;69:1370-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 110. | Tomiki Y, Ishiyama S, Sugimoto K, Takahashi M, Kojima Y, Tanaka M, Sakamoto K. Colorectal endoscopic submucosal dissection by using latex-band traction. Endoscopy. 2011;43 Suppl 2 UCTN:E250-E251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Parra-Blanco A, Nicolas D, Arnau MR, Gimeno-Garcia AZ, Rodrigo L, Quintero E. Gastric endoscopic submucosal dissection assisted by a new traction method: the clip-band technique. A feasibility study in a porcine model (with video). Gastrointest Endosc. 2011;74:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 112. | Neuhaus H, Costamagna G, Devière J, Fockens P, Ponchon T, Rösch T. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the “R-scope”). Endoscopy. 2006;38:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 113. | Yonezawa J, Kaise M, Sumiyama K, Goda K, Arakawa H, Tajiri H. A novel double-channel therapeutic endoscope (“R-scope”) facilitates endoscopic submucosal dissection of superficial gastric neoplasms. Endoscopy. 2006;38:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 114. | Uraoka T, Kato J, Ishikawa S, Harada K, Kuriyama M, Takemoto K, Kawahara Y, Saito Y, Okada H. Thin endoscope-assisted endoscopic submucosal dissection for large colorectal tumors (with videos). Gastrointest Endosc. 2007;66:836-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 115. | Morita Y, Masuda M, Tanaka S, Fujiwara M, Wakahara C, Toyonaga T, Azuma T. Approach to treating difficult cases of early gastric cancer. Development of a double scope-ESD using transnasal endoscope with a “Split Barrel” [Japanese with English abstract]. Endoscopia Digestiva. 2010;22:846-852. |
| 116. | Yahagi N, Neuhaus H, Schumacher B, Neugebauer A, Kaehler GF, Schenk M, Fischer K, Fujishiro M, Enderle MD. Comparison of standard endoscopic submucosal dissection (ESD) versus an optimized ESD technique for the colon: an animal study. Endoscopy. 2009;41:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |