Published online Jul 16, 2014. doi: 10.4253/wjge.v6.i7.304
Revised: May 26, 2014
Accepted: June 10, 2014
Published online: July 16, 2014
Processing time: 184 Days and 13.2 Hours
AIM: To analyze the effect of bipolar electrocoagulation and argon plasma coagulation on fresh specimens of gastrointestinal tract.
METHODS: An experimental evaluation was performed at Hospital das Clinicas of the University of São Paulo, on 31 fresh surgical specimens using argon plasma coagulation and bipolar electrocoagulation at different time intervals. The depth of tissue damage was histopathologically analyzed by single senior pathologist unaware of the coagulation method and power setting applied. To analyze the results, the mucosa was divided in superficial mucosa (epithelial layer of the esophagus and superficial portion of the glandular layer of the stomach and colon) intermediate mucosa (until the lamina propria of the esophagus and until the bottom of the glandular layer of the stomach and colon) and muscularis mucosa. Necrosis involvement of the layers was compared in several combinations of power and time interval.
RESULTS: Involvement of the intermediate mucosa of the stomach and of the muscularis mucosa of the three organs was more frequent when higher amounts of energy were used with argon plasma. In the esophagus and in the colon, injury of the intermediate mucosa was frequent, even when small amounts of energy were used. The use of bipolar electrocoagulation resulted in more frequent involvement of the intermediate mucosa and of the muscularis mucosa of the esophagus and of the colon when higher amounts of energy were used. In the stomach, these involvements were rare. The risk of injury of the muscularis propria was significant only in the colon when argon plasma coagulation was employed.
CONCLUSION: Tissue damage after argon plasma coagulation is deeper than bipolar electrocoagulation. Both of them depend on the amount of energy used.
Core tip: The best way of applying heat to hollow digestive organs during thermal endoscopic therapy has not been clearly established so far. This study analyzes the histophathological effect of bipolar electrocoagulation and argon plasma coagulation on fresh surgical specimens of the digestive tract. Tissue damage after argon plasma coagulation is deeper than bipolar electrocoagulation. Both of them depends on the amount of energy used.
- Citation: Garrido T, Baba ER, Wodak S, Sakai P, Cecconello I, Maluf-Filho F. Histology assessment of bipolar coagulation and argon plasma coagulation on digestive tract. World J Gastrointest Endosc 2014; 6(7): 304-311
- URL: https://www.wjgnet.com/1948-5190/full/v6/i7/304.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i7.304
The association of diathermy to endoscopy has provided significant advances in endotherapy, which became a valuable alternative to traditional surgery and therapeutic procedure of choice in several conditions (e.g., sphyncterotomy, polypectomy)[1-3]. Such a safe and cost-effective approach has justified the widespread of gastrointestinal endotherapy. However, reports of severe complications associated to endoscopic coagulation are common[4,5]. Pleural effusion, esophageal and colonic perforation and fistulae have followed argon plasma coagulation[6-8]. A case of colonic perforation has been associated to bipolar coagulation[9]. On the other hand, power setting and time interval of endoscopic coagulation can be very variable among authors[7,10-13]. The best way of applying heat to tissue has not been clearly established for hollow organs so far.
The aim of this study was to analyze the depth of coagulation necrosis caused by bipolar electrocoagulation and argon plasma coagulation on fresh gastrointestinal specimens, using different power settings and time intervals.
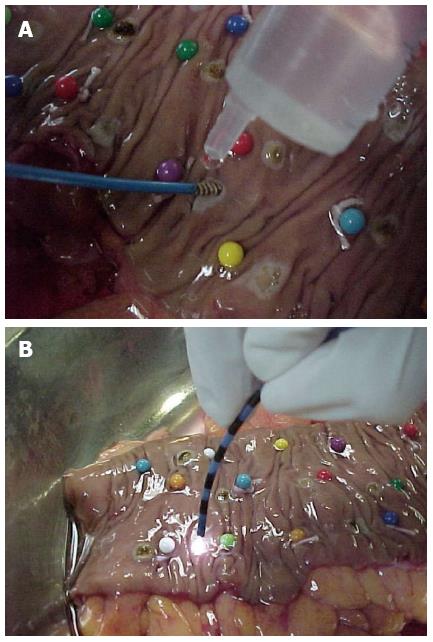
Nine fresh surgical specimens of esophagus, 11 of stomach and 11 of colon were submitted to bipolar electrocoagulation and argon plasma coagulation. Surgical specimens of esophagus, stomach and colon, resected for neoplasic diseases were given to the author in the surgical room, right after the end of surgery. The specimens were kept in saline solution from the time of its removal until its preparation for thermal appliance (median of 3 h). Bipolar electrocoagulation was applied with power settings of 20 W and 50 W, during 1, 3, 5 and 10 s. A 454A Kairos - DNI Nevada Inc.® equipment and 7Fr QuickSilver- COOK® probes were used for bipolar electrocoagulation (Figure 1A). The specimens were also coagulated by argon plasma, with power settings of 50, 70 and 90 W, during 1, 3 and 5 s. An ICC 300 - ERBE® equipment and 7Fr GIT - ERBE® probes were used for argon plasma coagulation. The argon gas flow was set to 2l/min. The probe was kept up to 2 cm from the tissue surface, in an angle of 90°. In the esophagus, the combination of 20 W × 1s for bipolar electrocoagulation and 70 W power setting for argon plasma coagulation were not applied due to less available tissue (Figure 1B).
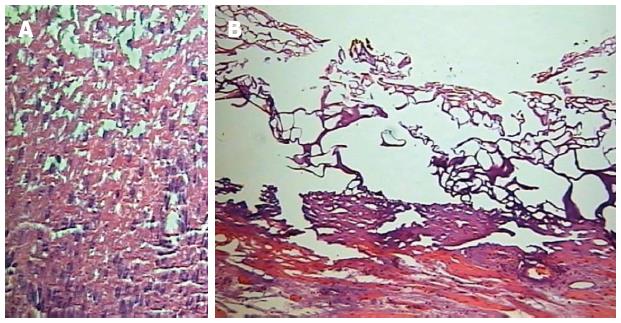
The depth of tissue damage was histopathologically analyzed by a single senior pathologist unaware of the coagulation method and power setting applied, with the help of an optic microscope (40 ×, 250 × and 400 ×). Citoplasmatic acidofilia, cellular picnosis and the presence of “ghost cells” were the histopathological parameters used to define cellular necrosis (Figure 2).
Necrosis involvement of the intermediate mucosa, the muscularis mucosa and the muscularis propria of the specimens was observed for the relevance of this stratification in clinical practice.
The intermediate mucosa was considered involved when necrosis was noted until the lamina propria of the esophagus or deep portion of the glandular layer of the stomach and colon. The muscularis mucosa was considered involved when necrosis was present through its whole extension. Muscularis propria was considered involved when any extension of necrosis was present. For both methods, necrosis involvement of the layers was compared in several combinations of power and time interval. Q-square and Fisher´s test were used for the statistical analysis and a level of significance < 5% was adopted.
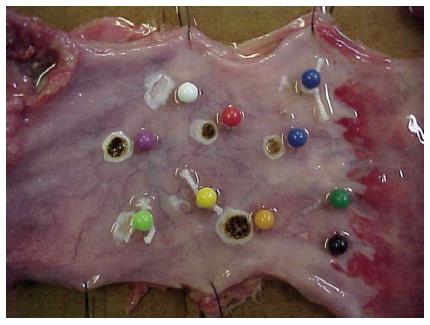
Macroscopically, coagulated spots from both methods resulted in depressed whitish lesions to brownish ulcerations associated to blisters (Figure 3).
The frequency of involvement of the layers in different combinations of power setting and time interval, in both methods, is shown in Tables 1-6. Involvement of the intermediate mucosa of the stomach and of the muscularis mucosa of the three organs was more frequent when higher amounts of energy were used with argon plasma. In the esophagus and in the colon, injury of the intermediate mucosa was frequent, even when small amounts of energy were used. The use of bipolar electrocoagulation resulted in more frequent involvement of the intermediate mucosa and of the muscularis mucosa of the esophagus and of the colon when higher amounts of energy were used. In the stomach, these involvements were rare. The risk of injury of the muscularis propria was significant only in the colon when argon plasma coagulation was employed.
| Time | 1 s | 3 s | 5 s | ||||||
| Power setting | 50 W | 70 W | 90 W | 50 W | 70 W | 90 W | 50 W | 70 W | 90 W |
| Energy amount | 50 J | 70 J | 90 J | 150 J | 210 J | 270 J | 250 J | 350 J | 450 J |
| M int | 89% | - | 78% | 89% | - | 89% | 78% | - | 89% |
| M M | 67% | - | 56% | 89% | - | 89% | 78% | - | 89% |
| M P | 0% | - | 11% | 0% | - | 0% | 11% | - | 0% |
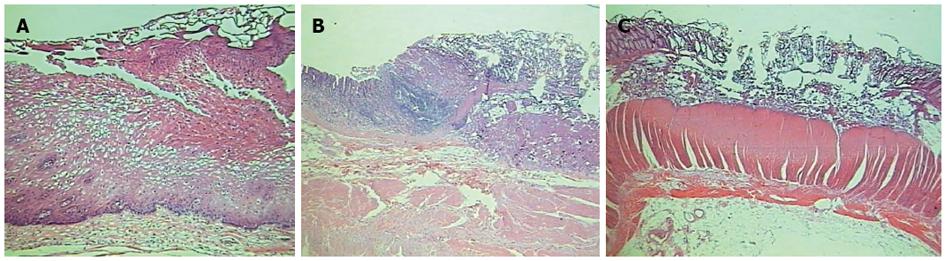
Figure 4 show the microscopic aspect of coagulated spots with different depths of coagulation necrosis.
The ideal way of applying thermal endoscopic methods to gastrointestinal wall should be deep enough to obtain the therapeutic purpose, as well as avoiding involvement of deeper layers which carries a risk of stenosis, due to healing of muscular layers or even perforation, when muscularis propria is involved.
In the esophageal specimens submitted to argon plasma coagulation, we observed a low incidence of involvement of the muscularis mucosa when the method was applied for short time, being 56% and 67% the frequencies of this involvement for appliances lasting 1 s and 78% to 89% for appliances lasting 3 and 5 s. No significant difference in depth was observed between 50 and 90 W coagulations. Watson et al[14] also have not noticed difference in depth related to power setting (from 40 to 99 W), applying the same method in three specimens of esophagus. The involvement of the entire mucosa (including the muscularis mucosa) was also less frequent when argon plasma was applied for shorter time, 52% and 76% for 1 and 3 s, respectively.
Damage to the intermediate mucosa (including the lamina propria) was frequently observed (78% to 89%), independently of the amount of energy used (from 50 to 450 J). As the destruction of the entire mucosa layer is, in theory, the purpose of the endoscopic ablation of Barrett´s metaplasic epithelium, it seems that application of smaller amounts of energy decreases the risk of involvement of the muscularis mucosa, maintaining a good therapeutic result. This is particularly relevant when Barrett involves the whole circumference of the organ, increasing the risk of stenosis. Using argon plasma with 60 W potency for 1 s to destroy Barrett´s Esophagus, Grade et al[15] observed intestinal metaplasia below repaired squamous epithelium in 20% of the cases. With this amount of energy they had no complications. Pereira-Lima et al[7], Pedrazzani et al[6], Schulz et al[16] and Ragunath et al[17] applied higher amounts of energy of argon plasma, treating patients with Barrett’s esophagus (65 to 70 W for 10 s vs 90 W for a short interval). Pereira-Lima et al[7], Pedrazzani et al[6] and Schulz et al[16] obtained complete eradication of the metaplastic epithelium, while Ragunath et al[17] obtained 65% eradication of the metaplastic epithelium. However, complications as stenosis, pleural infusion, one case of pneumoperitoneum and one case of hemorrhage for ulcer were observed in their series. Injury of the muscularis propria occurred in two coagulation points in our study, when 90 W × 1 s and 50 W × 5 s were used, representing 3.7% of all coagulation points. In Watson´s et al study[14], this damage occurred only in 5% of the cases when the time interval was 3 s and Heindorff et al[18] described just 1% of perforation when argon plasma was use to permeate esophageal cancer. This shows that despite uncommon, the risk of esophagus perforation with this method exists, even when small amounts of energy are used.
On the stomach wall, argon plasma coagulation resulted in involvement of the intermediate mucosa frequently (82% to 100%), when 90 J or more was applied (until 450 J). This energy interval also caused muscularis mucosa injury more often (64% to 100% of cases). In the other hand, the involvement of this layer until 70 J was 27% to 30%. Watson et al[14] also noted deeper involvement of the wall when higher power settings for longer intervals were used in three fresh surgical specimens of stomach. However, different stratification of the wall layers did not allow comparisons with our study. Eventual healing retractions of the stomach wall rarely result in clinical manifestation due to the amplitude of its lumen. Indeed, papers describing APC to treat Watermelon Stomach, using 60[19] to 100 W[12] were successful with no complications.
In a similar study, Johanns et al[20] described involvement of the muscularis mucosa when 75 W or more, for 5 or 10 s, were applied to the gastric wall. The difference found in our study may be consequent to the small number of specimens of the mentioned study (four). In both papers the involvement of the muscularis propria was rare, being observed only when 90 W × 5 s was applied in ours and 155 W × 10 s was applied in Johann’s. These results support the safety of the use of argon plasma coagulation for the treatment of gastric lesions. However as the intermediate mucosa is damaged with the same frequency with 90 J or more, application of higher amounts of energy seems to be unnecessary to treat lesions above the muscularis mucosa. Sebastian´s et al[21] results corroborate this theory.
Damage caused by APC to the muscularis mucosa of the colon was less frequent when up to 70 W × 1 s was applied (27% to 45%). The same interval of energy caused involvement of the intermediate mucosa frequently (82% of the cases). These findings are relevant as stenosis of the colon, similar to the esophagus’, usually are symptomatic. This consequence, however, can be minimized using lower amounts of energy, up to 70 J.
Damage of the muscularis propria of the colon occurred even when smaller amounts of energy were used. Although the frequency of this involvement was higher with bigger amounts of energy, reaching 45%, these findings alert to the care to be taken when APC is used in this organ, for the risk of perforation. Indeed, despite the use of a 40 W potency, Wahab et al[12] noticed one case of perforation in the cecum. Canard et al[22] used APC with 30 to 80 W to treat radiation proctitis and had three severe complications (extensive necrosis, perforation and hemorrhage), all of them when potency was above 45 W.
Vargo[8] reviewed eight papers (151 patients) dealing with the treatment of radiation proctitis with APC in potencies of 40 to 60 W. The incidence of success was high, independently of the power setting. In the other hand, major complications were observed in only three cases, a rectum-vaginal fistulae and two stenosis. These complications could be explained by the use of higher potencies, 50 W and 60 W, respectively. Our results differ from those written by Johanns et al[20], who noticed injury of the muscularis propria of the colon, similarly to the esophagus, only when 155 W for 10 s was applied. In their methodology, the authors report fibrosis and cellular picnosis below the coagulation zone. For us, these findings were considered cellular necrosis, justifying the deeper involvement observed here.
The application of bipolar electrocoagulation to esophageal specimens results in more frequent involvement of the intermediate mucosa (67% to 100%) when 100 J or more were used. Damage of the muscularis mucosa were less frequent (up to 44%) when 200 J or less were applied. Between 250 and 500 J this involvement was 67% to 78%. These findings suggest that the interval between 100 and 200 J may be best suited to ablation of the intermediate mucosa, especially in circumferential lesions with risk of healing retraction. Bipolar electrocoagulation did not resulted in damage of the muscularis propria of the esophagus in this study encouraging its use in clinical practice. Indeed, stenosis and perforation after Barrett´s treatment was significantly less frequently reported with the application of this method.
Kovacs et al[23] observed 5% of stenosis with the use of bipolar electro-coagulation on metaplasic epithelium in esophagus. Sampliner et al[24] observed only one case of stenosis out of 72 patients treated the same way. Sharma et al[25] and Sampliner et al[26] had no complications. However success rates were also lower, 81%, 78% and 73%, respectively. Montes et al[27] were successful in 100% of their cases by applying bipolar electrical current, with power of 20 W, on Barrett’s esophagus; unfortunately the study doesn’t specified electrocoagulation time employed. Electrocoagulation with higher power settings and for longer time might optimize the results of this method for the treatment of Barrett´s esophagus, keeping a lower risk for complications when compared to argon plasma. In the other hand, using argon plasma one can cover extensive areas faster than with the use of bipolar coagulation justifying the popularity of the first method. To overcome this limitation, Ganz et al[28] published the application of a new electrocoagulation probe with an adjustable balloon that allows contact to the entire circumference of the organ. The device has been used in three patients before surgery for esophageal cancer. Electrocoagulation was performed with 260 to 350 W power settings for 0.8 s (energy density of 10 to 12 J/cm2). There were no cases of perforation. A histological evaluation of these specimens showed mucosal ablation of 75% to 95% of the treated area in the two cases that the balloon contacted the whole circumference of the organ. The lamina propria was involved in all the three cases, being the muscularis mucosa totally involved in the majority of the coagulated areas. In a preliminary study using a porcine model (n = 12), electrocoagulation of healthy mucosa was performed with 350 W power setting and energy densities varying from 5 to 20 J/cm2. The application of more than 12 J/cm2 resulted in involvement of the submucosa and, above 15 J/cm2; damage of the muscularis propria was seen. There was one case of peri-esophageal effusion when 20 J/cm2 was used. This result is consistent with our findings, as, despite the ideal interval for these authors be 200 to 280 J, their target was the involvement of the muscularis mucosa. The concept of controlled deliverance of energy to the GI wall culminated with the introduction of radiofrequency ablation for the treatment of Barrett’s esophagus. In radiofrequency sessions both the amount of energy and the contact of the balloon-based probe with the mucosal surface are controlled which seem critical to the good results achieved with this technique[29].
In this study, when bipolar electrocoagulation was used, the frequency of involvement of the intermediate mucosa of the stomach was low, up to 45%, except when the combination 20 W × 3 s was used, raising its involvement to 64%. Damage of the muscularis mucosa varied between 0 and 18% in all combinations of power setting and time except with the combination 20 W × 3 s, when it was 27%. This combination was the only one that presented damage to the muscularis propria, in only one coagulated point (9%). There was no correlation between power setting or time of application and involvement of the intermediate mucosa or muscularis mucosa of the stomach. These findings suggest that bipolar electrocoagulation of the stomach surface can be safely applied, even with higher power settings and longer time, as the risk of muscularis mucosa damage is low and muscularis propria very rare.
Although characteristic features of antral vascular ectasia are found in the lamina propria of the mucosa, the variants mentioned above could explain the therapeutic success of bipolar electrocoagulation in the treatment of this condition, like the results observed by Binmoeller et al[30] and Jensen et al[10].
Morris et al[31] studied the effect of this method to the gastric wall of dogs. The animals were maintained alive for the next seven days, when the depth of the wall involvement was analyzed. They observed deeper involvement, using similar combinations of power setting and time than we did. However some considerations can be pointed out. The thickness of the specimens wall was not described, not allowing comparison and, histological analyses took place one week after coagulation. It is not established if this interval is responsible for healing or increasing the thermal lesion.
In the colon, electrocoagulation with smaller amounts of energy, up to 60J (20 W × 3 s), caused injury of the muscularis mucosa less frequently (9% to 30%), while the interval between 100 and 500 J provoked this involvement in 55% to 82%. In the other hand, less frequent muscularis mucosa involvement with less chance of stenosis was obtained with amounts of energy that provoked less damage to the intermediate mucosa (55% to 64% of the cases-up to 60 J and 82% to 100% of involvement-150 to 500 J). When the method was applied for longer interval (10 s), the muscularis propria was involved in 9% to 10% of the coagulated points, similarly to Jensen´s et al[32] findings. Although this could be considered a low incidence, this involvement should be pointed out for the risk of perforation. The application for short intervals (up to 5 s), even with 50 W power setting, did not caused muscularis propria damage in any coagulated point, offering better safety for clinical practice.
We would also like to emphasize that in this study as in Jensen´s et al[32], cecum specimens, known for presenting a thinner wall, were not used. Application of any thermal method on this colon segment should be performed more cautiously. Radiation lesions are also special situations for being located in ischemic, less resistant tissue.
In this study, electrocoagulation appeared safer than argon plasma also in the colon. Causing a more superficial damage, it seems to be adequate to lesions such as vascular ectasias. Nevertheless, Jensen et al[9] related one case of perforation after treating colonic vascular ectasias.
In conclusion, involvement of the intermediate mucosa of the stomach and of the muscularis mucosa of the stomach and the colon by argon plasma coagulation were more frequent when higher amounts of energy were used (above 90 J). The same tendency was observed in the esophagus samples for the involvement of the muscularis mucosa (above 150 J). In the esophagus and in the colon, injury of the intermediate mucosa caused by this method was frequent, even when small amount of energy was used (50 J). Injury of the muscularis propria was observed in 9% to 45% of the colon samples, depending on the amount of energy used. In the esophagus and in the stomach, the involvement of the muscularis propria was rare.
The use of bipolar electrocoagulation resulted in more frequent involvement of the intermediate mucosa and of the muscularis mucosa of the colon when higher amounts of energy were used (100 J or more). The same tendency was observed in the esophagus samples. In the stomach, the frequency of involvement of the intermediate mucosa and of the muscularis mucosa by the latter method was low, even when more energy was used (until 500 J). The risk of injury of the muscularis propria was low in the stomach and in the colon, not being observed in the esophagus.
Bipolar electrocoagulation seemed to cause more superficial injury to the specimens walls when compared to argon plasma coagulation, however the difference was statistically significant only for stomach specimens.
The association of diathermy to endoscopy has provided significant advances in endotherapy, which became a valuable alternative to traditional surgery and therapeutic procedure of choice in several conditions (e.g., sphyncterotomy, polypectomy). The best way of applying heat to tissue has not been clearly established for hollow organs so far.
The best way of applying heat to hollow digestive organs during thermal endoscopic therapy has not been clearly established so far. This study analyzes the histophathological effect of bipolar electrocoagulation and argon plasma coagulation on fresh surgical specimens of the digestive tract.
This study analyzes the histopathological effect of bipolar electrocoagulation and argon plasma coagulation on fresh surgical specimens of the digestive tract. Tissue damage after argon plasma coagulation is deeper than bipolar electrocoagulation. Both of them depend on the amount of energy used.
The use of argon plasma coagulation is popular in therapeutic endoscopy probably because it is easily available, has low cost, large surfaces of mucosa can be treated in one session and it causes allegedly superficial damage to the GI wall. These findings suggest that lower power settings are probably safer when argon plasma coagulation is employed at the colorectal and esophageal wall.
Bipolar coagulation: the passage of electrosurgical current occurs within the accessory. Argon plasma coagulation: the passage of monopolar electrosurgical current occurs through a cloud of argon gas.
This study deals with a topic that is very much valued by the endoscopic digestive surgeons, that is the laser argon versus the bipolar coagulator, which one the safer and more effective way of cauterization would be among them two.
P- Reviewer: La Torre F S- Editor: Song XX L- Editor: A E- Editor: Zhang DN
| 1. | Kawai K, Akasaka Y, Murakami K, Tada M, Koli Y. Endoscopic sphincterotomy of the ampulla of Vater. Gastrointest Endosc. 1974;20:148-151. |
| 2. | Cook DJ, Guyatt GH, Salena BJ, Laine LA. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: a meta-analysis. Gastroenterology. 1992;102:139-148. |
| 3. | Niezychowski W, Regula J, Fijuth J, Przytulski K, Butruk E. Argon plasma coagulation in palliative treatment of malignant dysphagia. Gut. 1996;39:A5. |
| 4. | Kumar P, Fleischer DE. Thermal therapy for gastrointestinal bleeding. Gastrointest Endosc Clin N Am. 1997;7:593-609. |
| 5. | Tam W, Moore J, Schoeman M. Treatment of radiation proctitis with argon plasma coagulation. Endoscopy. 2000;32:667-672. |
| 6. | Pedrazzani C, Catalano F, Festini M, Zerman G, Tomezzoli A, Ruzzenente A, Guglielmi A, de Manzoni G. Endoscopic ablation of Barrett’s esophagus using high power setting argon plasma coagulation: a prospective study. World J Gastroenterol. 2005;11:1872-1875. |
| 7. | Pereira-Lima JC, Busnello JV, Saul C, Toneloto EB, Lopes CV, Rynkowski CB, Blaya C. High power setting argon plasma coagulation for the eradication of Barrett’s esophagus. Am J Gastroenterol. 2000;95:1661-1668. |
| 8. | Vargo JJ. Clinical applications of the argon plasma coagulator. Gastrointest Endosc. 2004;59:81-88. |
| 9. | Jensen DM, Machicado GA, Kovacs TOG, Randall GM, Reedy T, Van Deventer G. Bleeding colonic angiomata: diagnosis, treatment and outcome. Gastrointest Endosc. 1989;35:173. |
| 10. | Jensen DM, Kovacs TOG, Randall G, Cheng S, Jensen ME. Prospective randomized study of patients with bleeding watermelon stomach (WMS) vs. other UGI angioma syndromes (UGAS) treated with bipolar or heater probe. Intestinal Dis. 1994;106:A241. |
| 11. | Laine L. Multipolar electrocoagulation in the treatment of peptic ulcers with nonbleeding visible vessels. A prospective, controlled trial. Ann Intern Med. 1989;110:510-514. |
| 12. | Wahab PJ, Mulder CJ, den Hartog G, Thies JE. Argon plasma coagulation in flexible gastrointestinal endoscopy: pilot experiences. Endoscopy. 1997;29:176-181. |
| 13. | Hauge T, Moum B, Sandvei P, Lerang F, Ravneng P. [Argon plasma coagulation--a new method in therapeutic endoscopy]. Tidsskr Nor Laegeforen. 2000;120:1413-1415. |
| 14. | Watson JP, Bennett MK, Griffin SM, Matthewson K. The tissue effect of argon plasma coagulation on esophageal and gastric mucosa. Gastrointest Endosc. 2000;52:342-345. |
| 15. | Grade AJ, Shah IA, Medlin SM, Ramirez FC. The efficacy and safety of argon plasma coagulation therapy in Barrett’s esophagus. Gastrointest Endosc. 1999;50:18-22. |
| 16. | Schulz H, Miehlke S, Antos D, Schentke KU, Vieth M, Stolte M, Bayerdörffer E. Ablation of Barrett’s epithelium by endoscopic argon plasma coagulation in combination with high-dose omeprazole. Gastrointest Endosc. 2000;51:659-663. |
| 17. | Ragunath K, Krasner N, Raman VS, Haqqani MT, Phillips CJ, Cheung I. Endoscopic ablation of dysplastic Barrett’s oesophagus comparing argon plasma coagulation and photodynamic therapy: a randomized prospective trial assessing efficacy and cost-effectiveness. Scand J Gastroenterol. 2005;40:750-758. |
| 18. | Heindorff H, Wøjdemann M, Bisgaard T, Svendsen LB. Endoscopic palliation of inoperable cancer of the oesophagus or cardia by argon electrocoagulation. Scand J Gastroenterol. 1998;33:21-23. |
| 19. | Chaves DM, Baba ER, Sakai P, Iriya K, Ishioka S. The argon beam plasma coagulation (ABPC) for treatment of gastric antral vascular ectasia (Watermelon Stomach). Endoscopy. 1999;31:E36. |
| 20. | Johanns W, Luis W, Janssen J, Kahl S, Greiner L. Argon plasma coagulation (APC) in gastroenterology: experimental and clinical experiences. Eur J Gastroenterol Hepatol. 1997;9:581-587. |
| 21. | Sebastian S, McLoughlin R, Qasim A, O’Morain CA, Buckley MJ. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia (watermelon stomach): long-term results. Dig Liver Dis. 2004;36:212-217. |
| 22. | Canard JM, Védrenne B, Bors G, Claude P, Bader R, Sondag D. [Long term results of treatment of hemorrhagic radiation proctitis by argon plasma coagulation]. Gastroenterol Clin Biol. 2003;27:455-459. |
| 23. | Kovacs BJ, Chen YK, Lewis TD, DeGuzman LJ, Thompson KS. Successful reversal of Barrett’s esophagus with multipolar electrocoagulation despite inadequate acid suppression. Gastrointest Endosc. 1999;49:547-553. |
| 24. | Sampliner RE, Faigel D, Fennerty MB, Lieberman D, Ippoliti A, Lewin K, Weinstein WM. Effective and safe endoscopic reversal of nondysplastic Barrett’s esophagus with thermal electrocoagulation combined with high-dose acid inhibition: a multicenter study. Gastrointest Endosc. 2001;53:554-558. |
| 25. | Sharma P, Bhattacharyya A, Garewal HS, Sampliner RE. Durability of new squamous epithelium after endoscopic reversal of Barrett’s esophagus. Gastrointest Endosc. 1999;50:159-164. |
| 26. | Sampliner RE, Fennerty B, Garewal HS. Reversal of Barrett’s esophagus with acid suppression and multipolar electrocoagulation: preliminary results. Gastrointest Endosc. 1996;44:532-535. |
| 27. | Montes CG, Brandalise NA, Deliza R, Novais de Magalhães AF, Ferraz JG. Antireflux surgery followed by bipolar electrocoagulation in the treatment of Barrett’s esophagus. Gastrointest Endosc. 1999;50:173-177. |
| 28. | Ganz RA, Utley DS, Stern RA, Jackson J, Batts KP, Termin P. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc. 2004;60:1002-1010. |
| 29. | Pouw RE, Bergman JJ. Radiofrequency ablation for Barrett’s esophagus, for whom and by whom? Clin Gastroenterol Hepatol. 2013;11:1256-1258. |
| 30. | Binmoeller KF, Katon RM. Bipolar electrocoagulation for watermelon stomach. Gastrointest Endosc. 1990;36:399-402. |
| 31. | Morris DL, Brearley S, Thompson H, Keighley MR. A comparison of the efficacy and depth of gastric wall injury with 3.2- and 2.3-mm bipolar probes in canine arterial hemorrhage. Gastrointest Endosc. 1985;31:361-363. |
| 32. | Jensen DM, Machicado GA, Tapia J, Mautner W. Comparison of argon laser photocoagulation and bipolar electrocoagulation for endoscopic hemostasis in the canine colon. Gastroenterology. 1982;83:830-835. |