Published online Sep 16, 2013. doi: 10.4253/wjge.v5.i9.440
Revised: May 28, 2013
Accepted: July 17, 2013
Published online: September 16, 2013
Processing time: 163 Days and 19.8 Hours
AIM: To analyze the diagnostic utility of a small-caliber endoscope (SC-E) and clinicopathological features of false-negative gastric cancers (FN-GCs).
METHODS: A total of 21638 esophagogastroduodenoscopy (EGD) gastric cancer (GC) screening examinations were analyzed. Secondary endoscopic examinations (n = 3352) were excluded because most secondary examinations tended to be included in the conventional endoscopy (C-E) group. Detection rates of GCs and FN-GCs were compared between SC-E and C-E groups. FN-GC was defined as GC performed with EGD within the past 3 years without GC detection. Macroscopic types, histopathological characteristics and locations of FN-GCs were compared with firstly found-gastric cancers (FF-GCs) in detail.
RESULTS: SC-E cases (n = 6657) and C-E cases (n = 11644), a total of 18301 cases, were analyzed. GCs were detected in 16 (0.24%) SC-E cases and 40 C-E (0.34%) cases (P = 0.23) and there were 4 FN-GCs (0.06%) in SC-E and 13 (0.11%) in C-E (P = 0.27), with no significant difference. FN-GCs/GCs ratio between SC-E and C-E groups was not significantly different (P = 0.75). The comparison of endoscopic macroscopic types of FN-GCs tended to be a less advanced type (P = 0.02). Histopathologically, 70.6% of FN-GCs were differentiated and 29.4% undifferentiated type. On the other hand, 43.0% of FF-GCs were differentiated and 53.8% undifferentiated type, so FN-GCs tended to be more differentiated type (P = 0.048).
CONCLUSION: The diagnostic utility of SC-E for the detection of GCs and FN-GCs was not inferior to that of C-E. Careful observation for superficially depressed type lesions in the upper lesser curvature region is needed to decrease FN-GCs.
Core tip: This is the first study to reveal that the screening performance for gastric cancers by a small caliber-endoscope might not be inferior to that of conventional endoscope. Superficially depressed type lesions in the upper lesser curvature region should be carefully observed in gastric cancer screening in order to decrease false-negative gastric cancers.
- Citation: Kataoka H, Mizuno K, Hayashi N, Tanaka M, Nishiwaki H, Ebi M, Mizoshita T, Mori Y, Kubota E, Tanida S, Kamiya T, Joh T. Diagnostic utility of small-caliber and conventional endoscopes for gastric cancer and analysis of endoscopic false-negative gastric cancers. World J Gastrointest Endosc 2013; 5(9): 440-445
- URL: https://www.wjgnet.com/1948-5190/full/v5/i9/440.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i9.440
Currently, transnasal esophagogastroduodenoscopy (EGD) using a small-caliber endoscope (SC-E) is being widely carried out as a screening examination for gastric cancer (GC) because EGD with an SC-E appears to be less stressful to the cardiovascular system and has good patient tolerance in several comparative analyses[1-5]. However, the diagnostic accuracy of SC-E has been thought to be low because of several weak points, including low resolution, low brightness and poor operational performance[6,7].
In this study, the diagnostic utility of SC-E and conventional endoscopy (C-E) in GC screening health checkups were compared from the viewpoints of GC detection rates and false-negative gastric cancers (FN-GCs). Furthermore, the clinicopathological features of FN-GCs were analyzed.
A total of 21638 patients who underwent EGD for screening of the upper gastrointestinal tract in Nagoya Toei Clinic from 2003 to 2011 were investigated. The SC-E group included 6831 subjects (4959 men, 1872 women; mean ± SD age 50.98 ± 9.89 years) and the C-E group included 14807 subjects (11000 men, 3807 women; age 52.05 ± 10.44 years), as shown in Table 1. EGD examinations by SC-E were performed using an EG 530N or EG530NW (Fuji Film, Tokyo, Japan), whereas EGD examinations by C-E were performed using an XQ240 and H260 (Olympus, Tokyo, Japan). The outer diameters of these scopes were 5.9 mm for the EG530N and EG530NW, 9.0 mm for the XQ240, and 9.8 mm for the H260. Viewing angles were 120° for the EG530N and 140° for the EG530NW, XQ240 and H260.
FN-GC was defined as GC that was missed on EGD examination within the past 3 years. FN-GCs were categorized into 4 groups by analyzing the previous EGD images of FN-GC patients: (1) undetected error; (2) incomplete visualization; (3) misdiagnosis as a benign lesion; or (4) no findings.
Descriptive statistics and simple analyses were carried out using the statistical package R version 2.4.1 (http://www.r-project.org). In the comparisons between any two subject groups, Student’s unpaired t test was used for continuous variables and the χ2 test and Fisher’s exact test were used to compare categorical variables. Analysis of variance was performed for comparisons among multiple groups. In all analyses, P values < 0.05 were considered significant.
As shown in Table 1, the SC-E group included 6831 subjects (4959 men, 1872 women; age 50.98 ± 9.89 years) and the C-E group included 14807 subjects (11000 men, 3807 women; age 52.05 ± 10.44 years); the SC-E group included more females and younger patients than the C-E group (P < 0.01).
The GC detection rate was significantly lower by SC-E than by C-E (P = 0.01). However, the FN-GC detection rate and the ratio of FN-GCs/GCs were not significantly different between the two groups (P = 0.48, P = 0.27) (Table 2). We found that some of these patients had some abnormalities in the stomach checked by X-ray barium studies before EGD examination. Furthermore, the majority of secondary endoscopic checks were included in the C-E group. A total of 3352 patients underwent EGD as secondary checks; these patients were excluded and the detection rates were re-calculated. As shown in Table 2, the SC-E group included 6657 subjects and the C-E group included 11644 subjects; 16 GCs were detected in the SC-E group (detection rate, 0.24%) and 40 GCs were detected in the C-E group (detection rate, 0.34%). There were no significant differences in the GC detection rates, FN-GC detection rates and FN-GCs/GCs ratios between the two groups. Thus, the GC and FN-GC detection rates were not different between SC-E and C-E.
As a next step, 17 FN-GCs were analyzed in detail (Table 3). Four FN-GCs were detected with SC-E and 13 with C-E, but there were no significant differences between the two groups in FN-GC detection rate and the ratio of FN-GCs/GCs, as shown in Table 2. The mean duration from the previous endoscopic examination to the day of cancer detection by EGD examination was 14.6 ± 8.2 months (mean ± SD). There were no significant differences in the mean duration between intramucosal GC cases (13.2 ± 3.0) and GC with submucosal layer cases (14.5 ± 2.5). Nine cases were intramucosal FN-GCs and 6 cases were FN-GCs with submucosal layer invasion, but there were no FN-GCs that invaded to the muscularis propria or deeper. Six cases were treated endoscopically (endoscopic mucosal resection and endoscopic submucosal dissection) and 9 cases were treated surgically. The previous endoscopic images were analyzed minutely and 3 cases were considered an “undetected error”, which means that the endoscopist missed the cancer lesion at the previous examination and the cancer lesions could be seen in the endoscopic images of previous EGD examinations. Six cases were considered “incomplete visualization”, which means that no images of the location of the cancer had been taken at the last examination or the image quality of the cancer location was low. Seven cases were considered “misdiagnosis as benign”. The endoscopic specimens of 7 cases were all re-checked by a pathologist and they were reconfirmed as benign. Thus, in the 7 “misdiagnosis as benign” cases, techniques of biopsy under endoscopy seemed to have been the major problem. There was only one case that was considered to be “no findings”. It was confirmed that there was no lesion in the previous clear image of the cancer location.
| Characteristics | Value |
| SC-E/C-E | 4/13 |
| Age (mean ± SD) | 57.6 ± 9.4 |
| Male/female | 14/3 |
| Duration1 (mean ± SD) | 14.6 ± 8.2 (M) |
| (m: 13.2 ± 3.0, sm: 14.5 ± 2.5) | |
| Depth of invasion | m: 9, sm: 6, mp: 0, unknown: 2 |
| Macroscopical types | Elevated type: 5, depressed type: 12 |
| Histopathological types | tub1: 8, tub2: 4, por/sig: 5 |
| Treatment | Endoscopic (EMR, ESD): 6 |
| Surgical: 9 | |
| Unknown: 2 | |
| The previous endoscopic findings | Undetected error: 3 |
| Incomplete visualization: 6 | |
| Misdiagnosis as benign: 7 | |
| No findings: 1 |
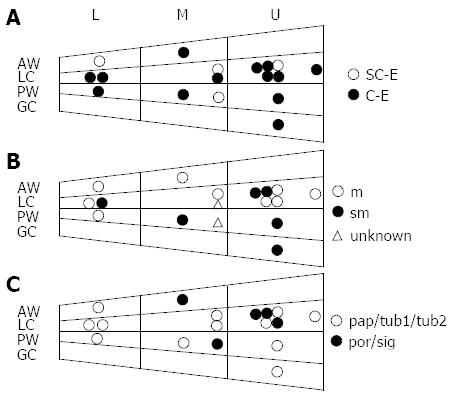
The locations of 17 FN-GC cases are shown in Figure 1. FN-GCs tended to be located in the upper (U) lesser curvature (LC) region. As shown in the lower panel (Figure 1C), GCs of differentiated type (pap/tub1/tub2) tended to be localized in the lower (L) region.
Finally, the macroscopic types of GCs were compared between the FN-GC group and the FF-GC group. As shown in Table 4, FF-GCs included more advanced type GCs (P < 0.05). In the early GCs, FN-GCs tended to include more IIc types (superficially depressed types) than FF-GCs, but the difference was not significant.
Finally, the histopathological features (pap/tub1/tub2, por/sig, unknown) of FN-GCs [12 (70.6%), 5 (29.4%), 0 (0%)] and FF-GCs [40 (43.0%), 50 (53.8%), 3 (3.2%)] were compared; FN-GCs included significantly more differentiated type GCs (pap/tub1/tub2) than FF-GCs (P = 0.048) (Table 5).
| Pap/Tub1/Tub2 | Por/Sig | Unknown | Total | |
| FN-GC | 12 (70.6%) | 5 (29.4%) | 0 (0%) | 17 |
| FF-GC | 40 (43.0%) | 50 (53.8%) | 3 (3.2%) | 93 |
GC is ranked as the second leading cause of global cancer mortality and the fourth most common cancer worldwide[8,9]. Japan is known as one of the countries of highest incidence and mortality of GC; approximately 110000 people develop GC each year, with 65000 estimated deaths. Detecting mucosal GC in asymptomatic people by high quality endoscopic GC screening is important for decreasing mortality of GC.
This is the first study to compare the detection rates of GCs and FN-GCs between an SC-E group and a C-E group in GC endoscopic screening. For GC screening, radiographic screening using upper gastrointestinal series has been performed nationwide in Japan, but the GC screening rate has gradually decreased due to a lack of human resources. Thus, several new methods are anticipated as alternative approaches for GC screening. Prescreening of a high-risk group for GC by serological testing for pepsinogen and Helicobacter pylori (H. pylori) antibody is one of the alternative methods, especially for the population at high risk of GCs[10-12]. Patients categorized as high-risk for GC are considered to be the candidates for endoscopic screening. Recently, although problems remain related to the confirmation of the validity of the evidence, several studies reported that endoscopic screening of the upper gastrointestinal tract significantly decreased the GC mortality rate[13,14].
Transnasal EGD with SC-E has been used more for GC screening because the tolerability, acceptability and safety are better for SC-E than for C-E[15,16]. However, the screening performance of SC-E for GC may be inferior to that of C-E due to low resolution, low luminous intensity and the narrow angle of view of SC-E.
In the present study, there were no significant differences in screening performance for GCs and FN-GCs between SC-E and C-E. Similar to the present results, some previous studies have reported that the diagnostic accuracy of SC-E is almost equivalent to that of C-E for the detection of upper gastrointestinal tract lesions, including GCs[17-21]. The present study has some weakness because it was a non-randomized retrospective study and the selection of endoscope (SC-E or C-E) was decided by patient’s choice. Further randomized controlled studies need to be carried out to achieve precise conclusions. Nakata et al[22] reported that the diagnostic performance of SC-E was inferior to that of C-E for GC screening, particularly in subjects with non-atrophic gastritis. In our study, the atrophic stages of the gastric mucosa were not significantly different between the FN-GC group and the FF-GC group. Between FN-GCs found by SC-E and FN-GCs found by C-E, there was no significant difference of gastric mucosal atrophic stages (data not shown).
Yoshida et al[15] reported no significant differences in the detection of early GC and adenoma between SC-E and C-E, but they pointed out that GCs might be overlooked by SC-E when performed by less experienced endoscopists. In the present study, almost all EGD examinations were performed by experienced endoscopists (over 10 years experience) and there was no laterality of endoscopists in experience who performed previous EGD examinations of FN-GCs.
Hayashi et al[23] analyzed the detection rates of early GCs and reported that SC-E was less efficient in screening for GCs located in the upper third of the stomach (U region) due to the narrower field of view and low luminous intensity. As shown in Figure 1A, although more FN-GC lesions tended to exist in the U region compared with the middle (M) and/or L regions, there was no laterality of FN-GCs in location detected by SC-E.
A literature search identified no previous studies that compared the detection rates of FN-GCs between an SC-E group and a C-E group. With respect to the ratio of FN-GCs/FF-GCs, Yoshimura et al[24] reported a ratio of 28.2% and Yoshikawa et al[25] reported 31.6% with SC-E; these are similar to the present false-negative rates (25.0% with SC-E and 32.5% with C-E). The clinicopathological features of FN-GCs detected by SC-E (4 cases) and FN-GCs by C-E (13 cases) were also investigated, but there were no significant differences between the two groups. However, in the previous endoscopic findings of FN-GC cases, 75% (3 out of 4 cases) of FN-GCs with SC-E was due to “incomplete visualization”. This finding may imply that improvement of the image quality of SC-E is necessary to achieve greater accuracy of GC screening by SC-E. A future study with a larger number of patients should be performed to analyze FN-GCs with SC-E in endoscopic screening.
The necessity of annual GC endoscopic screening is debatable from the viewpoint of not only mortality and morbidity rates but also cost-benefit. Chung et al[26] reported that endoscopic resection was performed more frequently in the annual screening group than in the biennial group (56.9% vs 33.3%; P = 0. 02) in an endoscopic screening study of 58849 subjects. As shown in Table 3 of the clinicopathological analyses of FN-GCs, the mean duration from the previous endoscopy to the day of cancer detection by endoscopy was 14.6 ± 8.2 mo and that of FN-GCs with submucosal invasion was 1.3 mo longer than that of FN-GCs in situ. These findings suggest that annual GC endoscopic screening is beneficial for decreasing the mortality rate of GCs by identifying FN-GCs in the early stages.
Finally, the histopathological analyses of FN-GCs revealed that differentiated type GC was significantly more common in the FN-GC group than in the FF-GC group. Yoshikawa et al[25] also reported that 66.6% of FN-GCs with SC-E were differentiated type GC. At this point, there is no apparent explanation for this result, but we should pay more attention to differentiated type GCs that are macroscopically superficial depressed type.
In conclusion, this is the first study to have compared the detection rates of FN-GCs and GCs between SC-E and C-E. The screening performance for GCs by SC-E might not be inferior to that of C-E. Superficially depressed type lesions in the upper lesser curvature region should be carefully observed in GC screening in order to decrease FN-GCs. In the near future, high-performance SC-E will surely be developed and used as the main endoscopy method for GC screening, with better tolerability, acceptability and safety than C-E.
Transnasal esophagogastroduodenoscopy (EGD) using a small-caliber endoscope (SC-E) is being widely carried out as a screening examination for gastric cancer (GC) because EGD with an SC-E appears to be less stressful to the cardiovascular system and has good patient tolerance in several comparative analyses. However, the diagnostic accuracy of SC-E has been thought to be low because of several weak points, including low resolution, low brightness and poor operational performance.
In this study, the diagnostic utility of SC-E and conventional endoscopy (C-E) in GC screening health checkups were compared from the viewpoints of GC detection rates and false-negative gastric cancers (FN-GCs). Furthermore, the clinicopathological features of FN-GCs were analyzed.
The results have clearly demonstrated that the screening performance for GCs by SC-E might not be inferior to that of C-E. FN-GCs tended to be located in the upper lesser curvature region, more differentiated type, more superficial depressed type and less advanced type.
The diagnostic utility of SC-E for the detection of GCs and FN-GCs was not inferior to that of C-E. Careful observation for superficially depressed type lesions in the upper lesser curvature region is needed to decrease FN-GCs.
This is a large monocentric retrospective study showing no difference for screening and diagnosis of gastric cancer between small-caliber and conventional endoscopes by expert endoscopists. Conclusions are nevertheless of great interest.
P- Reviewer Manfredi S S- Editor Gou SX L- Editor Roemmele A E- Editor Wang CH
| 1. | Zaman A, Hahn M, Hapke R, Knigge K, Fennerty MB, Katon RM. A randomized trial of peroral versus transnasal unsedated endoscopy using an ultrathin videoendoscope. Gastrointest Endosc. 1999;49:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Preiss C, Charton JP, Schumacher B, Neuhaus H. A randomized trial of unsedated transnasal small-caliber esophagogastroduodenoscopy (EGD) versus peroral small-caliber EGD versus conventional EGD. Endoscopy. 2003;35:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Birkner B, Fritz N, Schatke W, Hasford J. A prospective randomized comparison of unsedated ultrathin versus standard esophagogastroduodenoscopy in routine outpatient gastroenterology practice: does it work better through the nose? Endoscopy. 2003;35:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Yagi J, Adachi K, Arima N, Tanaka S, Ose T, Azumi T, Sasaki H, Sato M, Kinoshita Y. A prospective randomized comparative study on the safety and tolerability of transnasal esophagogastroduodenoscopy. Endoscopy. 2005;37:1226-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Kataoka H, Hayano J, Mizushima T, Tanaka M, Kubota E, Shimura T, Mizoshita T, Tanida S, Kamiya T, Nojiri S. Cardiovascular tolerance and autonomic nervous responses in unsedated upper gastrointestinal small-caliber endoscopy: a comparison between transnasal and peroral procedures with newly developed mouthpiece. Dig Endosc. 2011;23:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Walter T, Chesnay AL, Dumortier J, Mège-LeChevallier F, Hervieu V, Guillaud O, Lapalus MG, Lépilliez V, Fumex F, Ponchon T. Biopsy specimens obtained with small-caliber endoscopes have comparable diagnostic performances than those obtained with conventional endoscopes: a prospective study on 1335 specimens. J Clin Gastroenterol. 2010;44:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Kawai T, Yamamoto K, Fukuzawa M, Sakai Y, Moriyasu F. Ultra-thin transnasal esophagogastroduodenoscopy. Nihon Rinsho. 2010;68:1264-1267. [PubMed] |
| 8. | Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, Tan SH, Wu J, Lee MH, Ooi CH. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476-485, 485.e1-11. [PubMed] |
| 9. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2645] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 10. | Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 373] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Mizuno S, Miki I, Ishida T, Yoshida M, Onoyama M, Azuma T, Habu Y, Inokuchi H, Ozasa K, Miki K. Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci. 2010;55:3132-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, Doi H, Yoshida H, Kawabe T, Omata M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Hosokawa O, Miyanaga T, Kaizaki Y, Hattori M, Dohden K, Ohta K, Itou Y, Aoyagi H. Decreased death from gastric cancer by endoscopic screening: association with a population-based cancer registry. Scand J Gastroenterol. 2008;43:1112-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 14. | Matsumoto S, Yamasaki K, Tsuji K, Shirahama S. Results of mass endoscopic examination for gastric cancer in Kamigoto Hospital, Nagasaki Prefecture. World J Gastroenterol. 2007;13:4316-4320. [PubMed] |
| 15. | Yoshida Y, Hayami Y, Matuoka M, Nakayama S. Comparison of endoscopic detection rate of early gastric cancer and gastric adenoma using transnasal EGD with that of transoral EGD. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. 2008;20:184-189. |
| 16. | Tatsumi Y, Harada A, Matsumoto T, Tani T, Nishida H. Current status and evaluation of transnasal esophagogastroduodenoscopy. Dig Endosc. 2009;21:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Sorbi D, Gostout CJ, Henry J, Lindor KD. Unsedated small-caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study. Gastroenterology. 1999;117:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Murata A, Akahoshi K, Sumida Y, Yamamoto H, Nakamura K, Nawata H. Prospective randomized trial of transnasal versus peroral endoscopy using an ultrathin videoendoscope in unsedated patients. J Gastroenterol Hepatol. 2007;22:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Thota PN, Zuccaro G, Vargo JJ, Conwell DL, Dumot JA, Xu M. A randomized prospective trial comparing unsedated esophagoscopy via transnasal and transoral routes using a 4-mm video endoscope with conventional endoscopy with sedation. Endoscopy. 2005;37:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Catanzaro A, Faulx A, Isenberg GA, Wong RC, Cooper G, Sivak MV, Chak A. Prospective evaluation of 4-mm diameter endoscopes for esophagoscopy in sedated and unsedated patients. Gastrointest Endosc. 2003;57:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Saeian K, Staff DM, Vasilopoulos S, Townsend WF, Almagro UA, Komorowski RA, Choi H, Shaker R. Unsedated transnasal endoscopy accurately detects Barrett’s metaplasia and dysplasia. Gastrointest Endosc. 2002;56:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Nakata H, Enomoto S, Maekita T, Inoue I, Ueda K, Deguchi H, Shingaki N, Moribata K, Maeda Y, Mori Y. Transnasal and standard transoral endoscopies in the screening of gastric mucosal neoplasias. World J Gastrointest Endosc. 2011;3:162-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Hayashi Y, Yamamoto Y, Suganuma T, Okada K, Nego M, Imada S, Imai M, Yoshimoto K, Ueki N, Hirasawa T. Comparison of the diagnostic utility of the ultrathin endoscope and the conventional endoscope in early gastric cancer screening. Dig Endosc. 2009;21:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Yoshimura R, Shiga N, Yoshimura D, Nasu S, Nakamura K. A retrospective single-center study on false negative cases of gastric cancer screening. Stomach and Intestine. 2012;47:948-956. |
| 25. | Yoshikawa H, Aida K, Hamada R. Investigation of false-negative cases in gastric cancer screening with transnasal endoscopy-a retrospective study on opportunistic screening. Stomach and Intestine. 2012;47:957-965. |
| 26. | Chung SJ, Park MJ, Kang SJ, Kang HY, Chung GE, Kim SG, Jung HC. Effect of annual endoscopic screening on clinicopathologic characteristics and treatment modality of gastric cancer in a high-incidence region of Korea. Int J Cancer. 2012;131:2376-2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |