Published online Sep 16, 2013. doi: 10.4253/wjge.v5.i9.433
Revised: July 26, 2013
Accepted: August 17, 2013
Published online: September 16, 2013
Processing time: 101 Days and 13.2 Hours
AIM: To evaluate the efficacy, tolerability, acceptability and feasibility of bisacodyl plus low volume polyethyleneglycol-citrate-simeticone (2-L PEG-CS) taken the same day as compared with conventional split-dose 4-L PEG for late morning colonoscopy.
METHODS: Randomised, observer-blind, parallel group, comparative trial carried out in 2 centres. Out patients of both sexes, aged between 18 and 85 years, undergoing colonoscopy for diagnostic investigation, colorectal cancer screening or follow-up were eligible. The PEG-CS group received 3 bisacodyl tablets (4 tablets for patients with constipation) at bedtime and 2-L PEG-CS in the morning starting 5 h before colonoscopy. The control group received a conventional 4-L PEG formulation given as split regimen; the morning dose was taken with the same schedule of the low volume preparation. The Ottawa Bowel Preparation Scale (OBPS) score was used as the main outcome measure.
RESULTS: A total of 164 subjects were enrolled and 154 completed the study; 78 in the PEG-CS group and 76 in the split 4-L PEG group. The two groups were comparable at baseline. The OBPS score in the PEG-CS group (3.09 ± 2.40) and in the PEG group (2.39 ± 2.55) were equivalent (difference +0.70; 95%CI: -0.09-1.48). This was confirmed by the rate of successful bowel cleansing in the PEG-CS group (89.7%) and in the PEG group (92.1%) (difference -2.4%; 95%CI: -11.40- 6.70). PEG-CS was superior in terms of mucosa visibility compared to PEG (85.7% vs 72.4%, P = 0.042). There were no significant differences in caecum intubation rate, time to reach the caecum and withdrawal time between the two groups. The adenoma detection rate was similar (PEG-CS 43.6% vs PEG 44.7%). No serious adverse events occurred. No difference was found in tolerability of the bowel preparations. Compliance was equal in both groups: more than 90% of subjects drunk the whole solution. Willingness to repeat the same bowel preparations was about 90% for both regimes.
CONCLUSION: Same-day PEG-CS is feasible, effective as split-dose 4-L PEG for late morning colonoscopy and does not interfere with work and daily activities the day before colonoscopy.
Core tip: The timing of bowel preparation is fundamental for high quality colonoscopy and also for patient satisfaction. Split-dose preparation improves the rate of adequate cleansing and patient compliance. This study shows that the same-day low volume polyethyleneglycol-citrate-simeticone (PEG-CS) plus bisacodyl tablets is feasible, and as effective as split 4-L PEG. The low volume bowel preparation taken the same day of the exam may be an attractive option for late morning colonoscopy as it reduces the overall time for bowel preparation with no loss of work time and impact on daily activities the day before the exam.
-
Citation: Leone A, Tamayo D, Fiori G, Ravizza D, Trovato C, Roberto GD, Fazzini L, Fante MD, Crosta C. Same-day 2-L PEG-citrate-simethicone plus bisacodyl
vs split 4-L PEG: Bowel cleansing for late-morning colonoscopy. World J Gastrointest Endosc 2013; 5(9): 433-439 - URL: https://www.wjgnet.com/1948-5190/full/v5/i9/433.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i9.433
Optimal bowel preparation is an essential component of high quality colonoscopy. A clean colon free of residual stool or brown liquid over the mucosa minimizes the risk of missing a flat adenoma or other small lesions[1,2] .
The ideal preparation for colonoscopy should effectively and rapidly remove all residual content from the large bowel, without inducing macroscopic or histologic alterations of the colonic mucosa. It should be safe with no risk for causing significant shifts in fluids or electrolytes, easy and pleasant to take in terms of volume and taste and should minimally interfere with daily activities.
To date, no bowel preparation meets all the requirements though important, advancements have been made with the low-volume[3-7] and split-dose bowel preparations. There is still a need to increase the overall acceptability of bowel preparation for colonoscopy and reduce the burden and impact on productivity and daily living with the ultimate objective to improve the attitude toward colonoscopy within the colon cancer screening programs[8].
A new low volume isotonic sulphate-free formulation of polyethyleneglycol-citrate-simeticone (PEG-CS) plus bisacodyl tablets has been designed to be as effective as high volume conventional PEG bowel preparation before colonoscopy and to improve patient satisfaction and compliance. Split-dose administration has been shown to provide better cleansing and reduce patient discomfort compared with a traditional administration on the day before[9-13]. Same-day low volume bowel preparation may provide a further option for people who desire no or minimum impact on their work and daily activities on the day before the endoscopic procedure.
The present study was intended to compare the same-day PEG-CS with the split-dose conventional 4-L PEG for late morning colonoscopy. The primary endpoint was to compare the efficacy and the feasibility of both regimens. The secondary endpoints included adverse events, tolerability, acceptability and compliance and colonoscopy quality indicators.
This was a randomised, observer-blind and parallel group trial. Data were collected over an 11-mo period (from April 2011 to March 2012) at two Endoscopy Units. The trial was registered at Clinical Trials Gov site with number NCT01685853. The study was performed in accordance with the Declaration of Helsinki. The protocol was carried out according to the general principles of Good Clinical Practices and was approved by the Local Ethical Committee.
Adult out-patients of both sexes, aged between 18 and 85 years, undergoing colonoscopy for diagnostic investigation, colorectal cancer screening or follow-up were eligible. Patients with known or suspected gastrointestinal obstruction or perforation, severe acute inflammatory bowel disease or toxic megacolon, ileus or gastric retention, ileostomy, hypersensitivity to any of the ingredients, pregnancy and lactation and/or at a risk of becoming pregnant, were excluded. Patients unable to reach the Endoscopy Units in less than 1 h were not included in the study.
Eligible patients were informed about the aims, procedures, benefits and possible risks of the study prior to signing the informed consent form from day -30 to day -3. In the same visit a baseline evaluation, including medical history, physical examination and collection of demographic data, was performed by a study physician other than the study endoscopist (blinded for patient’s preparation). The same physician instructed the patients how to take the preparation in both oral and written forms and gave to the patient a diary to record the timing of preparation intake, the number and the time of bowel movements, any adverse event, impact of daily life and any additional comments. In the last three days before colonoscopy, patients had to follow a free fibre diet, i.e., without pasta, rice, bread, vegetables and fruits (fruit juices allowed). They could eat meats, fish, eggs and dairy products. The day before the examination, the subjects had to follow a clear liquid diet (e.g., tea, milk, coffee, fruit juices, soft drinks and soup).
Patients were assigned to receive one of the two bowel preparations according to a computer generated block-randomisation list. One group received PEG-CS (2-L LoVOL®-esse) + bisacodyl tablets (Lovol-dyl®). The main active ingredient of the new formulation is macrogol 4000. The other important ingredients are citric acid, sodium citrate and simethicone. The product is available as sachets containing powder for oral solution. Each sachet must be dissolved in 500 mL of water and taken every 30 min. The dosing schedule in detail was as follows: (1) 3 bisacodyl tablets (4 tablets for patients with an history of chronic or occasional constipation) at bedtime; and (2) 2-L PEG-CS in the morning of colonoscopy – starting 5 h before colonoscopy. It was estimated that about 3 h were needed for drinking the solution and for bowel movements, up to an 1 h for the journey to hospital and 30 min in the waiting room). The control group received a conventional PEG-ELS formulation (SELG®1000) given as split regimen: 2-L + 2-L with the morning dose taken with the same schedule of the low volume preparation. The main active ingredients are macrogol 4000 and sodium sulphate. Each sachet of powder must be dissolved in 1L of water and taken as 250 mL every 15 min. The dosing schedule in detail was as follows: (1) 2-L at 6:00 pm the evening before the exam; and (2) 2-L the morning of colonoscopy, starting 5 h before colonoscopy.
Patients returned to the Endoscopy Unit for colonoscopy and gave back the completed diary to the Physician who asked them about tolerability, adverse events, acceptance compliance and impact on daily activities. The colonoscopy was performed by experienced Endoscopists who perform more than 500 colonoscopy/year and have familiarity with the bowel preparation scoring scale used in this study [the validated Ottawa Bowel Preparation Scale, Ottawa Bowel Preparation Scale (OBPS)][14]. The endoscopists were unaware of the bowel preparation taken by the patient and scored the colon cleansing according to the aforementioned scale.
The cleanliness of each section of the colon, i.e., the right, the mid and the rectosigmoid colon was rated according to the 5-point Ottawa scale. The overall colonic fluid was rated according to a 3-point scale. The total score (bowel cleansing total score; primary endpoint) may range from 0 (best) to 14 (worst).
A total OBPS score < 7 was considered a successful bowel preparation.
In addition, we also measured the amount of foam and bubbles in terms of overall impact on mucosal visibility, as follows: (1) Excellent: clear imaging, no or minimal amount of bubbles or foam, which can be easily removed = 0; Fair: modest amount of bubbles and foam, which can be cleared, with loss of some time = 1; and (2) Insufficient: a great amount of foam and bubbles, which reduce significantly the clear visualization of the mucosa = 2.
The occurrence, time of onset and severity of gastrointestinal (GI) symptoms, i.e., nausea, bloating, abdominal pain/cramps, anal irritation, during and after bowel preparation were collected by means of a 3-point Likert scale (2 = severe distress, 1 = mild distress, 0 = no distress).
Pre-determined questions were addressed to each patient with regard to: (1) difficulty to take the preparation within scheduled times; (2) urgency and incontinence episodes during the trip to the hospital; (3) sleep lost (yes/no); (4) ease of taking the preparation (none, mild and severe distress); and (5) patient preference as compared to previous bowel preparations [willingness to use the same product in the future (yes/no)].
Compliance was scored on a 3-grade scale specifying the percentage of drunk solution: (1) Optimal: intake of the whole solution = 0; (2) Good: intake of at least 75% of the solution = 1; and (3) Poor: intake of < 75% of the solution = 2.
Any adverse event reported by any subject or observed by the Physician, independently from its seriousness and its relation to the study formulations, were recorded including time of onset, nature, duration, severity and any action taken.
Caecum intubation rate, time to reach the caecum (intubation time), withdrawal time and adenoma detection rate were recorded.
Taking into account a drop-out rate of 15%, 164 patients (82 per treatment group) had to be enrolled and randomised to obtain 138 evaluable subjects. Such sample size was determined assuming a standard deviation value for the bowel cleansing score equal to 3 points and using an equivalence margin of 1 point, so that the two-sides 95%CI of the mean score difference was expected to lie between ± 1.5 points with 80% power. The data were summarized by treatment using classical descriptive statistics: mean, standard deviation, minimum and maximum values (for quantitative variables) and by frequencies and percentages (qualitative variables). The efficacy analysis was performed on both intention to treat (ITT) and per protocol (PP) populations (patients having drunk at least 75% of the solution) by building the 95%CI for the difference of the mean Ottawa bowel cleansing score in the two groups. Other analysis were performed on ITT populations.
Treatments were compared using z test for bowel cleansing score and other quantitative variables while using chi-square test for qualitative variables. All tests were considered two-tailed with significance level set to 5%.
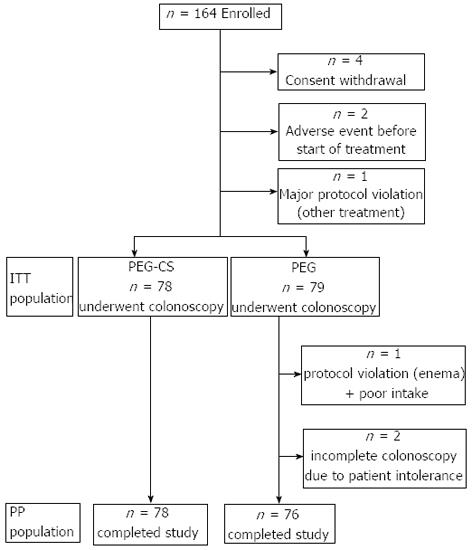
One hundred and sixty-four subjects were enrolled and randomly assigned to the two groups: seven subjects were excluded before colonoscopy (5 for consent withdrawal, 2 for adverse events before starting the treatment). A total of 157 patients underwent colonoscopy (ITT), 78 randomized to PEG-CS and 79 to PEG (Figure 1). The demographic data of the two groups at baseline were comparable (Table 1).
| Variable | PEG-CS + Bis (n = 78) | PEG (n = 79) |
| Male | 30 (38.5) | 27 (34.2) |
| Age (yr) | 61.8 ± 10.8 | 60.9 ± 12.0 |
| Height (cm) | 166.2 ± 9.1 | 165.0 ± 8.1 |
| Weight (kg) | 68.4 ± 14.5 | 68.6 ± 13.4 |
| BMI (kg/m2) | 24.6 ± 3.8 | 25.1 ± 4.1 |
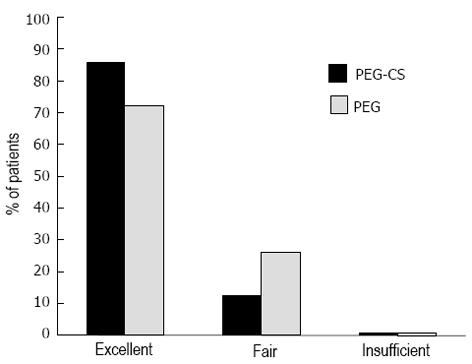
The mean OBPS score was 3.09 ± 2.40 in the PEG-CS group and 2.39 ± 2.55 in the PEG group. The difference between the mean OBPS score in the two groups was not statistically significant for both PP (+0.70; 95%CI: -0.09-1.48) and ITT populations (+0.63; 95%CI: -0.18-1.43). As the confidence intervals are within the predefined interval range (-15%-15%), the two bowel preparations were equivalent for efficacy (Table 2). The rates of successful bowel preparation (OBPS < 7) were similar between the two groups (89.7% vs 92.1%). The rate of excellent visibility (no or minimal amount of bubbles or foam) was greater in the PEG-CS group (85.7%) as compared with 72.4% in the split PEG 4-L group (P value = 0.042) (Figure 2). There were no significant differences in the caecum intubation rate, time to reach the caecum and withdrawal time between the two groups (Table 2).
| PEG-CS + Bis (n = 78) | PEG (n = 76)1 | |
| Overall OBPS score | 3.09 ± 2.40 | 2.39 ± 2.55 |
| Caecal intubation rate | 76 (97.4) | 75 (98.7) |
| Time (min) to reach the caecum | 10.90 ± 6.1 | 9.80 ± 3.6 |
| Adenoma detection | 34 (43.6) | 34 (44.7) |
A significant association between subjects aged > 60 years and adenoma detection rate was found (P = 0.04).
No serious adverse event occurred and no subject discontinued bowel preparation for an adverse event or poor tolerability. No difference was found in terms of tolerability between bowel preparations. There was no significant difference in terms of GI symptom associated with bowel preparation (Table 3).
| PEG-CS + Bis (n = 78) | PEG (n = 79) | |
| GI tolerability | ||
| Nausea (no or mild) | 73 (93.6) | 72 (91.1) |
| Bloating (no or mild) | 77 (98.7) | 78 (98.7) |
| Abdominal pain/cramps (no or mild) | 73 (93.6) | 77 (97.5) |
| Anal irritation (no or mild) | 75 (96.1) | 77 (97.5) |
| Adverse events | ||
| Vomiting | 6 (7.7) | 2 (2.5) |
| Sweating | 2 (2.6) | 0 (0.0) |
| Headache | 3 (3.8) | 3 (3.8) |
| Shivering | 2 (2.6) | 1 (1.3) |
| Pre-syncope | 2 (2.6) | 0 (0.0) |
| Acceptability | ||
| Easy of intake (no distress) | 51 (65.4) | 48 (60.8) |
| Willingness to repeat the same regimen | 67 (85.9) | 71 (89.9) |
| Preference to current regimen1 | 23 (82.1) | 26 (68.4) |
| Urgency during the journey (no or mild) | 78 (100.0) | 79 (100.0) |
| Interference with sleeping (no or mild) | 71 (91.0) | 76 (96.2) |
Ninety percent of subjects, in both groups of treatment, drunk the whole solution with no difference in compliance. The majority of subjects in both groups had no distress during bowel preparation, was willing to repeat the future colonoscopy with the same bowel preparation and preferred the present preparation to the previous one with no significant difference between the two preparations. No patient had severe urgency or a need to stop for bowel movement or incontinence during the journey to the hospital. Only few subjects reported moderate to severe interference with sleeping, with no significant difference between the two groups (Table 3).
In this trial the combined regimen of bisacodyl tablets given at bedtime the day before and 2-L of the new isotonic sulphate-free PEG-CS taken in the morning 5 h before the scheduled colonoscopy was compared with the split-dose 4-L PEG in which the morning dose was given with the same timing. We have shown that the same day schedule is feasible and as effective as the split-dose conventional PEG regimen for late morning colonoscopy.
As a matter of fact, the means of Ottawa Bowel Cleansing Score of the two treatment groups were statistically equivalent. This finding was confirmed by the rates of patients with successful bowel, preparation, which were similar between the two preparations. Similarly, the adenoma detection rate and caecum intubation rate, two indicators of the quality of colonoscopy, were comparable between PEG-CS and PEG. It is important to note that PEG-CS was superior than PEG in terms of mucosal visibility. This is explained by the anti-foam action of simethicone[15-18] which is contained only in PEG-CS.
The clinical rationale of same-day bowel preparation is the same as that of split-dosing, i.e., to shorten the interval between the completion of bowel preparation and colonoscopy[19]. It has been demonstrated that the quality of bowel preparation improves when the interval between the last dose of bowel preparation and colonoscopy does not exceed 8 h[20-22]. After that period a viscous bile-stained mucous enters the colon and distributes over the colonic mucosa of the right colon with the potential to cover small or flat lesions containing high dysplasia. These lesions are considered a great challenge for the endoscopist having a high potential to remain missed at colonoscopy[23,24]. The morning dose of the same day as well as split dose clears away this material and may increase the performance rate of colonoscopy in terms of detection of small adenomas.
Our study shows that same-day bowel preparation with a low-volume PEG-CS plus bisacodyl tablets is feasible and well accepted by subjects who are referred for colonoscopy. No subject had to stop the journey to hospital for urgency or arrived late in the hospital.
There was no significant difference for sleep interference between the two preparations. No patient in the PEG-CS group (and in the PEG group) complained nocturnal awakenings for bowel movements or pain/cramps. This suggests that sleep difficulty is more likely to be attributed to the anxiety for the day-after procedure. Bowel movements induced by bisacodyl taken at bedtime occurred after the wake-up.
We were unable to find differences for tolerability and acceptability between the two bowel preparations even if the new PEG-CS solution was considered in a panel of subjects more palatable than conventional PEG, which contains sodium sulphate. The subjects in our study were thoroughly instructed how to use the bowel preparation and its importance for a quality colonoscopy. This increased the motivation of the patients in the study and contributed to the high compliance rates in both groups. In routine clinical practice the motivation and compliance to the high volume PEG solution appear to be lower.
In addition to a 2-d low-fibre diet, the patients followed a clear fluid diet all the day before and this may have increased the rates of successful bowel cleansing. As the clear fluid diet is not well accepted, it would be interesting to evaluate whether same results can be obtained with a low-fibre diet extended to the day before, which is better accepted.
We were unable to show substantial advantages in terms of tolerability and acceptability for this new low volume bowel preparation which requires to drink only 2-L of bowel preparation solution: this was probably due to the low sensitivity of our measuring tools. We have shown that both PEG-CS and PEG bowel preparation can be used to substantially shorten the runway time, that is the time between the end of bowel preparation and colonoscopy.
A limit of this study was to evaluate only bowel preparation for late morning colonoscopy, i.e., the period from 10:00-10:30 am and 1:00-1:30 pm. Therefore our results cannot be extrapolated to early morning colonoscopy. Another limit is that we did not randomize patients according to factors such as age, indication to colonoscopy, bowel habits or comorbidities (for instance diabetes) which may influence bowel cleansing. However the two groups were relatively comparable in terms of indications for colonoscopy and comorbidities. Patients with constipation received an additional tablet of bisacodyl. No differences were found in terms of colon cleansing between patients with constipation and those with normal habits. The most common co-morbidity was hypertension (controlled by drug therapy) followed by diabetes, both well balanced between the two groups. No patients had heart failure or renal failure or other conditions which predispose to electrolyte imbalance. The age (cut-off 60 years) showed a significant association with adenoma detection rate; however this finding was largely expected because patients older than 60 years have a higher prevalence of adenomas.
The most important advantage of the PEG-CS preparation in comparison to the PEG regimen is the lack of any impact on work activity and quality of life the day before. This is important for the clinical practice as today healthy subjects have a full working and free time life and are reluctant to lose their time. A faster and easier bowel preparation method such as PEG-CS plus bisacodyl may increase the adherence to the colonoscopy.
In this study we maintained our current practice method, i.e., 48-h low fibre diet, which is usually well accepted followed by 24-h clear fluid diet which is bothersome for most patients. Considering the high rates of successful bowel cleansing in our study, it is time to reconsider the value of this practice which was introduced long time ago. It is likely that with the improved bowel cleansing regimens which are performed more closely to colonoscopy, a more patient-friendly diet can be adopted. Only the low fibre diet for one day may be sufficient to achieve satisfactory bowel preparation[25].
In our study bisacodyl was taken at bedtime and the PEG-CS preparation 5 h before the scheduled colonoscopy. Some patients started to take the morning dose as early as 5:00-5:30 am without great inconvenience. Most patients started drinking at 7:00 am to be ready for colonoscopy at 12:00 am In all patients colonoscopy was performed no later than 3-4 h after finishing bowel preparation. Most colonoscopies were scheduled between 1:00 and 2:00 in patients who started taking PEG-CS at 7:00 am and finishing at 9:00 am.
We are aware that the same day dosing of low-volume PEG (as well as split-dosing) cannot be proposed for early morning colonoscopy (e.g., before 10:00 am).
Our study has also implications for the organisation of Endoscopic Unit. Patients having a long journey to reach the hospital should be scheduled late in the morning or in the afternoon to exploit the advantage of the split or same day bowel preparation. This approach could be proposed for late morning and afternoon colonoscopies, especially within colorectal cancer screening programs, with the aim to increase the compliance to colonoscopy.
A relevant aspect of this study is that the proposed low-volume bowel cleansing regimen had a good acceptability by the patients. The low rate of mild adverse events, the high proportion of patients who drank the whole solution and the willingness to repeat the same modality of bowel preparation, suggest that the same day regimen can be proposed as an attractive alternative to the split high volume PEG. In this context the co-operation of the patient which is influenced positively by the extent and quality of oral and written instructions provided by health professionals and the patient preference for the type of bowel preparation remain important.
However future larger multicenter studies encompassing the evaluation of the patient characteristics are warranted to confirm our results and to establish if compliance to colonoscopy could be really increased.
Bowel preparation is fundamental for high quality colonoscopy. Colon cleansing varies inversely with the time interval between the end of bowel preparation and endoscopic examination. The split-dose preparation has demonstrated to significantly improve the rate of adequate cleansing and patient compliance. The disadvantages are represented by the ingestion of a high volume and the burden for the long bowel preparation.
Same-day bowel preparations are recommended for afternoon colonoscopy. The low volume bowel preparation may also be used for late morning colonoscopy but no clinical studies are available.
The low volume polyethyleneglycol-citrate-simethicone (PEG-CS) given same-day plus bisacodyl is feasible and as effective as split PEG 4-L. PEG-CS plus bisacodyl may be an attractive option for late morning colonoscopy. It reduces the overall time for bowel preparation with no loss of work time and impact on daily activities the day before the exam.
PEG-CS plus bisacodyl may represent an attractive option compared to split-dose PEG 4 L for late morning colonoscopy.
It is a good manuscript with a concise methodology and clearness of the results. A real difference between both preparations was not found but patient compliance. There seems to be no bias in the results and discussion.
P- Reviewers Amornyotin S, Teramoto-Matsubara OT S- Editor Zhai HH L- Editor A E- Editor Wu HL
| 1. | Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16-S28. [PubMed] |
| 2. | Hassan C, Fuccio L, Bruno M, Pagano N, Spada C, Carrara S, Giordanino C, Rondonotti E, Curcio G, Dulbecco P. A predictive model identifies patients most likely to have inadequate bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2012;10:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum. 1994;37:229-233; discussion 233-234. [PubMed] |
| 4. | Sharma VK, Chockalingham SK, Ugheoke EA, Kapur A, Ling PH, Vasudeva R, Howden CW. Prospective, randomized, controlled comparison of the use of polyethylene glycol electrolyte lavage solution in four-liter versus two-liter volumes and pretreatment with either magnesium citrate or bisacodyl for colonoscopy preparation. Gastrointest Endosc. 1998;47:167-171. [PubMed] |
| 5. | DiPalma JA, Wolff BG, Meagher A, Cleveland Mv. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol. 2003;98:2187-2191. [PubMed] |
| 6. | Ker TS. Comparison of reduced volume versus four-liter electrolyte lavage solutions for colon cleansing. Am Surg. 2006;72:909-911. [PubMed] |
| 7. | DiPalma JA, McGowan J, Cleveland MV. Clinical trial: an efficacy evaluation of reduced bisacodyl given as part of a polyethylene glycol electrolyte solution preparation prior to colonoscopy. Aliment Pharmacol Ther. 2007;26:1113-1119. [PubMed] |
| 8. | Parente F, Marino B, Crosta C. Bowel preparation before colonoscopy in the era of mass screening for colo-rectal cancer: a practical approach. Dig Liver Dis. 2009;41:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Aoun E, Abdul-Baki H, Azar C, Mourad F, Barada K, Berro Z, Tarchichi M, Sharara AI. A randomized single-blind trial of split-dose PEG-electrolyte solution without dietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc. 2005;62:213-218. [PubMed] |
| 10. | El Sayed AM, Kanafani ZA, Mourad FH, Soweid AM, Barada KA, Adorian CS, Nasreddine WA, Sharara AI. A randomized single-blind trial of whole versus split-dose polyethylene glycol-electrolyte solution for colonoscopy preparation. Gastrointest Endosc. 2003;58:36-40. [PubMed] |
| 11. | Marmo R, Rotondano G, Riccio G, Marone A, Bianco MA, Stroppa I, Caruso A, Pandolfo N, Sansone S, Gregorio E. Effective bowel cleansing before colonoscopy: a randomized study of split-dosage versus non-split dosage regimens of high-volume versus low-volume polyethylene glycol solutions. Gastrointest Endosc. 2010;72:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Kilgore TW, Abdinoor AA, Szary NM, Schowengerdt SW, Yust JB, Choudhary A, Matteson ML, Puli SR, Marshall JB, Bechtold ML. Bowel preparation with split-dose polyethylene glycol before colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc. 2011;73:1240-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Enestvedt BK, Tofani C, Laine LA, Tierney A, Fennerty MB. 4-Liter split-dose polyethylene glycol is superior to other bowel preparations, based on systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2012;10:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482-486. [PubMed] |
| 15. | McNally PR, Maydonovitch CL, Wong RK. The effectiveness of simethicone in improving visibility during colonoscopy: a double-blind randomized study. Gastrointest Endosc. 1988;34:255-258. [PubMed] |
| 16. | Lazzaroni M, Petrillo M, Desideri S, Bianchi Porro G. Efficacy and tolerability of polyethylene glycol-electrolyte lavage solution with and without simethicone in the preparation of patients with inflammatory bowel disease for colonoscopy. Aliment Pharmacol Ther. 1993;7:655-659. [PubMed] |
| 17. | Wu L, Cao Y, Liao C, Huang J, Gao F. Systematic review and meta-analysis of randomized controlled trials of Simethicone for gastrointestinal endoscopic visibility. Scand J Gastroenterol. 2011;46:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Park JJ, Lee SK, Jang JY, Kim HJ, Kim NH. The effectiveness of simethicone in improving visibility during colonoscopy. Hepatogastroenterology. 2009;56:1321-1325. [PubMed] |
| 19. | Gurudu SR, Ratuapli S, Heigh R, DiBaise J, Leighton J, Crowell M. Quality of bowel cleansing for afternoon colonoscopy is influenced by time of administration. Am J Gastroenterol. 2010;105:2318-2322. [PubMed] |
| 20. | Siddiqui AA, Yang K, Spechler SJ, Cryer B, Davila R, Cipher D, Harford WV. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Aisenberg J. Bowel preparation for colonoscopy: shortening the “runway time”. Gastrointest Endosc. 2009;69:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Varughese S, Kumar AR, George A, Castro FJ. Morning-only one-gallon polyethylene glycol improves bowel cleansing for afternoon colonoscopies: a randomized endoscopist-blinded prospective study. Am J Gastroenterol. 2010;105:2368-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Gurudu SR, Ramirez FC, Harrison ME, Leighton JA, Crowell MD. Increased adenoma detection rate with system-wide implementation of a split-dose preparation for colonoscopy. Gastrointest Endosc. 2012;76:603-608.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 24. | Johnson ME, Feinn R, Anderson JC. Clinical factors associated with non-polypoid colonic adenomas ≥ 6 mm: a prospective study in an asymptomatic population using a high-definition colonoscope. Am J Gastroenterol. 2011;106:2018-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Wu KL, Rayner CK, Chuah SK, Chiu KW, Lu CC, Chiu YC. Impact of low-residue diet on bowel preparation for colonoscopy. Dis Colon Rectum. 2011;54:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |