Published online Sep 16, 2013. doi: 10.4253/wjge.v5.i9.428
Revised: July 30, 2013
Accepted: August 4, 2013
Published online: September 16, 2013
Processing time: 89 Days and 20.7 Hours
Diagnosis of gastric subepithelial tumor (SET) has shown a rapid increase worldwide. Although, until now, endoscopic ultrasound guided procedures such as fine needle aspiration have shown relatively high accuracy in diagnosis of SET, the most important modality for diagnosis and treatment of SETs is complete resection such as endoscopic or surgical resection. However, endoscopic resection or laparoscopic wedge resection alone also has some limitations. Endoscopic resection is difficult to perform in cases of gastric SET located within deep portion of the gastric layer or a relatively large (larger than 25 mm diameter). On the other hand, gastric SET in a difficult location, such as the gastroesophageal junction or pyloric ring is challenging for laparoscopic surgical resection. The hybrid natural orifice transluminal endoscopic surgery (NOTES) technique is a combined method, including the advantages of both laparoscopic resection and endoscopic resection for gastric SETs. This method may be performed safely with reasonable operation times, less bleeding, and adequate resection margin and regardless of tumor size. In particular, in the case of a difficult location for resection, such as the esophagogastric junction or pyloric ring, hybrid NOTES is currently believed to be an ideal treatment method.
Core tip: Hybrid natural orifice transluminal endoscopic surgery is thought to be an ideal method for treatment of gastric subepithelial tumor with adequate resection margin, regardless of tumor size and location, such as the esophagogastric junction or pyloric ring.
- Citation: Heo J, Jeon SW. Hybrid natural orifice transluminal endoscopic surgery in gastric subepithelial tumors. World J Gastrointest Endosc 2013; 5(9): 428-432
- URL: https://www.wjgnet.com/1948-5190/full/v5/i9/428.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i9.428
Diagnosis of gastric subepithelial tumor (SET) has shown a rapid increase worldwide in accordance with increasing performance of endoscopy for screening. The SETs occupy approximately 5% of total gastric tumors, showing various aspects from benign, such as lipoma, to malignancy, such as gastrointestinal stromal tumor[1].
For diagnosis of SETs, the use of endoscopic ultrasonography (EUS) and EUS guided fine needle aspiration or Tru-Cut biopsy has shown a recent increase. However, the average accuracy rate of EUS guided fine needle aspiration (EUS-FNA) for diagnosis of SETs is only 60% to 80%. In a recent study, Mekky et al[2] reported on the diagnostic utility of EUS-FNA in gastric SETs. The sampling adequacy was 83%, with an average of 2.5 passes. EUS-FNA results were diagnostic in 43.3%, suggestive in 39% and non-diagnostic in 17.7%. EUS-FNA results showed 95.6% accuracy in differentiation of potential malignant lesions. Another study validated the unroofing technique for diagnosis of SETs in 16 patients[3]. Use of the unroofing technique provided specimens that were sufficient for diagnosis and assessment of risk for malignancy in 15 out of 16 cases [diagnostic yield 93.7% (95%CI, 80.4%-100.0%)]. However, the indication for use of the unroofing technique should be confined to liquid SETs, such as lipoma and cystic lymphangioma. In addition, EUS guided biopsy or unroofing technique is limited to use as a diagnostic tool rather than a treatment modality.
Therefore, until now, the most important modality for diagnosis and treatment of SETs has been complete resection. Because the characteristics of SETs are mostly benign in nature and are rarely malignant in nature with hematogenous spread rather than lymphatic metastasis, lymph node dissection is not necessary for treatment of SETs. Therefore, SETs are a good indication for resection of tumors using endoscopy or laparoscopy.
According to development of endoscopic technology, a non-invasive method is currently preferred. In this article, we will provide validation for endoscopic treatment, surgical treatment and hybrid natural orifice transluminal endoscopic surgery (NOTES) for treatment of gastric SETs.
Various endoscopic resection techniques have recently been reported for treatment of SETs. However, there are two major limitations of endoscopic resection alone. One is for SETs originating within a deep portion of the gastric layer and another is for SETs of large size. Endoscopic mucosal resection (EMR), including EMR with a cap and eEMR with ligation is a simple method for resection of small SETs originating from the mucosal and submucosal layer with low complication rates. However, for lesions originating from the muscularis propria, endoscopic resection has a main drawback of a risk of perforation. Therefore, SETs located deeper below the submucosal lesion are usually managed by surgery.
To overcome this limitation, some new techniques have been developed. Endoscopic submucosal tunnel dissection was validated for upper gastric SETs. In 12 patients who presented with an upper gastrointestinal SET of ≤ 40 mm located in the esophagus or cardia, a submucosal tunnel was created endoscopically starting at approximately 5 cm proximal to the lesion. SETs had a mean size of 19.5 mm (range, 10-40 mm), eight were located in the esophagus and four in the cardia[4]. SET resection was successful in 10 patients (83.3%) who underwent en bloc resection and the two remaining patients who underwent resection in two pieces. However, endoscopic tunnel dissection is difficult to perform for a large SET. The size of piecemeal resected SETs was 25 mm and 40 mm, which were larger than en bloc resected SETs (median 15 mm, range 10-25 mm). In addition, there is a risk for perforation during or after treatment.
In another Chinese study, 26 patients with gastric SETs originating from the muscularis propria were treated by endoscopic full thickness resection (EFR)[5]. Briefly, the EFR procedure is as follows: (1) a circumferential incision as deep as muscularis propria around the lesion by the endoscopic submucosal dissection (ESD) technique; (2) incision into the serosal layer around the lesion using a knife; (3) completion of full-thickness incision to the tumor, including the serosal layer using a knife or snare by gastroscopy without laparoscopic assistance; and (4) closure of the gastric-wall defect with metallic clips. The complete resection rate was 100%, and the mean resected lesion size was 2.8 cm (range, 1.2-4.5 cm). The key to the EFR procedure is the successful closure of wall defects after resection for prevention of peritonitis and surgical intervention. Because the size of a wall defect after resection should be smaller than the width of the open clips, performance of the EFR procedure for large SETs (large than 25 mm) is difficult.
In summary, endoscopic resection alone has a limitation of complete resection for gastric SETs located within a deep portion of the gastric layer and is difficult to perform for a relatively large size (larger than 25 mm diameter).
Traditionally, the basis for complete resection of SETs has been surgical resection. Recently, laparoscopic wedge resection has commonly been used as a non-invasive modality[6,7]. The surgical techniques can be selected according to location and characteristics of the tumor[8,9]. The location and aspect of SETs is also a limitation of laparoscopic resection. The exogastric approach is the most popular technique for SETs located at the anterior wall, particularly those that exhibit extraluminal growth[7,10]. However, because it is associated with excessive resection of healthy tissue of the gastric wall, there is a possibility of stenosis or deformity with this procedure[9]. Therefore, this approach is not considered suitable for SETs at or near the gastric inlet or outlet, such as the area near the gastroesophageal junction and pyloric ring. Tumors located at the posterior wall of the stomach can usually be treated using a transgastric or intragastricapproach[8,9,11,12]. The intragastric approach is the preferred method for lesions located at the posterior wall and for the tumors of the esophagogastric junction[13-16]. Use of this procedure carries little possibility of deformity and stenosis. However, it cannot be applied to anterior wall lesions or large tumors. In addition, after completion of this procedure, repair of two or three stab wounds of the anterior wall of the stomach must be performed.
Tumors located near the pylorus and the lesser curvature of the stomach are challenging. The usual approach to submucosal tumors of the stomach is wedge resection with an adequate margin. In order to ensure patency of the gastric lumen and to prevent vagus nerve injury, special precautions must be taken during resection of tumors located near the pylorus and the lesser curvature of the stomach. A application of the intragastric or transgastric approach in this area is very difficult because of the small space available for handling or introduction of the instrument. Use of linear staplers in the prepyloric antrum is not recommended because surgeons cannot guarantee penetration to the luminal side and because the inevitable removal of healthy tissue from the gastric wall results in luminal compromise[17].
NOTES implies the use of empty organs as an access to the peritoneal cavity using an endoscope, completely avoiding skin incisions[18]. In order to overcome current technical limitations, investigators have combined NOTES with the conventional laparoscopic approach in the so-called hybrid NOTES technique.
Wilhelm et al[19] reported that three different methods are available for laparoscopic-endoscopic ‘‘rendez-vous’’ resection. In the case of laparoscopic assisted endoscopic resection, the lesion is resected with diathermy; larger lesions demand that resection be performed as a wedge resection for tumors located in the anterior aspect of the stomach and as a transgastric resection for posterior wall lesions.
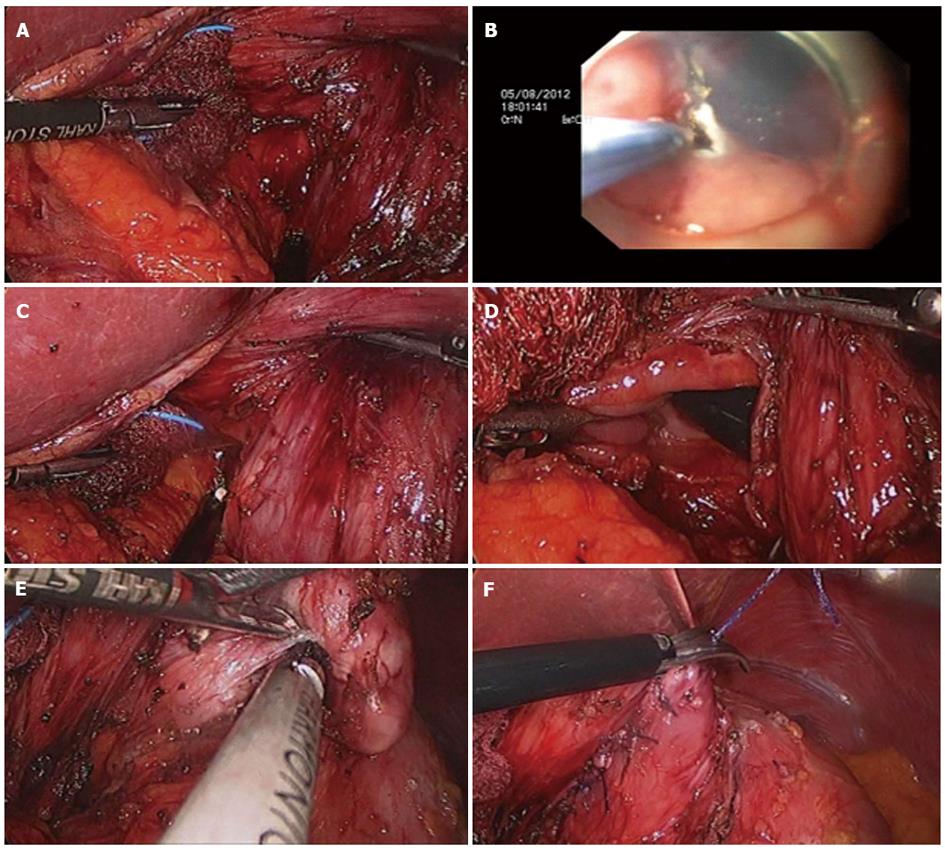
Hiki et al[20] reported on 7 cases of laparoscopic and endoscopic cooperative surgery using endoscopic submucosal dissection with laparoscopic wedge resection as the technique developed for hybrid NOTES. The procedure for hybrid NOTES was as follows: both mucosal and submucosal layers around the tumor were circumferentially dissected using endoscopic submucosal dissection via intraluminal endoscopy. Subsequently, the seromuscular layer was laparoscopically dissected on the exact three-fourths cut line around the tumor. The submucosal tumor was then exteriorized to the abdominal cavity and dissected using a standard endoscopic stapling device (Figure 1). Endoscopic approach using using the ESD technique can provide the precise cut line as a marker for laparoscopic resection. During performance of the resection, use of an intragastric endoscopic and extragastric laparoscopic approach can allow for observation of both sides of the resection margin. These dual approaches can allow for attainment of an appropriate resection margin. In addition, this method can provide an easy approach for the difficult location of SET resection and minimize the stricture or deformity after resection of gastric SETs at the esophagogastric junction or pyloric ring. In addition, as always, hybrid NOTES has the advantage of external wedge resection for large sized subepithelial tumors.
In the study reported by Hiki et al[20], in all cases, the laparoscopic and endoscopic cooperative surgery (LECS) procedure was successful for dissecting out the gastric submucosal tumor. In four of seven cases, the tumor was located in the upper gastric portion near the esophagogastric junction. The three remaining tumors were located in the posterior gastric wall. In two cases, the tumors were more than 5 cm in diameter, and one was a gastrointestinal tumor (GIST) of the remnant stomach. The mean operation time was 169 ± 17 min, and the estimated blood loss was 7 ± 2 mL. The postoperative course was uneventful in all cases. In another study, 20 consecutive patients underwent LECS for resection of gastric SETs. In all cases, dissection of the gastric SET was successful using the LECS procedure. The tumor was located in the upper third of the stomach in eight cases, in the middle third in eight cases, and in the lower third in four cases[21]. A summary of some published series on hybrid NOTES is shown in Table 1[20-22].
| Author, year and number of patients | Operation time, min | Intraoperative bleeding, mL | Tumor size, mm | Number of linear staplers used | Postoperative complications | Hospital stay, d | Tumor location | Type of growth | Pathologic diagnosis, n |
| Hiki et al[20], n = 7 | 169.0 ± 17.0 | 7.0 ± 2.0 | 46.0 ± 3.0 (35-60) | 2.2 ± 0.1 | 0 | 7.4 ± 8.1 | U4M1L1Remnant stomach, posterior 1 | Extragastric type 1Intragastric type 6 | GIST, 6Schwannoma, 1 |
| Tsujimoto et al[21], n = 20 | 157.5 ± 68.4 (89-316) | 3.5 ± 6.4 (0-20) | 37.9 ± 11.0 (18-63) | 2.7 ± 0.5 (2-3) | 0 | 11.6 ± 9.5 (6-13) | U8 (40%)M8 (40%)L4 (20%) | Extragastric type 2 (15%)Intragastric type 17 (85%) | GIST, 16 (80%)Inflammation for parasite, 1 (5%), leiomyoma, 1 (5%),glomus tumor, 1 (5%), aberrant pancreas, 1 (5%) |
| Abe et al[22], n = 4 | 221.5 ± 129.4 | 38.0 ± 46.7 | 38.0 ± 7.1 (22-43) | NA | 0 | 7.5 ± 0.7 | U1M3 | NA | GIST, 1Lipoma, 1Ectopic pancreas, 1Schwannoma, 1 |
The hybrid NOTES procedure for treatment of gastric SET should be performed carefully. Accidental rupture of a gastric SET, such as GIST, during resection with peritoneal seeding is theoretically possible. Therefore, hybrid NOTES may be contraindicated for ulcerated or bleeding tumor. Removal of tumors from the abdomen into a specimen retrieval bag is also important for prevention of seeding of the tumor to the peritoneum and port-site wound.
The hybrid NOTES technique is a combined method including the advantages of laparoscopic resection and endoscopic resection for gastric SETs. This method may be performed safely with reasonable operation times, less bleeding, and adequate resection margin regardless of tumor size. In particular, in cases of difficult location for resection, such as the esophagogastric junction or pyloric ring, hybrid NOTES is currently believed be an ideal treatment method.
P- Reviewers dos Santos JS, Dilek FH S- Editor Gou SX L- Editor A E- Editor Wu HL
| 1. | Eckardt AJ, Wassef W. Diagnosis of subepithelial tumors in the GI tract. Endoscopy, EUS, and histology: bronze, silver, and gold standard? Gastrointest Endosc. 2005;62:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Mekky MA, Yamao K, Sawaki A, Mizuno N, Hara K, Nafeh MA, Osman AM, Koshikawa T, Yatabe Y, Bhatia V. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Lee CK, Chung IK, Lee SH, Lee SH, Lee TH, Park SH, Kim HS, Kim SJ, Cho HD. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010;71:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. 2012;44:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 6. | Cheng HL, Lee WJ, Lai IR, Yuan RH, Yu SC. Laparoscopic wedge resection of benign gastric tumor. Hepatogastroenterology. 1999;46:2100-2104. [PubMed] |
| 7. | Aogi K, Hirai T, Mukaida H, Toge T, Haruma K, Kajiyama G. Laparoscopic resection of submucosal gastric tumors. Surg Today. 1999;29:102-106. [PubMed] |
| 8. | Tagaya N, Mikami H, Kogure H, Kubota K, Hosoya Y, Nagai H. Laparoscopic intragastric stapled resection of gastric submucosal tumors located near the esophagogastric junction. Surg Endosc. 2002;16:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Tagaya N, Mikami H, Kubota K. Laparoscopic resection of gastrointestinal mesenchymal tumors located in the upper stomach. Surg Endosc. 2004;18:1469-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Cugat E, Hoyuela C, Rodríguez-Santiago JM, Marco C. Laparoscopic ultrasound guidance for laparoscopic resection of benign gastric tumors. J Laparoendosc Adv Surg Tech A. 1999;9:63-67. [PubMed] |
| 11. | Hepworth CC, Menzies D, Motson RW. Minimally invasive surgery for posterior gastric stromal tumors. Surg Endosc. 2000;14:349-353. [PubMed] |
| 12. | Ibrahim IM, Silvestri F, Zingler B. Laparoscopic resection of posterior gastric leiomyoma. Surg Endosc. 1997;11:277-279. [PubMed] |
| 13. | Sekimoto M, Tamura S, Hasuike Y, Yano M, Murata A, Inoue M, Shiozaki H, Monden M. A new technique for laparoscopic resection of a submucosal tumor on the posterior wall of the gastric fundus. Surg Endosc. 1999;13:71-74. [PubMed] |
| 14. | Taniguchi E, Kamiike W, Yamanishi H, Ito T, Nezu R, Nishida T, Momiyama T, Ohashi S, Okada T, Matsuda H. Laparoscopic intragastric surgery for gastric leiomyoma. Surg Endosc. 1997;11:287-289. [PubMed] |
| 15. | Uchikoshi F, Ito T, Nishida T, Kitagawa T, Endo S, Matsuda H. Laparoscopic intragastric resection of gastric stromal tumor located at the esophago-cardiac junction. Surg Laparosc Endosc Percutan Tech. 2004;14:1-4. [PubMed] |
| 16. | Walsh RM, Ponsky J, Brody F, Matthews BD, Heniford BT. Combined endoscopic/laparoscopic intragastric resection of gastric stromal tumors. J Gastrointest Surg. 2003;7:386-392. [PubMed] |
| 17. | Hwang SH, Park do J, Kim YH, Lee KH, Lee HS, Kim HH, Lee HJ, Yang HK, Lee KU. Laparoscopic surgery for submucosal tumors located at the esophagogastric junction and the prepylorus. Surg Endosc. 2009;23:1980-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117. [PubMed] |
| 19. | Wilhelm D, von Delius S, Burian M, Schneider A, Frimberger E, Meining A, Feussner H. Simultaneous use of laparoscopy and endoscopy for minimally invasive resection of gastric subepithelial masses - analysis of 93 interventions. World J Surg. 2008;32:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (2)] |
| 21. | Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. 2012;36:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Abe N, Takeuchi H, Yanagida O, Masaki T, Mori T, Sugiyama M, Atomi Y. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc. 2009;23:1908-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |