Published online Jun 16, 2013. doi: 10.4253/wjge.v5.i6.293
Revised: April 8, 2013
Accepted: April 13, 2013
Published online: June 16, 2013
Processing time: 102 Days and 21.7 Hours
The colorectal mucosa includes two quantitatively, structurally and functionally dissimilar areas: one, built with columnar and goblet cells, covers the vast majority of the mucosa, and the other consists of scattered minute gut-associated lymphoid tissue (GALT). The overwhelming majority of colorectal carcinomas evolve in GALT-free mucosal areas and very rarely in GALT aggregates. Remarkably, the colonic mucosa in patients with ulcerative colitis (UC) displays a high number of newly formed GALT-aggregates. The patient here described is a 68-year-old female with a history of UC since 1984. At surveillance colonoscopy in 2012, one of two detected polyps was a tubular adenoma with high-grade dysplasia. Beneath this adenoma, a well-circumscribed GALT sheltering a carcinoma was found. Serial sections revealed no connection between the villous adenoma and the GALT-carcinoma. The GALT-carcinoma here reported seems to have evolved in a newly formed, UC-dependent, GALT complex. This notion is substantiated by the fact that 27% or 4 out of the 15 cases of GALT-carcinomas in the colon reported in the literature (including the present case) evolved in patients with UC.
Core tip: Of the 15 cases of gut-associated lymphoid tissue (GALT)-carcinomas in the colon reported in the literature (including the present case) 27% (n = 4) have evolved in patients with ulcerative colitis. The possibilities that the advanced adenoma on top had invaded the GALT-complex underneath or that the GALT-carcinoma was a metastasis from the adenoma on top were rejected, since serial sections revealed neither continuity between the adenoma and the GALT-carcinoma, nor invasive growth in the adenoma.
- Citation: Rubio CA, Befrits R, Ericsson J. Carcinoma in gut-associated lymphoid tissue in ulcerative colitis: Case report and review of literature. World J Gastrointest Endosc 2013; 5(6): 293-296
- URL: https://www.wjgnet.com/1948-5190/full/v5/i6/293.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i6.293
The colorectal mucosa can be divided into two quantitatively, structurally and functionally dissimilar areas[1]. One comprises the vast majority of the colorectal mucosa: it is built with mucus producing goblet cells and columnar cells exhibiting microvilli covered with glycocalix. The function of this huge mucosal area is to protect the underlying structures, to allow free passage into the host, of water and other fluids (encouraged by aquaporin 8, a water channel protein[2]), ions, vitamins and some nutrients, as well as to produce lysozyme, the innate antibacterial enzyme that annihilates pathogenic bacteria[3]. The other mucosal area, called gut-associated lymphoid tissue (GALT), is composed of tiny mucosal fractions scattered in the colorectal mucosa. O’Leary et al[4] found only 36 GALT aggregates (also called cryptopatches or lymphoglandular complexes) per colectomy in 27 specimens without ulcerative colitis. A single layer of cubic cells and few or no goblet cells build the epithelium covering GALT aggregates. Electron-microscopic studies show an epithelium with a poorly developed brush border, but clear-cut micro-ridges (thereof the M designation). In addition, invaginations in the surface of M cells create intraepithelial pockets[5]. The function of M cells is to absorb luminal antigens such as macromolecules and microorganisms via clathrin-mediated endocytosis[6] and to haul these antigens into the underlying collection of gut-indigenous, thymus-independent lymphoid tissue for immediate immunological processing. Hence, the M cell-lymphoid tissue assemblage (that is GALT) is a lymphoepithelial immunological unit that coordinates antigen recognition and processing in the gut mucosa[5].
Nearly all-colorectal carcinomas (CRC), the third most frequent cancer worldwide[7], evolve in GALT-free mucosal areas. In contrast, CRC arising in GALT-associated mucosa are very rare.
Patients with extensive ulcerative colitis (UC) are at increased risk of developing a CRC[7]. It is generally accepted that CRC in UC also originates in GALT-free colorectal mucosa: either from UC-related non-protruding dysplastic crypts (known as dysplasia in flat mucosa[8]), from protruding, or non-protruding adenomatous lesions, or from age-dependent, UC-unrelated, sporadic adenomas[9].
Dukes[10] described in colitic patients a histological lesion, usually in the submucosa, characterized by “misplaced” colonic epithelium surrounded by nodular lymphoid tissue. Dukes[10] believed that this epithelium was the result of mucosal repair following regeneration of a mucosal ulcer and that the epithelium detached and buried in the submucosa encouraged cancer development. Hultén et al[11] also considered this phenomenon, a precancerous lesion. Their descriptions fit well with the notion of GALT-mucosa.
Searching for a confirmation of the hypothesis of Cuthbert Dukes, we reported and illustrated in 1984, the first case of GALT-carcinoma of the colon in the literature[12]. In 2002, Rubio and Talbot reported another case of GALT-carcinoma in a patient with UC[13]. Of note, of the two cases of GALT-carcinoma reported by Stewart et al[14], one occurred in a patient with UC.
de Petris et al[15] reported a case of sporadic GALT-carcinoma in the colon of a patient without UC. Because of its protruding shape, these authors proposed to call it dome carcinoma (DC). Since then, six new cases of sporadic DC in patients without UC appeared in the literature[14,16-19] (Table 1). In addition 3 DC were found in a colectomy specimen in a patient with Lynch syndrome[20].
The purpose of this communication is to report a new case of GALT-carcinoma in a patient with UC.
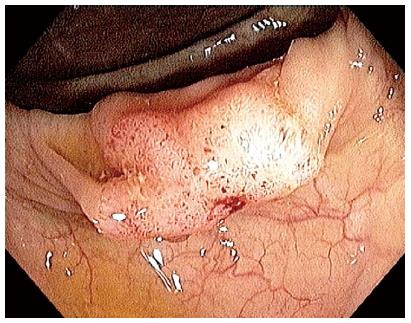
The patient is a 68-year-old female, with a history of UC since 1984. She has been under colonoscopic-histologic surveillance since 1985. In 2004 one of 11 biopsies exhibited low-grade dysplasia (LGD) in flat mucosa. In 2005, an aggressive breast ductal cancer was diagnosed and treated with surgery and chemotherapy. Despite treatment, the disease progressed, and several skeletal metastases were detected. In September 2011, numerous polyps in the right colon were found at a colonoscopic-histologic séance; two of these polyps were reported as tubular adenomas with LGD. A new colonoscopy in February 2012 revealed two new polyps, this time in the transverse colon (Figure 1).
Biopsies were stained with hematoxylin and eosin (HE), and immuno-histochemically stained with MNF 116, Actin SM (Leica Microsystems AB, Bromma, Sweden), Ki67 (clone MIB1, Leica Microsystems AB, Bromma, Sweden), p53 (BD Products, Franklin Lakes, United States), p21WAF1 (Oncogene Science, Chicago, United States), and histochemically stained with Alcian blue pH 2.5, periodic acid-Schiff (PAS) and PAS-D.
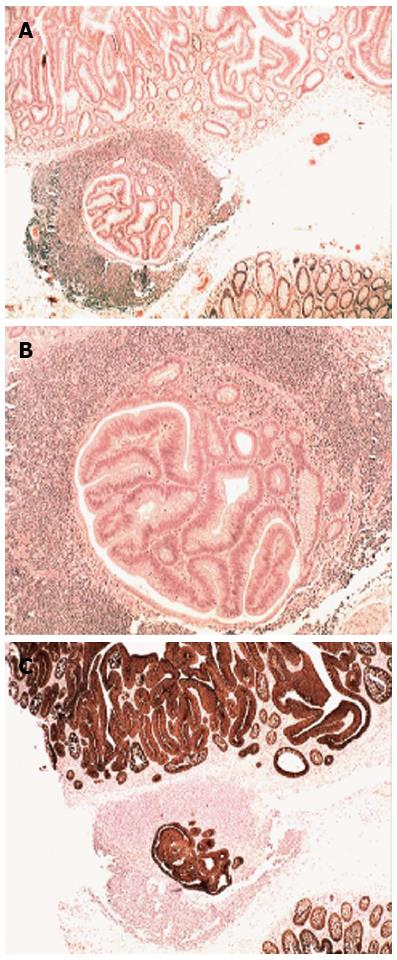
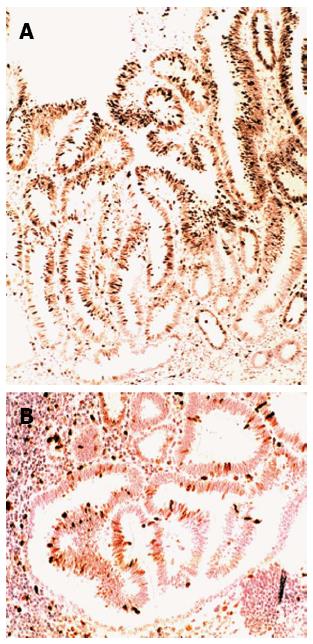
The histological examination showed in one of the two polyps in the transverse colon a GALT-carcinoma roofed by a tubular adenoma with high-grade dysplasia (Figure 2A). Beneath the adenoma, a well-circumscribed GALT-carcinoma was found (Figure 2B). Serial sections revealed no connection between the villous adenoma and the GALT-carcinoma. MNF 116 immunostain labelled all epithelial cells in the villous adenoma on top and in the subjacent GALT-carcinoma (Figure 2C). MIB1 disclosed high cell proliferation in the villous adenoma (Figure 3A); cell proliferation was comparatively lower in the GALT-carcinoma (Figure 3B).
Neither the GALT-carcinoma nor the advanced adenoma expressed p53. The neoplastic cells displayed sialomucins (Alcian blue stain) and mucopolysaccharides (PAS stain) were demonstrated, both in the villous adenoma and in the GALT-carcinoma.
The lymphoid tissue in the colorectal mucosa is found in three different compartments: in the epithelium, in the lamina propria mucosa, and in GALT aggregates. GALT aggregates may be found as minute lymphoid collections or larger collections of lymphoid tissues, known as Peyer’s patches. It goes without saying that the possibility for a neoplasia to evolve in the minute mucosal area that covers a GALT aggregate might be a fortuitous event.
While investigating colorectal neoplasias in Japanese patients[21] we found GALT aggregates underneath 38% of non-protruding adenomas. Puzzlingly, GALT-carcinomas are a common finding in the colon of rats treated with 1,2-dimethylhydrazine[22]. Following 27 wk treatment, subjacent lymphoid aggregates were found in as many as 36% of the flat (non-protruding) colon adenomas and early flat adenocarcinomas in rats[22]. In contrast, only 9% subjacent lymphoid aggregates were found in exophytic (protruding) colon adenomas and early flat adenocarcinomas. When only adenomas were considered, subjacent lymphoid aggregates were present in 50.0% of the flat adenomas, but only in 14.0% of the 50 protruding adenomas[22]. This is surprising, considering that in these animals, only a mean of 1.9 GALT aggregates per colon was recorded. Thus, it would appear that in humans and in rats, non-protruding colonic adenomas evolve not only in the GALT-free colonic mucosa but also in the GALT-associated mucosa.
Table 1 shows that 27% (4/15) of the reported cases of GALT-carcinoma of the colon evolved in patients with UC. In this context, O’Leary et al[23] found, 36 GALT foci per colectomy in patients without UC, but as many as 168 GALT foci per colectomy in patients with UC that is 4.7 times more frequently. Obviously, in the colon of patients with UC, newly GALTs are being formed. It is therefore not inconceivable that the GALT-carcinoma here reported might have evolved in a newly formed, UC-dependent, GALT complex.
Immunohistochemistry showed that cell proliferation was lower in the GALT-carcinoma than in the villous adenoma on top. These findings are in concert with those obtained by Anjomshoaa et al[24]. These authors found decreased tumour proliferation in metastatic lymph nodes from colon carcinomas.
This report is limited by the rarity of these tumors. Notwithstanding, the awareness that colonic carcinomas may evolve in mucosa-associated lymphoid tissue should encourage endoscopists to methodically examine areas with GALT complexes, particularly in patients with UC.
The possibilities that the advanced adenoma on top had invaded the GALT-complex underneath or that the GALT-carcinoma was a metastasis from the adenoma on top were rejected, since serial sections revealed neither continuity between the adenoma and the GALT-carcinoma, nor invasive growth in the adenoma. In light of these considerations it is submitted that the GALT-carcinoma here described evolved in a newly formed GALT aggregate in a patient with UC. A similar conclusion was drawn in 1984, when searching for a confirmation of the hypothesis of Cuthbert Dukes[10], the first case of GALT-associated carcinoma was detected[12].
P- Reviewer Amornyotin S S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Owen D. Stomach. Histology for Pathologists. 2nd ed. Philadelphia: Lippincott-Raven 1998; 481-493. |
| 2. | Fischer H, Stenling R, Rubio C, Lindblom A. Differential expression of aquaporin 8 in human colonic epithelial cells and colorectal tumors. BMC Physiol. 2001;1:1. |
| 3. | Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1452-1459. |
| 4. | O’Leary AD, Sweeney EC. Lymphoglandular complexes of the normal colon: histochemistry and immunohistochemistry. Ir J Med Sci. 1987;156:142-148. |
| 5. | Sminia T, Wilders MM. Antigen-processing cells in gut associated lymphoid tissue (GALT). Cell Biol Int Rep. 1983;7:677. |
| 6. | Bittner MA, Aikman RL, Holz RW. A nibbling mechanism for clathrin-mediated retrieval of secretory granule membrane after exocytosis. J Biol Chem. 2013;288:9177-9188. |
| 7. | Ferlay J, Bray F, Pisani P. GLOBOCAN 2002: Cancer Incidence. Mortality and Prevalence Worldwide. IARC Cancer-Base No.5, Version 2.0. Lyon: IARC Press 2004; . |
| 8. | Lennard-Jones JE, Melville DM, Morson BC, Ritchie JK, Williams CB. Precancer and cancer in extensive ulcerative colitis: findings among 401 patients over 22 years. Gut. 1990;31:800-806. |
| 9. | Rubio CA. Serrated neoplasias and de novo carcinomas in ulcerative colitis: a histological study in colectomy specimens. J Gastroenterol Hepatol. 2007;22:1024-1031. |
| 10. | Dukes CE. The surgical pathology of ulcerative colitis. Ann R Coll Surg Engl. 1954;14:389-400. |
| 11. | Hultén L, Kewenter J, Ahrén C. Precancer and carcinoma in chronic ulcerative colitis. A histopathological and clinical investigation. Scand J Gastroenterol. 1972;7:663-669. |
| 12. | Rubio CA. Ectopic colonic mucosa in ulcerative colitis and in Crohn’s disease of the colon. Dis Colon Rectum. 1984;27:182-186. |
| 13. | Rubio CA, Talbot I. Lymphoid-associated neoplasia in herniated colonic mucosa. Histopathology. 2002;40:577-579. |
| 14. | Stewart CJ, Hillery S, Newman N, Platell C, Ryan G. Dome-type carcinoma of the colon. Histopathology. 2008;53:231-234. |
| 15. | De Petris G, Lev R, Quirk DM, Ferbend PR, Butmarc JR, Elenitoba-Johnson K. Lymphoepithelioma-like carcinoma of the colon in a patient with hereditary nonpolyposis colorectal cancer. Arch Pathol Lab Med. 1999;123:720-724. |
| 16. | Jass JR, Constable L, Sutherland R, Winterford C, Walsh MD, Young J, Leggett BA. Adenocarcinoma of colon differentiating as dome epithelium of gut-associated lymphoid tissue. Histopathology. 2000;36:116-120. |
| 17. | Clouston AD, Clouston DR, Jass JR. Adenocarcinoma of colon differentiating as dome epithelium of gut-associated lymphoid tissue. Histopathology. 2000;37:567. |
| 18. | Asmussen L, Pachler J, Holck S. Colorectal carcinoma with dome-like phenotype: an under-recognised subset of colorectal carcinoma? J Clin Pathol. 2008;61:482-486. |
| 19. | Yamada M, Sekine S, Matsuda T. Dome-Type Carcinoma of the Colon Masquerading a Submucosal Tumor. Clin Gastroenterol Hepatol. 2012;S1542-3565. |
| 20. | Rubio CA, Lindh C, Björk J, Törnblom H, Befrits R. Protruding and non-protruding colon carcinomas originating in gut-associated lymphoid tissue. Anticancer Res. 2010;30:3019-3022. |
| 21. | Rubio CA, Kumagai J, Kanamori T, Nakamura K. Apoptosis in flat neoplasias of the colorectal mucosa. In Vivo. 1995;9:173-176. |
| 22. | Rubio CA, Shetye J, Jaramillo E. Non-polypoid adenomas of the colon are associated with subjacent lymphoid nodules. An experimental study in rats. Scand J Gastroenterol. 1999;34:504-508. |
| 23. | O’Leary AD, Sweeney EC. Lymphoglandular complexes in the diseased colon. Ir J Med Sci. 1987;156:353-360. |
| 24. | Anjomshoaa A, Nasri S, Humar B, McCall JL, Chatterjee A, Yoon HS, McNoe L, Black MA, Reeve AE. Slow proliferation as a biological feature of colorectal cancer metastasis. Br J Cancer. 2009;101:822-828. |