Published online May 16, 2013. doi: 10.4253/wjge.v5.i5.231
Revised: March 5, 2013
Accepted: April 10, 2013
Published online: May 16, 2013
Processing time: 113 Days and 13.4 Hours
AIM: To determine whether topical lidocaine benefits esophagogastroduoduenoscopy (EGD) by decreasing propofol dose necessary for sedation or procedure-related complications.
METHODS: The study was designed as a prospective, single centre, double blind, randomised clinical trial and was conducted in 2012 between January and May (NCT01489891). Consecutive patients undergoing EGD were randomly assigned to receive supplemental topical lidocaine (L; 50 mg in an excipient solution which was applied as a spray to the oropharynx) or placebo (P; taste excipients solution without active substance, similarly delivered) prior to the standard propofol sedation procedure. The propofol was administered as a bolus intravenous (iv) dose, with patients in the L and P groups receiving initial doses based on the patient’s American Society of Anaesthesiologists (ASA) classification (ASA I-II: 0.50-0.60 mg/kg; ASA III-IV: 0.25-0.35 mg/kg), followed by 10-20 mg iv dose every 30-60 s at the anaesthetist’s discretion. Vital signs, anthropometric measurements, amount of propofol administered, sedation level reached, examination time, and the subjective assessments of the endoscopist’s and anaesthetist’s satisfaction (based upon a four point Likert scale) were recorded. All statistical tests were performed by the Stata statistical software suite (Release 11, 2009; StataCorp, LP, College Station, TX, United States).
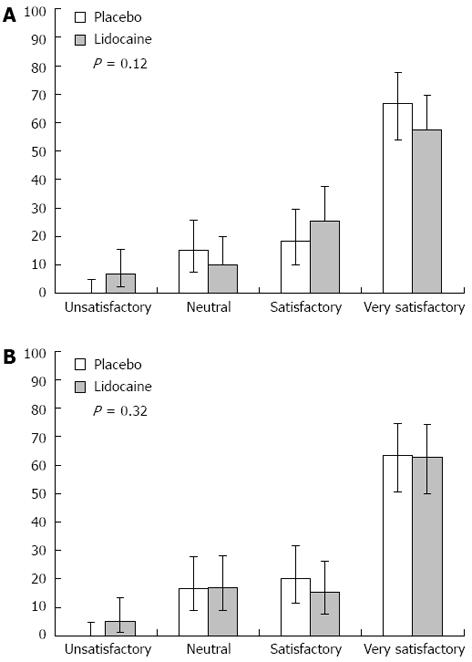
RESULTS: No significant differences were found between the groups treated with lidocaine or placebo in terms of total propofol dose (310.7 ± 139.2 mg/kg per minute vs 280.1 ± 87.7 mg/kg per minute, P = 0.15) or intraprocedural propofol dose (135.3 ± 151.7 mg/kg per minute vs 122.7 ± 96.5 mg/kg per minute, P = 0.58). Only when the L and P groups were analysed with the particular subgroups of female, < 65-year-old, and lower anaesthetic risk level (ASA I-II) was a statistically significant difference found (L: 336.5 ± 141.2 mg/kg per minute vs P: 284.6 ± 91.2 mg/kg per minute, P = 0.03) for greater total propofol requirements). The total incidence of complications was also similar between the two groups, with the L group showing a complication rate of 32.2% (95%CI: 21.6-45.0) and the P group showing a complication rate of 26.7% (95%CI: 17.0-39.0). In addition, the use of lidocaine had no effect on the anaesthetist’s or endoscopist’s satisfaction with the procedure. Thus, the endoscopist’s satisfaction Likert assessments were equally distributed among the L and P groups: unsatisfactory, [L: 6.8% (95%CI: 2.2-15.5) vs P: 0% (95%CI: 0-4.8); neutral, L: 10.1% (95%CI: 4.2-19.9) vs P: 15% (95%CI: 7.6-25.7)]; satisfactory, [L: 25.4% (95%CI: 10-29.6) vs P: 18.3% (95%CI: 15.5-37.6); and very satisfactory, L: 57.6% (95%CI: 54-77.7) vs P: 66.6% (95%CI: 44.8-69.7)]. Likewise, the anaesthetist’s satisfaction Likert assessments regarding the ease of maintaining a patient at an optimum sedation level without agitation or modification of the projected sedation protocol were not affected by the application of lidocaine, as evidenced by the lack of significant differences between the scores for the placebo group: unsatisfactory, L: 5.8% (95%CI: 1.3-13.2) vs P: 0% (95%CI: 0-4.8); neutral, L: 16.9% (95%CI: 8.9-28.4) vs P: 16.7% (95%CI: 8.8-27.7); satisfactory, L: 15.2% (95%CI: 7.7-26.1) vs P: 20.3% (95%CI: 11.3-31.6); and very satisfactory, L: 62.7% (95%CI: 49.9-74.3) vs P: 63.3% (95%CI: 50.6-74.7).
CONCLUSION: Topical pharyngeal anaesthesia is safe in EGD but does not reduce the necessary dose of propofol or improve the anaesthetist’s or endoscopist’s satisfaction with the procedure.
Core tip: We are pleased to report the second study in the literature about the possible efficacy of using an adjuvant topical anaesthesia, in this case lidocaine applied as a spray to the oropharynx, during esophagogastroduodenoscopy performed under sedation with propofol. This study is unique, however, in that it is the first randomized controlled trial demonstrating that this routine application has no beneficial effect on reduction of propofol dose or procedure-related complications, or on improved satisfaction of the endoscopist or anaesthetist. These findings may help to improve and streamline the current procedures used for endoscopy sedation, saving resources such as time during surgery and monetary costs for the topical agent.
- Citation: de la Morena F, Santander C, Esteban C, de Cuenca B, García JA, Sánchez J, Moreno R. Usefulness of applying lidocaine in esophagogastroduodenoscopy performed under sedation with propofol. World J Gastrointest Endosc 2013; 5(5): 231-239
- URL: https://www.wjgnet.com/1948-5190/full/v5/i5/231.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i5.231
Sedation in gastrointestinal endoscopy was traditionally performed with benzodiazepines in isolation or in combination with opioids. However, since the introduction of propofol nearly two decades ago, this very powerful ultra-short action hypnotic agent has emerged as the primary method for sedation in digestive endoscopy[1-4]. Nevertheless, its use is not without risk[5], such as serious cardiorespiratory consequences[6], and the ability to resolve cases of over-sedation is hindered by the lack of antagonists.
Previous studies of non-sedated esophagogastroduodenoscopy (EGD) have shown that the use of topical pharyngeal anaesthesia improves the patients’ perceived satisfaction with the procedure[7,8]. Another study of patients undergoing EGD with sedation via the traditional drugs indicated that administration of topical anaesthesia facilitated the endoscopic examination and increased patients’ tolerance[9]. However, this beneficial effect has not been sufficiently researched in patients sedated via propofol[10]. Therefore, the purpose of this study was to establish whether application of topical pharyngeal anaesthesia benefits patients undergoing EGD by reducing total propofol dosage required for sedation or affecting the rate of procedure-related adverse effects. In addition, this study assessed whether the use of topical lidocaine impacts the quality of the endoscopic examination as perceived by the endoscopist/anaesthetist.
Consecutive patients over 18-year-old referred to the Endoscopy Unit of the Infanta Cristina Hospital for diagnostic or therapeutic EGD with sedation were recruited for the study. Patients were excluded from enrolment according to the following criteria: undergoing urgent endoscopy; presence of encephalopathy; refusal of cooperation for the treatment or study procedures; refusal to provide informed consent; not having fasted; having a history of or predisposition to methemoglobinemia (NADH reductase, pyruvate kinase, or glucose-6-phosphate dehydrogenase deficiency); women who were pregnant or lactating; or presence of known allergies to propofol and/or lidocaine (or the amide group of local anaesthetics). All enrolled study participants provided informed consent prior to the treatment procedure. The study was approved by the Clinical Trials and Research Committee, the Spanish lidocaine drug manufacturer (Inibsa, Spain), and the Spanish Medical Products Agency (AEMPS 2012-01-02).
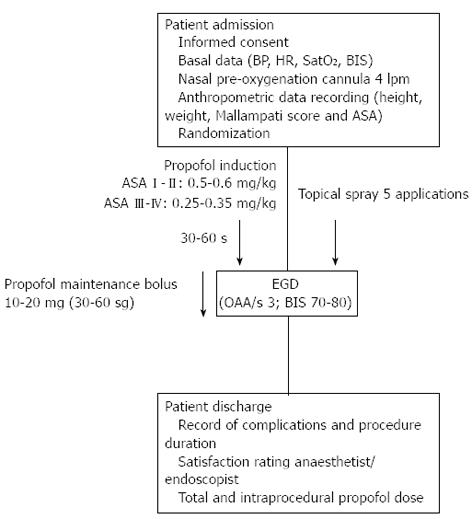
Designed as a double blind, randomised, prospective trial, this study was conducted with patients from a single centre (Infanta Cristina Hospital Endoscopy Unit in Parla, Madrid, Spain) treated between January 2012 and May 2012. The 120 enrolled patients were randomised by computer-generated numerical codes that were marked on spray devices containing lidocaine (L) or placebo (P) and enclosed in opaque envelopes that were unsealed for use during the surgical procedure. Thus, the patient, endoscopist, and anaesthetist were all “blinded” to the group assignment. The spray application and subsequent sedation procedure are illustrated in Figure 1 and described in the proceeding section.
To guarantee the integrity of the treating physicians being blinded to the group assignment during the physical application of the spray, eight pressurized phials with controlled dosage release mechanisms were used, four of them contained 10% lidocaine (10 mg = 1 puff; Xilonibsa, Inibsa, Spain) mixed with excipients (menthol, saccharine, banana aroma, macrogol 600, and ethanol) and the other four contained the excipient solution without lidocaine (for use as placebo, so that the patient could not distinguish the two by taste). In the event of an adverse reaction and the need to unmask, only the number of the phial concerned would be identified, so that the study could continue.
A single endoscopist and anaesthetist, both experts in their fields, performed the respective procedures on all study participants. All endoscopies were performed with a EG-2990K video-esophagogastroduodenscope equipped with a 9 mm diameter insertion tube (Pentax Corporation, Tokyo, Japan).
The topical pharyngeal anaesthesia or placebo was administered 180 s prior to endoscope insertion. The various spray dispensers administered a controlled volume of 10 mg per spray. A total 50 mg of lidocaine or placebo was administered to each patient by five sequential spray applications. Gentle tongue traction was used to expose the targeted spray area: the posterior wall of the oropharynx, tonsillar pillars, soft palate and base of tongue. Between each spray, patients were asked to swallow in order to maximize the anaesthetic effect on the hypopharynx. The spray procedure was performed in a room adjoining the endoscopy unit and by trained nurses who were not involved in the subsequent endoscopy and sedation procedures, thereby further ensuring masking.
Sedation was administered by bolus intravenous (iv) injection of 1% propofol at various dosages adjusted by patient weight and corresponding to the patient’s physical status classification according to the American Society of Anaesthesiologists (ASA) guidelines[11]. ASA I-II patients received an initial dose of 0.5-0.6 mg/kg, followed by sequential 10-20 mg maintenance doses every 30-60 s given at the anaesthetist’s discretion. ASA III-IV patients received an initial dose of 0.25-0.35 mg/kg, followed by the same maintenance protocol. This regimen aimed to achieve and maintain an optimum level of moderate sedation for the EGD procedure, which was defined as a score of 3 on the observer alertness assessment scale (OAA/S3) and estimated values of 70-80 for the bispectral range (BIS) measured by four frontal electrodes and the BIS View monitoring system (Aspect Medical System Inc., Norwood, MA, United States). Once the desired sedation level was reached, the endoscopic examination began. Regulation of maintenance propofol doses and administration frequency fluctuated according to three factors: patient’s tolerance as perceived by the anaesthetist (indicated by movement, coughing, nausea, agitation), sedation level (to maintain OAA/S3), and pre-determined physical characteristics and individual factors of each patient (including age, weight, and toxic habits).
All patients were fitted with a nasal cannula prior to the procedure to deliver oxygen at 4 lpm, which was initiated at least 180 s prior to the endoscopy procedure and continuing until completion. Pulsoxymetry, electrocardiography and blood pressure measurements were taken and recorded every 120 s.
Occurrence of the following adverse effects was recorded: hypoxemia (SatO2 < 90%, or a > 4% drop relative to the baseline value if it was ≤ 93%), bradycardia (< 60 bpm, or a > 10% drop in relation to the baseline value), hypotension (systolic blood pressure < 90 mmHg or diastolic blood pressure < 60 mmHg), anaphylactic reaction, bronchoaspiration (clinical diagnosis based on coughing, fever and/or lung infiltrations up to 48 h after the endoscopic examination), or methemoglobinemia. Suspicion of methemoglobinemia secondary to lidocaine or cyanosis with normal oxygen saturation was addressed by sampling the arterial blood for assessment by CO-oxymetry to determine the necessary treatment.
This protocol is registered at ClinicalTrials.gov under identifier number NCT01489891.
The following data were recorded for each patient: age, sex, weight (kg), height (m), ASA classification, medical recommendation, Mallampati score, prior history of EGD under sedation, history of or on-going alcohol/drug abuse, total propofol dose administered (mg), initial and maintenance propofol doses administered (mg), total and partial examination time (defined as the period from endoscope insertion to removal, in s), average BIS level reached, complete or incomplete examination, and complications. In addition, the endoscopist recorded a global satisfaction rating for the ease of performing each examination and the anaesthetist recorded a rating on the ease of reaching and maintaining the desired sedation level; these subjective ratings were based on a Likert-type 4-element scale of very satisfactory, satisfactory, neutral, and unsatisfactory.
The primary study objective was to determine whether use of lidocaine reduced the subsequent need for total propofol without increasing adverse effects or incomplete endoscopies, or causing significant variations in the subjective rating scales of the endoscopist and the anaesthetist. The secondary objectives were to determine the precise differences in adverse effect incidence between the lidocaine and placebo groups, and to establish the existing differences between the procedure-related satisfaction ratings awarded by the endoscopist and the anaesthetist.
Continuous variables are expressed as average ± SD and were compared between groups using the Student’s t-test. Categorical variables are expressed as percentage and were compared between groups using the Pearson’s χ2 test. The threshold of statistical significance was set at 0.05. Stratification analysis was carried out to control for effects by potentially confounding variables. All statistical tests were performed by the Stata statistical software suite (Release 11, 2009; StataCorp, LP, College Station, TX, United States).
Sample size was calculated based on achieving a reduction of the total average propofol dose by at least 30 mg to reach and maintain the same level of objective sedation in the lidocaine group as in the placebo group. It was estimated that at least 59 patients were required for each study section (L and P) to detect statistically significant differences, admitting a risk α of 0.05 and a statistical power of 90%.
A total of 127 patients were prospectively recruited between January and May 2012. After applying the exclusion criteria, three patients were denied enrolment: two for age < 18 years and one for history of sensitivity to amide group anaesthetics. Four additional patients refused to participate in the study. Thus, 120 patients were initially enrolled. One enrolled patient from the lidocaine group was subsequently excluded from analysis due to a technical problem that occurred in the endoscopy room during the examination procedure.
The randomization process assigned 59 patients to the L group and 60 patients to the P group. Comparison of the two groups showed no statistically significant differences in anaesthetic risk, age, sex, Mallampati scale, drug abuse, and prior experience regarding endoscopies under sedation. However, the average weight of individuals in the placebo group was significantly higher: 5.8 kg [95%CI: (-0.1)-(-11.4)] higher than those in the lidocaine group. The results are summarized in Table 1.
| Lidocaine | Placebo | Diff1 | P2 | |
| n | 59 | 60 | ||
| Age, yr | 49.7 ± 15.81 | 51.7 ± 14.9 | -2.0 (-7.6, 3.5) | 0.47 |
| Male sex | 51.10% | 48.60% | 1.10% | 0.85 |
| (37.0-65.0) | (37.6-51.8) | (0.5-2.3) | ||
| Weight, kg | 70.8 ± 14.0 | 76.6 ± 17.0 | -5.8 (-0.1, -11.4) | 0.04 |
| Height, cm | 162.1 ± 9.0 | 162.2 ± 11.0 | 0.10 (-3.6, 3.7) | 0.50 |
| ASA I-II | 50.4 (40.5-60.5) | 46.1 (28.7-64.5) | 1.10 (0.7-1.7) | 0.60 |
| Mallampati I-II | 51 (41.2-60.7) | 48.9 (24.4-66.5) | 0.84 (0.5-1.4) | 0.49 |
| Drug abuse | 50 (26.3-76.3) | 49.5 (40.3-58.7) | 1.00 (0.5-1.9) | 1.00 |
| Previous sedated EGD | 47.6 (28.3-67.6) | 50 (40.2-59.7) | 0.95 (0.6-1.5) | 0.80 |
As shown in Table 2, no statistically significant differences were found between the L and P groups in total or partial propofol doses, sedation level reached by BIS, or average total or partial examination time. However, there was a trend towards longer examination time for the L group. Stratification analysis of the increased examination time (using patient weight) indicated that the differences for total values (mg/kg per minute) and for time from endoscope insertion to removal were not significant.
| Lidocaine | Placebo | Diff1 | P | |
| Average BIS | 68.1 ± 7.5 | 68.8 ± 7.6 | 0.76 (-2.0, 3.5) | 0.58 |
| Total examination values | ||||
| Total examination time, s | 405.0 ± 134.8 | 387.0 ± 127.6 | 18.6 (-29.0, 66.2) | 0.44 |
| Total propofol dose, mg | 134.9 ± 42.5 | 129.2 ± 40.4 | 5.6 (-9.4, 20.7) | 0.45 |
| Total propofol dose adjusted weight and time, mg/kg per minute | 310.7 ± 139.2 | 280.1 ± 87.7 | 30.6 (-11.5, 72.7) | 0.15 |
| Intraprocedural examination values2 | ||||
| Partial examination time, s | 281.8 ± 137.3 | 265.5 ± 122.3 | 16.3 (-30.8, 63.5) | 0.49 |
| Partial propofol dose, mg | 40.9 ± 33.7 | 38.9 ± 31.4 | 2 (-9.7, 13.8) | 0.73 |
| Partial propofol dose adjusted weight and time, mg/kg per minute | 135.3 ± 151.7 | 122.7 ± 96.5 | 12.6 (-33.5, 58.7) | 0.58 |
Table 3 summarizes the results of stratification analyses to assess the influences of potentially cofounding factors on the propofol dose. Statistically significant differences were found between the L and P groups for greater total propofol requirements among patients who were female, < 65-years old, and with lower anaesthetic risk level (ASA I-II). The latter two factors were found to be related, with the low ASA groups having a significantly greater proportion of young patients [relative risk (RR) = 3.2 (95%CI: 1.75-6.01)]. The significance of these differences was lost, however, when only the patients receiving partial doses were considered in each of these categories.
| Diff1 | P2 | |||
| Age, yr | ||||
| < 65 | > 65 | |||
| Total propofol dose | 315.3 ± 118.9 | 223.9 ± 73.7 | 91.4 (49.3, 133.5) | < 0.001 |
| Partial3 propofol dose | 138.2 ± 135.5 | 96.0 ± 81.4 | 42.2 (-5.6, 90.0) | 0.08 |
| Sex | ||||
| Male | Female | |||
| Total propofol dose | 263.7 ± 87.9 | 314.6 ± 127.9 | -59.9 (-93.8, -8.0) | 0.02 |
| Partial propofol dose | 111.5 ± 101.9 | 139.6 ± 139 | -28.1 (-75.4, 19.2) | 0.20 |
| ASA classification | ||||
| I-II | III-IV | |||
| Total propofol dose | 310.8 ± 121.3 | 239.8 ± 77.7 | 71.0 (21.2, 120.8) | < 0.001 |
| Partial propofol dose | 135.9 ± 136.3 | 104.1 ± 80.1 | 31.9 (-23.6, 87.4) | 0.20 |
| Mallampati classification | ||||
| I-II | III-IV | |||
| Total propofol dose | 302.4 ± 119.5 | 262.4 ± 98.6 | 40.0 (-15.3, 95.3) | 0.10 |
| Partial propofol dose | 127.1 ± 133.9 | 137.7 ± 85.9 | -10.6 (-71.5, 49.9) | 0.70 |
| Drug abuse | ||||
| Yes | No | |||
| Total propofol dose | 320.5 ± 92.1 | 293.0 ± 118.7 | 27.5 (-48.9, 103.9) | 0.40 |
| Partial propofol dose | 120.3 ± 87.1 | 129.8 ± 129 | -9.5 (-92.6, 73.6) | 0.80 |
| Previous sedated EGD | ||||
| Yes | No | |||
| Total propofol dose | 260.2 ± 102.7 | 302.8 ± 118.6 | -42.6 (-97.4, 12.2) | 0.10 |
| Partial propofol dose | 128.9 ± 108.4 | 129.0 ± 130.6 | -0.1 (-60.1, 59.9) | 0.90 |
Table 4 summarizes the results of stratification analyses to assess the influence of lidocaine on the propofol dose variations according to the potential confounding factors. Lidocaine only produced a significant modifying effect on the amount of total or partial propofol administered in any the ASA I-II patients, for whom lidocaine administration prior to endoscopy appeared to have a pernicious effect, with greater total doses of propofol required as compared to the corresponding patients in the P group. However, there were no significant differences between the ASA I-II patients in the L and P groups in terms of age (45.3 ± 13.5 years vs 48.7 ± 14.8 years), female sex [66.6% vs 67.4%; RR = 0.98 (95%CI: 0.74-1.31)], and BIS level (67.4 ± 7.5 vs 69.6 ± 7.6), and the underlying influential factor remains unknown. Nevertheless, the significance of the pernicious effect in the ASA I-II group was lost when only the patients receiving partial propofol doses were considered for each category.
| Lidocaine | Placebo | Diff1 | P2 | ||
| Age, yr | |||||
| < 65 | |||||
| Total propofol dose | 338.1 ± 138.7 | 292.0 ± 90.3 | 46.1 (-2.2, 94.4) | 0.06 | |
| Partial3 propofol dose | 147.2 ± 165.6 | 128.9 ± 96.5 | 18.3 (-37.7, 74.3) | 0.51 | |
| > 65 | |||||
| Total propofol dose | 203.6 ± 77.8 | 241.1 ± 68.0 | -37.5 (-96.5, 21.5) | 0.20 | |
| Partial propofol dose | 88.7 ± 61.6 | 102.3 ± 97.1 | -13.6 (-80.8, 53.6) | 0.67 | |
| Sex | |||||
| Male | |||||
| Total propofol dose | 280.0 ± 101.3 | 246.7 ± 69.6 | 33.3 (-19.2, 85.8) | 0.20 | |
| Partial propofol dose | 97.3 ± 95.5 | 125.0 ± 108.0 | -27.7 (-33.7, 89.1) | 0.36 | |
| Female | |||||
| Total propofol dose | 330.4 ± 157.0 | 299.6 ± 92.1 | 30.8 (-28.5, 90.0) | 0.30 | |
| Partial propofol dose | 141.9 ± 175.2 | 137.5 ± 95.2 | 4.4 (-60.5, 69.3) | 0.89 | |
| ASA classification | |||||
| I-II | |||||
| Total propofol dose | 336.5 ± 141.2 | 284.6 ± 91.2 | 51.9 (2.8, 100.9) | 0.03 | |
| Partial propofol dose | 149.6 ± 164.5 | 122.1 ± 99.6 | 27.5 (-28.6, 83.6) | 0.16 | |
| III-IV | |||||
| Total propofol dose | 209.7 ± 70.0 | 265.7 ± 76.7 | 56.0 (-3.8, 115.8) | 0.06 | |
| Partial propofol dose | 79.4 ± 63.3 | 125.0 ± 88.9 | -45.6 (-19.3, 110.5) | 0.16 | |
| Mallampati classification | |||||
| I-II | |||||
| Total propofol dose | 319.6 ± 140.0 | 284.3 ± 91.4 | 35.4 (-12.2, 83.0) | 0.14 | |
| Partial propofol dose | 136.1 ± 159.2 | 117.7 ± 102.1 | 18.4 (-35.4, 72.2) | 0.49 | |
| III-IV | |||||
| Total propofol dose | 260.8 ± 130.7 | 263.6 ± 72.3 | -2.8 (-96.0, 90.5) | 0.95 | |
| Partial propofol dose | 130.8 ± 108.0 | 142.8 ± 69.9 | -12.0 (-93.2, 69.2) | 0.76 | |
| Drug abuse | |||||
| Yes | |||||
| Total propofol dose | 313.5 ± 115.2 | 327.6 ± 75.4 | -14.1 (-156.1, 127.9) | 0.82 | |
| Partial propofol dose | 96.1 ± 100.8 | 144.4 ± 73.8 | -48.3 (-177.1, 80.5) | 0.41 | |
| No | |||||
| Total propofol dose | 310.5 ± 142.2 | 275.9 ± 88.2 | 34.6 (-10.2, 79.4) | 0.12 | |
| Partial propofol dose | 138.9 ± 155.1 | 120.8 ± 98.6 | 18.1 (-31.1, 67.3) | 0.46 | |
| Previous sedated EGD | |||||
| Yes | |||||
| Total propofol dose | 297.6 ± 116.3 | 226.1 ± 79.1 | 71.6 (-18.4, 161.7) | 0.11 | |
| Partial propofol dose | 124.4 ± 129.5 | 132.9 ± 91.5 | -8.5 (-110.1, 93.1) | 0.86 | |
| No | |||||
| Total propofol dose | 313.4 ± 144.4 | 292.3 ± 85.7 | 21.1 (-26.9, 69.1) | 0.38 | |
| Partial propofol dose | 137.5 ± 157.0 | 120.4 ± 98.4 | 17.1 (-35.8, 70.0) | 0.52 | |
Minor complications occurred in 29.4% of the endoscopic examinations, none of which necessitated suspension of the procedure. None of the patients showed signs of methemoglobinemia. There were no significant differences between the L and P groups for total complication rates or incidence rates of the various types of adverse events (Table 5). Furthermore, stratification analysis of the complication incidences and the various risk factors (i.e., advanced age, ASA level, female sex, Mallampati score, previous drug abuse, previous endoscopy, total propofol dose administered, and BIS depth) revealed no significant differences between the groups (Table 6).
| Lidocaine | Placebo | Diff1 | P | |
| Complications | 32.2 (21.6-45.0) | 26.7 (17.0-39.0) | 1.2 (0.7-2.1) | 0.50 |
| Desaturation | 57.1 (25.0-84.2) | 54.5 (38.0-70.1) | 1.0 (0.5-2.1) | 0.90 |
| Hypotension | 63.6 (42.9-80.3) | 66.6 (43.6-84.0) | 0.95 (0.6-1.5) | 0.80 |
| Bradycardia | 13.6 (3.9-34.2) | 25.0 (6.3-55.9) | 0.5 (0.1-2.7) | 0.46 |
| Aspiration | 0 (0-17.4) | 5.5 (0-27.6) | - | 0.26 |
| Bronchospasm | 9.0 (1.3-29.0) | 0 (0-20.7) | - | 0.19 |
| Diff1 | P | |||
| Age, yr | ||||
| < 65 | > 65 | |||
| 22.8 (11.8-39.2) | 17.6 (10.8-27.2) | 1.3 (0.6-2.7) | 0.25 | |
| Sex | ||||
| Male | Female | |||
| 31.4 (18.4-48.1) | 41.2 (31.3-51.8) | 0.7 (0.4-1.3) | 0.15 | |
| ASA classification | ||||
| I-II | III-IV | |||
| 22.8 (11.8-39.2) | 21.2 (13.7-31.1) | 1.1 (0.5-2.2) | 0.41 | |
| Mallampati classification | ||||
| I-II | III-IV | |||
| 17.1 (7.7-33.0) | 17.6 (10.9-27.2) | 0.9 (0.4-2.3) | 0.47 | |
| Drug abuse | ||||
| Yes | No | |||
| 2.8 (0-15.8) | 10.6 (5.4-19.1) | 0.2 (0.01-2.0) | 0.08 | |
| Propofol dose2 | ||||
| > 277 | < 277 | |||
| 30.5 (20.2-43.2) | 26.7 (17.0-39.1) | 1.1 (0.6-2.0) | 0.32 | |
| Average BIS | ||||
| < 70 | > 70 | |||
| 33.3 (24.0-44.2) | 21.0 (10.8-36.6) | 1.6 (0.8-3.1) | 0.08 | |
| Previous sedated EGD | ||||
| Yes | No | |||
| 17.1 (7.7-33.0) | 20.0 (12.4-30.5) | 0.8 (0.3-2.0) | 0.36 | |
Finally, the systematic use of lidocaine in EGDs under propofol sedation did not significantly affect the endoscopist’s or anaesthetist’s perception of satisfaction with the procedure (Figure 2, respectively).
This study shows the ineffectiveness of lidocaine as a standard sedation coadjuvant to propofol in EGDs; specifically, the systematic use of lidocaine did not reduce total or partial doses of propofol, lower incidence of adverse effects, nor increase the treating physician’s satisfaction with the performance of endoscopic or anaesthetic procedures. Our data generally coincided with those of the only other study reported to date on clinical application and utility of lidocaine with propofol[12]. In addition to the main conclusions stated above, the previous study also showed that the application of lidocaine may help to reduce the gag reflex. Some important differences that exist between their study design and our own may explain their unique result. First, the previous study used a lower dose of lidocaine (40 mg vs 50 mg in our current study). Second, the previous study did not consider dosage as an objective, and did not monitor sedation levels using objective methods. These differences may affect the comparative interpretation of the previous and current studies’ results.
In the current study, univariate stratified analysis indicated that advanced age, male sex, and elevated anaesthetic risk were independent factors related to reduced total propofol, but not partial, dose required during an EDG examination. In concordance with these results, both advanced age and male sex are factors that have been previously demonstrated as related to need for a lower dose of sedatives during endoscopy[13]. It is important to note here that the patients in our study with a higher ASA classification were administered lower total propofol doses for the sedation induction. Neither the patient’s Mallampati score, drug abuse history, nor previous endoscopy under sedation affected the propofol dose. In relation to the Mallampati score, two previous studies have shown modifications in the tolerance perceived by patients from the subgroup with less retropharyngeal space (Mallampati III-IV). It has been suggested that occlusive morphology of the oropharynx may be related to greater endoscope friction on the posterior wall and tonsillar pillars, possibly explaining the lower tolerance of non-sedated EGD observed in this subgroup of patients[14]. Indeed, lidocaine has been shown to have a beneficial effect in Mallampati III-IV patients undergoing non-sedated EGD[7]. Likewise, factors such as not having undergone a previous endoscopy with sedation or drug abuse have been previously identified as factors predisposing to poorer patient tolerance of the EGD procedure[15,16]; the fact that these patients in our study cohort did not require greater propofol doses may suggest a marginal influence of these factors.
The significant difference found in greater total propofol dose requirements for patients with ASA I-II who received lidocaine were did not exist when intraprocedural doses were considered for the analysis. Therefore, the essential difference between these groups lies in the different induction doses that were used to reach an OAA/S3 sedation level prior to the start of the endoscopic examination. Subordinate analysis of the potential factors that may have explained this different response in ASA I-II patients (such as the Mallampati score, drug abuse, age, sex, or average BIS level) did not identify any as significantly associated. Only the variation between individuals in relation to the necessary propofol doses and uncontrollable randomization of the study groups for the above-mentioned patient factors might explain the differences found.
Although not statistically significant, the differences observed regarding the increase in the necessary dose (both partial and total) in the lidocaine group as compared to the placebo group may be explained by several factors. First, we propose that the greater average weight of patients in the placebo group, and uncontrollable effect of the randomization process, may have contributed to the results. The patient’s individual weights affected the propofol dose administered in the initial bolus as per the protocol used (such that an obese patient received an initially higher dose which may have caused a quicker and more effected sedation level than in the non-obese or thin patients). These individual responses to propofol doses and dosage administration might paradoxically explain the greater induction phase dose requirement in the lidocaine group (characterized as thinner) as compared to the placebo group (characterized as heavier). Thus, while the placebo group received a bolus with a higher initial dose, the lidocaine group received a lower overall dose.
One of the most important advantages of our study design is the quality control of sedation levels during endoscopic procedures. The optimum sedation level for upper digestive endoscopy has been defined by consensus as moderate in ASA III-IV patients and moderate-deep in ASA I-II patients[17]. This sedation level is roughly equivalent to level 3 on the OAA/S alert-sedation scale[18]. We believe that the use of a single anaesthetist, who specialises in endoscopic sedation, for all of the examinations performed in this study cohort benefitted the quality of this study by helping to achieve a possibly homogenous sedation level across the patient population. In addition, however, we made objective measurements of the sedation levels reached and performed analysis with the average BIS index of the groups and subgroups. It is known that moderate sedation in correlation with the Ramsay scale at levels 3-4 encompasses BIS values 70-80[19], which was found in 65 of our patients.
Our study showed a greater overall incidence of side effects arising from sedation with propofol as compared to previous reports, but with no significant differences between the lidocaine and placebo groups[20,21]. The most frequent adverse effects observed were hypotension and desaturation, both of which occurred in minor ranges. No serious adverse reactions occurred in any of the 119 participants. In our study, only 5.8% of cases experienced a hypoventilation incident (as defined in endoscopic procedures under sedation with propofol at 50%-84%, with repercussions in mild transitory hypoxemia between 4%-7%[2,20,21]), none of which required ventilation with a mask bag (data not shown). Hypotension occurred in 21.8% of patients, but there was no difference between the incidence in the lidocaine and placebo groups. The incidence of this complication in our study cohort was greater than previously reported in the literature, which ranges between 3%-7%[21]. The possibility of incidentally recording blood pressure figures very close to the initial induction bolus may explain our results, as the method of bolus administration has known risk for causing hypotension, as compared to the continual infusion methods[22,23].
Regarding procedural satisfaction perceived by the treating physician, a Likert scale of four elements was designed for use by the anaesthetist and the endoscopist immediately after the procedure completion to assess the ease of attaining and maintaining an appropriate sedation level for the former and the ease of achieving examination objectives for the latter. Such results may overlap with those recently obtained by Heuss et al[12], who also demonstrated the inefficacy of lidocaine to improve the satisfaction of endoscopists.
Our study has three relevant limitations that must be considered when interpreting our findings. The first is the absence of a patient satisfaction assessment. In our opinion, the greater depth of sedation reached with propofol might affect these results and their comparability with results from the older protocols with lower doses. The second limitation is the sedation level achieved, which, while sufficient and subjectively monitored by an expert anaesthetist, had recorded BIS levels at the lower limit of the interpolation validated as OAA/S3. This raises the question as to whether possible over-sedation in some patients might interfere with the conclusions of our study, and whether different results might have been obtained with more superficial sedation. Lastly, the use of patients from a single centre, treated by a single endoscopist, a single anaesthetist and a single nursing team, may have caused some bias.
In conclusion, the use of topical pharyngeal anaesthesia does not reduce the propofol dose required to maintain optimum sedation levels in EGD. While its use does not increase the incidence or type of adverse effects, it also does not improve the treating physician’s satisfaction with the procedure itself. This lack of benefit suggests that topical lidocaine application may be removed from the EDG procedure carried out with propofol sedation, and further studies should consider this option.
Application of topical pharyngeal anaesthesia has been shown to improve patient tolerance of and satisfaction with both non-sedated and traditional sedated endoscopy procedures. However, this effect has not yet been demonstrated specifically with propofol sedation protocols.
Lidocaine is a common topical aesthetic applied routinely and frequently as coadjuvant with sedation agents in endoscopy procedures, such as esophagogastroduodenoscopies (EGDs), performed without sedation. However, no systematic investigations have yet reported on its utility in propofol-based sedation protocols in terms of reduction of doses or of side effects. This study demonstrates that the systematic use of lidocaine in esophagogastroduodenoscopy with propofol sedation is ineffective for reducing the doses required for or side effects related to propofol sedation.
Potential pitfalls of using a procedure or coadjuvant agent - such topical lidocaine application with propofol-sedated endoscopy - without evidence of actual clinical utility or benefit include unnecessary increases in monetary costs and risk and discomfort to the patient. This is the first study to report that application of topical lidocaine does not decrease the dose of propofol necessary to reach and maintain an optimal level of sedation during an esophagogastroduodenoscopic procedure. Furthermore, the results suggest that its use may increase the propofol dosage required in certain patients.
Topical pharyngeal anaesthesia neither reduces the necessary doses of propofol nor improves the endoscopist’s or anaesthetist’s satisfaction with the procedure’s performance. However, its use does not increase the incidence or type of adverse effects related to the propofol-sedated esophagogastroduodenoscopy. Therefore, the authors suggest that the routine use of lidocaine in all EGDs performed with propofol sedation be reconsidered.
The bispectral index was introduced by Aspect Medical Systems, Inc. in 1994 as a novel measure of the level of a consciousness while under general anaesthesia by using algorithmic analysis of a patient’s electroencephalogram. This measurement is used in conjunction with other physiologic monitoring procedures, such as electromyography, to estimate the dose and administration of anaesthesia in order to minimize the possibility of intraoperative awareness. Meanwhile, the observer’s assessment of alertness/sedation scale was developed to measure the level of alertness in subjects who are sedated.
In this randomized controlled trial, de la Morena et al compare the potential benefit of topical lidocaine as a coadjuvant to propofol sedation during esophagogastroduodenoscopy. In particular, they investigate whether the lidocaine application may reduce the dose and/or side effects of propofol. The study is designed as a single centre, double blinded, prospective trial, in which 119 patients received propofol-sedated EGDs with or without lidocaine. Comparative analysis of quantitative and qualitative variables revealed that the lidocaine application may be safe but unnecessary, providing neither increased risk of complications nor clinical benefit to the patient or the treating physicians.
P- Reviewers Maluf F, Wong KKY S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Heuss LT, Froehlich F, Beglinger C. Changing patterns of sedation and monitoring practice during endoscopy: results of a nationwide survey in Switzerland. Endoscopy. 2005;37:161-166. |
| 2. | Rex DK, Deenadayalu VP, Eid E, Imperiale TF, Walker JA, Sandhu K, Clarke AC, Hillman LC, Horiuchi A, Cohen LB. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009;137:1229-137; quiz 1229-137;. |
| 3. | Riphaus A, Rabofski M, Wehrmann T. Endoscopic sedation and monitoring practice in Germany: results from the first nationwide survey. Z Gastroenterol. 2010;48:392-397. |
| 4. | Thomson A, Andrew G, Jones DB. Optimal sedation for gastrointestinal endoscopy: review and recommendations. J Gastroenterol Hepatol. 2010;25:469-478. |
| 5. | Goulson DT, Fragneto RY. Anesthesia for gastrointestinal endoscopic procedures. Anesthesiol Clin. 2009;27:71-85. |
| 6. | Ellett ML. Review of propofol and auxiliary medications used for sedation. Gastroenterol Nurs. 2010;33:284-95; quiz 296-7. |
| 7. | Jiménez-Puente G, Hidalgo-Isla M. [Use of topical pharyngeal anaesthesia in esophagogastroduodenoscopy in unsedated patients]. Enferm Clin. 2011;21:30-34. |
| 8. | Amornyotin S, Srikureja W, Chalayonnavin W, Kongphlay S, Chatchawankitkul S. Topical viscous lidocaine solution versus lidocaine spray for pharyngeal anesthesia in unsedated esophagogastroduodenoscopy. Endoscopy. 2009;41:581-586. |
| 9. | Evans LT, Saberi S, Kim HM, Elta GH, Schoenfeld P. Pharyngeal anesthesia during sedated EGDs: is “the spray” beneficial? A meta-analysis and systematic review. Gastrointest Endosc. 2006;63:761-766. |
| 10. | Dumonceau JM, Riphaus A, Aparicio JR, Beilenhoff U, Knape JT, Ortmann M, Paspatis G, Ponsioen CY, Racz I, Schreiber F. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2010;42:960-974. |
| 11. | Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011;55:111-115. |
| 12. | Heuss LT, Hanhart A, Dell-Kuster S, Zdrnja K, Ortmann M, Beglinger C, Bucher HC, Degen L. Propofol sedation alone or in combination with pharyngeal lidocaine anesthesia for routine upper GI endoscopy: a randomized, double-blind, placebo-controlled, non-inferiority trial. Gastrointest Endosc. 2011;74:1207-1214. |
| 13. | Wong RC. The menu of endoscopic sedation: all-you-can-eat, combination set, á la carte, alternative cuisine, or go hungry. Gastrointest Endosc. 2001;54:122-126. |
| 14. | Huang HH, Lee MS, Shih YL, Chu HC, Huang TY, Hsieh TY. Modified Mallampati classification as a clinical predictor of peroral esophagogastroduodenoscopy tolerance. BMC Gastroenterol. 2011;11:12. |
| 15. | Lee SY, Son HJ, Lee JM, Bae MH, Kim JJ, Paik SW, Yoo BC, Rhee JC, Kim S. Identification of factors that influence conscious sedation in gastrointestinal endoscopy. J Korean Med Sci. 2004;19:536-540. |
| 16. | Peña LR, Mardini HE, Nickl NJ. Development of an instrument to assess and predict satisfaction and poor tolerance among patients undergoing endoscopic procedures. Dig Dis Sci. 2005;50:1860-1871. |
| 17. | de la Morena Madrigal E, Acosta Cacho G. Sedación en la endoscopia digestiva. Edimsa. 2011;1:1-199. |
| 18. | Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675-701. |
| 19. | Agrawal D, Feldman HA, Krauss B, Waltzman ML. Bispectral index monitoring quantifies depth of sedation during emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2004;43:247-255. |
| 20. | Qadeer MA, Vargo JJ, Khandwala F, Lopez R, Zuccaro G. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1049-1056. |
| 21. | Lichtenstein DR, Jagannath S, Baron TH, Anderson MA, Banerjee S, Dominitz JA, Fanelli RD, Gan SI, Harrison ME, Ikenberry SO. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:205-216. |
| 22. | Hug CC, McLeskey CH, Nahrwold ML, Roizen MF, Stanley TH, Thisted RA, Walawander CA, White PF, Apfelbaum JL, Grasela TH. Hemodynamic effects of propofol: data from over 25,000 patients. Anesth Analg. 1993;77:S21-S29. |
| 23. | Steinbacher DM. Propofol: a sedative-hypnotic anesthetic agent for use in ambulatory procedures. Anesth Prog. 2001;48:66-71. |