Published online Mar 16, 2013. doi: 10.4253/wjge.v5.i3.95
Revised: November 15, 2012
Accepted: January 5, 2013
Published online: March 16, 2013
Processing time: 184 Days and 0.3 Hours
AIM: To compare the quality and tolerance of esophagogastroduodenoscopy (EGD)-assisted and conventional split-dose polyethylene glycol electrolyte solution for inpatient colonoscopy.
METHODS: The study was a randomized controlled trial in hospitalized patients. Hospitalized patients undergoing colonoscopy the day following EGD for evaluation of gastrointestinal (GI) bleeding or other symptoms. Patients randomized to either EGD-assisted bowel prep [2 L polyethylene glycol (PEG) administered endoscopically into distal duodenum at time of EGD, plus 1 L PEG orally the following day] or conventional-PEG (2 L PEG orally the evening prior and 1 L PEG orally the following day). The main outcome measurements are bowel preparation quality and patient tolerance of bowel prep.
RESULTS: Forty-two patients randomized to EGD-assisted bowel prep and 40 patients to conventional-PEG. Overall mean ± SD preparation quality was superior for EGD-PEG (4.1 ± 2.8) vs conventional-PEG (6.5 ± 3.1; P = 0.0005). Seventy-four percent of patients rated EGD-PEG as easy or slightly difficult to tolerate compared to 46% for standard-PEG (P = 0.0133). Mean EGD-procedural time was greater for EGD-assisted subject (24 ± 10 min) compared to conventional-PEG prep subjects (15 ± 7 min; P < 0.0001). Conscious sedation requirements did not differ between groups. There were no significant prep-related adverse events in either group.
CONCLUSION: In selected hospitalized patients, compared to a conventional split-dose regimen, use of EGD to administer the majority of PEG solution improves patient tolerance and quality of bowel preparation for colonoscopy.
- Citation: Barclay RL. Esophagogastroduodenoscopy-assisted bowel preparation for colonoscopy. World J Gastrointest Endosc 2013; 5(3): 95-101
- URL: https://www.wjgnet.com/1948-5190/full/v5/i3/95.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i3.95
Adequate bowel preparation is of critical importance for colonoscopy. Insufficient colon cleansing may compromise the safety, accuracy and therapeutic potential of the procedure[1]. Particularly among hospitalized patients, inadequate bowel preparation for colonoscopy may arise due to patient intolerance to the prescribed laxative regimen. In contrast to the ambulatory population, hospitalized patients are more often elderly and more likely to have co-existing conditions that impair their ability to ingest a large-volume laxative regimen[2,3]. Suboptimal bowel preparation may in turn lead to repeat endoscopic procedures, invasive interventions such as nasogastric tube insertion for administration of purgative agents, and additional days of hospitalization[4]. Thus, improvements in bowel preparation for colonoscopy in hospitalized patients would likely improve patient care and reduce hospital costs. The purpose of this study was to determine if administering a portion of the bowel purgative via esophagogastroduodenoscopy (EGD) could improve colonoscopy preparation in hospitalized patients.
Bowel preparation for colonoscopy can be challenging under certain circumstances. For example, advanced age, hospital setting and comorbid illnesses have been demonstrated as factors that reduce the quality of bowel preparation[2,3]. Suggestions to improve patient tolerance of bowel preparation include reducing the volume of purgative ingested, splitting the amount of purgative into two separate doses, and administering adjuvant agents to improve gastric emptying and reduce nausea and vomiting associated with ingestion of purgatives[5]. Patients who are unable to tolerate oral ingestion of a sufficient quantity of purgative pose a particular challenge to adequate colon cleansing. In this situation, one approach is to place a nasogastric tube for administration of the purgative solution. However, in addition to the inherent drawbacks of nasogastric (NG) tube placement, NG-assisted bowel preparation carries the potential for pulmonary aspiration if large volumes of solution accumulate in the stomach[6].
On the other hand, experience in other clinical situations has demonstrated the utility and an acceptable safety profile with rapid administration of large-volume PEG solution. Rapid whole gut lavage with large volumes of polyethylene glycol (PEG) electrolyte solution has been used for decades in the acute management of drug overdoses[7]. Others have employed rapid PEG lavage via nasogastric tube for bowel preparation in the setting of acute lower gastrointestinal (GI) bleeding[8]. One purported advantage of this approach is the resultant high quality of colonic mucosal visualization, which may improve the diagnostic or therapeutic yield of colonoscopy. The current study sought to extend this experience by evaluating a novel method of bowel preparation for colonoscopy: Rapid luminal infusion of PEG solution into the duodenum during EGD.
This study was conducted at OSF St. Anthony’s Medical Center and SwedishAmerican Hospital, community-based hospitals in Rockford, Illinois. Patients were enrolled from August, 2009 to July, 2011. The study was approved by the institutional review boards of the participating institutions. Adult inpatients were offered to participate in the study at the time of EGD if, depending on the EGD results, there was a possibility that colonoscopy would be performed the following day and during the hospital stay. Patients were excluded if there was evidence of bowel obstruction, suspicion of a diffuse GI motility disorder (patients with suspected gastroparesis were not excluded), inability to ingest oral bowel preparation, or if outpatient rather than inpatient colonoscopy was anticipated following EGD.
The study was a randomized controlled trial in hospitalized patients. In order to test the concept of EGD-assisted prep administration in routine clinical practice, patients in whom colonoscopy was anticipated the day following EGD comprised the study population. In this scenario the most common indication for EGD was GI bleeding but other indications were permitted. The non-bleeding indications included abdominal pain, positive fecal occult blood test with associated upper GI symptoms, and metastatic cancer of unclear origin. Patients provided written informed consent for the study at the time of EGD. They were informed that, if the upper endoscopy proved non-diagnostic or inconclusive (e.g., no convincing source of bleeding identified), colonoscopy would be recommended and would be scheduled the following morning. Participating patients were randomized (in a 1:1 ratio) at the time of EGD to either the control arm or the intervention arm (see details below). Randomization was performed using a set of random numbers, which corresponded to assignments of conventional prep (control) or EGD-assisted subjects. Eligible subjects were randomized at the time of (negative) EGD, while the scope was still in the stomach. Sealed envelopes concealed the prep assignment until the time of randomization. Endoscopic procedures were performed by 15 experienced, board-certified GI physicians during day-to-day hospital rotations. Patients received conscious sedation with IV midazolam and IV fentanyl to achieve a moderate level of sedation. Left-lateral position was used for EGD. Monitoring included continuous measurement of heart rate, respiratory rate and SaO2, and intermittent BP monitoring. When possible, the endoscopist was blinded to the subject’s prep assignment. However, physician blinding was not possible in situations in which the endoscopist performing the colonoscopy had also performed the EGD the day before. The study’s author performed 24/42 (57%) of EGD-assisted procedures; the remainder was distributed evenly among other physicians. In 19/42 (45%) cases, the endoscopist who performed the EGD-assisted procedure also performed the subsequent colonoscopy. There was no significant difference in the distributions of physicians scoring the bowel preps of EGD-assisted and conventional-PEG groups (data not shown).
Following (non-diagnostic) EGD, control subjects received routine instructions and management for spit-dose PEG bowel preparation for colonoscopy the following day. They were prescribed a clear liquid diet over the day prior to colonoscopy, oral ingestion of two liters of PEG solution (Nulytely, Braintree Laboratories Inc, Braintree, MA) at 5 PM the evening prior to colonoscopy, and an additional 1 L of Nulytely 4 h prior to colonoscopy the next day. A 10 mg IV dose of metoclopramide was given 30 min prior to administration of PEG solution. Tap water enemas were administered 1 h prior to colonoscopy. The total volume of PEG prescribed was 3 L because of our clinical experience that hospitalized patients rarely tolerate greater volumes of PEG solution. Recognizing that a 4-L PEG regimen may be used more commonly in clinical practice, the 2 plus 1 L split-dose PEG regimen falls within recent guidelines elaborated by a multi-society task force document on bowel preparation for colonoscopy[5].
At the completion of non-diagnostic upper endoscopy, subjects randomized to the intervention group received a 10 mg IV dose of metoclopramide. With the endoscope tip advanced as distally as possible in the post-bulbar duodenum, a 2-L volume of Nulytely solution was instilled through the channel of the endoscope, either with repeated injections of a 60 cc syringe or with a foot-pedal activated pump (Endogator, Byrne Medical Inc., Conroe, TX) attached directly to the container of PEG solution. The PEG solution was instilled slowly, typically over 10 to 15 min, depending on the individual patient’s ability to accommodate the fluid load. The total duration of the EGD procedure was recorded but time to instill the prep solution was not recorded separately. Patients were positioned in the left-lateral position with the head elevated 30 degrees. As a further safety precaution, PEG infusion was continued only if there was sufficient bowel motility to propel the fluid distally from the duodenum. Based on early experience with this method, endoscopists were given instruction to observe the presence of duodenal contractions and the effect that this had on the ability to instill more fluid. For example, if there was adequate motility to clear the lumen of fluid to such a degree as to appreciate an air-fluid interface, as opposed to a lumen completely full of fluid, then additional PEG solution could be instilled. Fluid that refluxed back into the stomach was suctioned out through the endoscope. However, small volumes (i.e., < 50 cc) of fluid that pooled in the fundus were not suctioned out. Care was taken to keep the stomach decompressed by suctioning out air. Other than instructions to administer fluid slowly over 10 to 15 min, there was no written protocol to direct physicians. If fluid was obviously being propelled distally, the rate of administration was more rapid than if fluid pooled in the duodenum. The endoscope was retracted into the stomach every 3 to 5 min to check for proximal fluid accumulation. The most practical observation that informed the appropriate rate of fluid instillation was observing peristaltic contractions followed by air in the lumen after fluid was propelled distally. Following EGD, patients were prescribed a clear liquid diet over the remainder of the day. The following morning, 4 h prior to colonoscopy, they were prescribed one liter of Nulytely to be ingested orally. Tap water enemas were administered 1 h prior to colonoscopy. Subjects who “failed” EGD-assisted prep administration, i.e., were unable to tolerate endoscopic administration of the full 2-L fluid volume were not crossed over into an orally-ingested PEG first dose.
The mean ± SD or median and corresponding range and inter-quartile range (IQR) were used to summarize data for continuous variables and percentages for categorical variables. Continuous variables with normal distributions were compared using student’s t-test; variables with non-normal distributions were analyzed using the Mann-Whitney test. Differences in categorical variables were analyzed using the Fisher exact test and χ2 test. A P value < 0.05 was considered statistically significant. Reported P values are two-tailed. Statistical tests were performed with the use of Analyse-it software (version 1.73, Leeds, United Kingdom). The sample size was calculated based on prior studies of bowel preparation[9] in which approximately 50% of patients who received PEG solution for colonoscopy had a fair or poor quality preparation. For the current study, to detect a 33% difference in this rate of suboptimal bowel preparation, with 80% power and α = 0.05, it was estimated that approximately 40 subjects would be required in each treatment arm.
The primary outcome measure was the quality of bowel preparation as assessed by the Ottawa bowel preparation scale[10]. The secondary outcome was patient tolerance of bowel preparation, which was assessed via a questionnaire administered just prior to sedation for colonoscopy, used in previous studies at our center[11,12]. Other variables measured included duration of procedures, amounts of sedative medications administered for EGD, and adverse events.
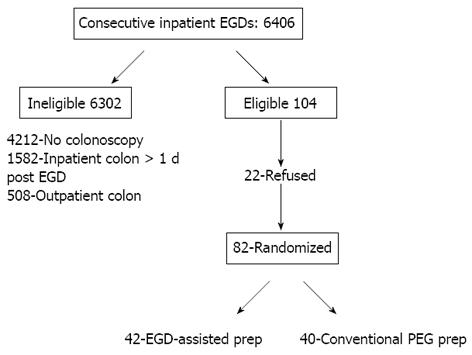
During the 23-mo study period a total of 6406 EGDs were performed in 4058 hospitalized patients. Of these procedures, 4212 were considered diagnostic and/or therapeutic such that follow-up colonoscopy was not indicated. Of the remaining patients, 1582 underwent inpatient colonoscopy more than one day following the EGD and 508 patients underwent outpatient colonoscopy, generally within 4 wk. The predominant reason for deferring inpatient colonoscopy following EGD related to managing concomitant medical conditions to achieve clinical stability to permit colonoscopy. The decision to enroll or not to enroll patients for EGD-assisted prep study was based on the clinical assessment of the physician at the time of EGD. Twenty-two patients either refused participation or were considered ineligible for the study. Of the remaining 82 subjects who comprised the study population, 42 were randomized to EGD-PEG and 40 to conventional-PEG. Thus, only 82/6406 (1.2%) of inpatient EGDs qualified for inclusion (Figure 1). Table 1 shows the baseline characteristics of these study subjects. There were no statistically-significant differences in the baseline characteristics of control and intervention subjects. The median age of subjects was 73 ± 13 years. Congestive heart failure, renal failure and diabetes mellitus were common, with roughly one-third of study subjects having at least one of these major comorbid disorders. The predominant indication for EGD was overt GI bleeding (74% of subjects). Other indications included abdominal pain, iron deficiency anemia and abnormal results of imaging studies (detailed data not shown).
| EGD-assisted(n = 42) | Conventional-PEG(n = 40) | |
| Age [median (range (IQR)] | 73 [42-99 (19.25)] | 73.5 [45-97 (17.5)] |
| Males/females | 15/27 | 20/20 |
| Bleeding as indication for EGD | 28 (67) | 33 (83) |
| Diabetes mellitus | 12 (29) | 13 (33) |
| Congestive heart failure | 11 (26) | 10 (25) |
| Stroke | 10 (24) | 4 (10) |
| Azotemia | 16 (38) | 10 (25) |
| Opiate/anticholinergic medications | 16 (38) | 13 (33) |
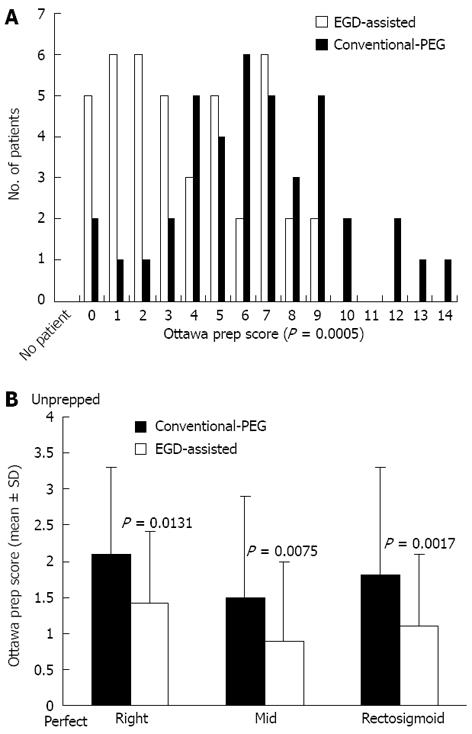
Figure 2A shows frequency distributions of Ottawa preparation scale scores for EGD-assisted and conventional split-dose PEG groups. With this scale, the numerical value is inversely related to the overall quality of the preparation. Overall mean preparation quality was superior for EGD-assisted bowel prep (4.1 ± 2.8) vs conventional-PEG (6.5 ± 3.1; P = 0.0005). Bowel preparation quality in EGD-assisted bowel prep subjects was also superior to conventional-PEG subjects when analyzed by specific colonic segment (Figure 2B). Four subjects (10%) in the conventional-PEG group required repeat colonoscopy due to inadequate preparation compared with zero patients in the EGD-assisted bowel prep group (P = 0.0523). Two of these subjects who required repeat colonoscopy because of inadequate prep had their procedures scored by the study author. Within each group, there appeared to be a trend toward poorer preparation of the right colon compared to distal segments, but these differences were not statistically significant. For example, the most pronounced differences were between the right- and mid-colon segments (P = 0.0614 for EGD-assisted and P = 0.0629 for conventional-PEG subjects).
When the analysis was confined to cases in which the endoscopist who performed the colonoscopy had not performed the prior EGD, there were 23 subjects who received EGD-assisted bowel preparation and 24 subjects who received conventional split-dose PEG preparation. Among these subjects, bowel prep was superior in EGD-assisted subjects (mean overall Ottawa prep score 4.2 ± 2.9) compared to conventional-PEG subjects (6.0 ± 2.8; P = 0.0361).
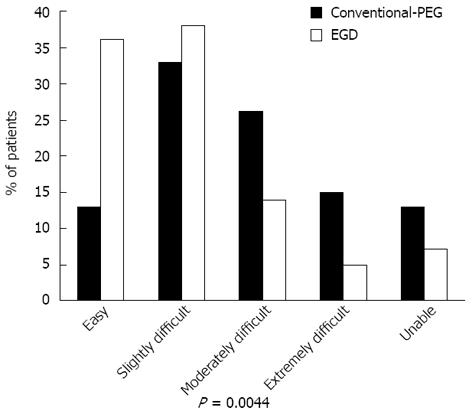
Figure 3 shows the overall level of patient tolerance of EGD-assisted vs standard split-dose PEG. The overall trend for tolerance of prep was significantly in favor of EGD-assisted prep vs the conventional-PEG protocol (P = 0.0044). 74% of patients rated EGD-assisted prep as easy or slightly difficult to tolerate compared to 46% for standard-PEG (P = 0.0133). Three patients in the EGD-assisted PEG group were unable to complete the orally-ingested portion of the prep compared to 6 conventional-PEG patients (P = 0.02963), with failure defined as inability to consume the prescribed fluid volume. Table 2 shows individual symptom profiles among EGD-assisted vs conventional-PEG subjects. In comparison to EGD-assisted subjects, conventional-PEG subjects had significantly greater rates of nausea, taste intolerance and a greater inclination to try an alternative prep for future procedures.
| Symptom | None | Mild | Moderate | Severe | Intolerable | P value |
| Bloating | 26/21 | 8/9 | 3/1 | 1/2 | 0/0 | 0.83252 |
| Dizzy | 37/30 | 1/4 | 0/0 | 0/0 | 0/0 | 0.12802 |
| Nausea | 30/18 | 7/10 | 0/6 | 0/1 | 1/0 | 0.00502 |
| Vomiting | 35/30 | 2/2 | 0/1 | 0/1 | 0/0 | 0.33592 |
| Pain | 28/21 | 8/12 | 1/1 | 1/0 | 0/0 | 0.27882 |
| Poor sleep | 22/13 | 9/8 | 11/9 | 0/3 | 0/1 | 0.19152 |
| Taste | 8/4 | 27/20 | 2/9 | 1/2 | 0/0 | 0.01762 |
| Complete | Yes 35/29 | No 3/6 | - | - | - | 0.29633 |
| Other prep | Yes 10/21 | No 28/14 | - | - | - | 0.00473 |
| Refuse | Yes 1/5 | No 37/30 | - | - | - | 0.09823 |
Median EGD-procedural time was greater for EGD-assisted subjects [20 min; range 6-45 min (IQR 15)] than for conventional-PEG subjects [15 min; 3-25 min (10); P = 0.0036]. Six subjects in the EGD-assisted prep group had EGD procedural times of 10 min or less: Two did not tolerate the endoscopic infusion of prep solution and 4 in whom the entire volume of prep solution was able to be instilled in less than 10 min. After eliminating these outliers from the analysis of EGD procedure times, the mean EGD-time in EGD-assisted prep subjects was 24 ± 10 min, compared to 15 ± 7 min in conventional-PEG prep subjects (P < 0.0001). There was no significant difference in conscious sedation requirements between the two study groups: Median [range (IQR)] doses of midazolam and fentanyl in EGD-assisted subjects were, respectively, 3 [1-8 (3)] mg and 50 [25-150 (50)] mg; corresponding values for conventional-PEG subjects were 3 [1-6 (3)] mg and 100 [25-150 (50)] mg (P = 0.9805 and 0.2932, respectively). Two subjects in the EGD-assisted bowel prep group were administered a minimal volume of PEG solution during EGD due to poor gastric emptying and a tendency of the prep to reflux back into the stomach. One of these subjects had a history of chronic use of opiate analgesics but no established history of GI dysmotility. In all other EGD-assisted prep subjects, only a small amount of prep solution was suctioned from the stomach, so that nearly all subjects received a standardized 2-L infusion of prep solution. One elderly woman in the EGD-assisted group developed hypoxemia immediately following EGD. This corrected quickly following administration of reversal agents (flumazenil and naloxone) and noninvasive (bipap-assisted) ventilation. Chest radiographs showed no evidence of pulmonary aspiration. There were no additional significant prep-related adverse events in either group. The majority of EGD-assisted subjects began passing liquid stools within 2 h, after they had been transferred from the GI endoscopy unit to the hospital floor. However, two subjects (5%) began passing watery stools during EGD-assisted administration of the PEG solution.
The median colonoscopy procedure time was significantly longer for conventional-PEG subjects [33.0 min; range 14-65 min (IQR 12)] compared to EGD-assisted subjects [28 min; 8-56 min (15); P = 0.0324].
Hospital-based colonoscopy typically is performed in older, acutely ill patients, in contrast to ambulatory colonoscopy, which is weighted towards a younger and generally healthy population undergoing screening procedures[13,14]. Advanced age, comorbid illness and other factors contribute to a decreased ability of hospitalized patients to comply with oral preparation for colonoscopy[2,3]. In particular, a substantial number of patients are unable to consume the most commonly-prescribed regimen, large-volume PEG electrolyte solution, for colonoscopy preparation. In an attempt to address the issue of inadequate preparation due to intolerance of a large-volume prep among hospital patients undergoing colonoscopy, this study tested a novel and unconventional approach to bowel preparation: Direct administration of the majority of the purgative solution into the small bowel lumen through the endoscope at the time of EGD. Judging by superior patient tolerance (e.g., significantly better nausea and taste profiles) and improved bowel preparation quality when compared to conventional split-dose PEG solution prep, EGD-assisted bowel preparation appears to be a promising approach in selected hospitalized patients. Using a careful technique emphasizing precautions to minimize risks of aspiration, there were no significant adverse events directly attributable to the prep administration. Though EGD-assisted preparation added a small amount of time to standard EGD, conscious sedation medication needs were similar for EGD-assisted and standard EGD examinations. The mean incremental increase in EGD-procedure time for EGD-assisted patients was only nine minutes, but this may not be an accurate reflection of the actual time required for prep infusion, which was not measured separately. There was a reciprocal decrease in the observed colonoscopy procedure times among EGD-assisted bowel prep subjects compared to conventional-PEG subjects, which would be consistent with less time required for washing and suctioning to improve visualization in the superiorly prepped EGD-assisted subjects. Furthermore, the use of EGD-assisted bowel preparation in this study obviated repeat colonoscopy due to inadequate preparation, which was required in 10% of patients who received conventional-PEG preparation.
Although EGD-assisted bowel preparation would be impractical and unnecessary for all hospitalized patients, such an approach could be considered in selected patients who were unable to ingest any (or especially a large) volume of oral purgative solution. Given that this study excluded patients unable to ingest an oral prep and included younger healthier patients better able to tolerate a conventional oral prep, the results likely under-estimate the true benefit of EGD-assisted bowel preparation. In patients unable to ingest an oral prep, the alternative of nasogastric tube placement to facilitate bowel preparation has drawbacks of significant patient discomfort and the potential for serious risks, including nasopharyngeal trauma, inadvertent tube misplacement and pulmonary aspiration[6]. Indeed, based on the results of the current study, one could speculate that the aspiration risk of an NG-administered prep might exceed that of EGD-assisted prep given the ability of the latter approach to directly visualize the stomach during fluid administration, thereby ensuring that a large volume of fluid does not accumulate in the stomach. However, further prospective study is needed to clarify the balance of risks and benefits of methods such as NG-assisted or EGD-assisted administration of bowel preparation in patients with extreme difficulty ingesting sufficient prep volumes. Although American Society of Anesthesia guidelines[15] prohibit oral fluid intake within two hours prior to sedated upper endoscopy, there are particular clinical situations in which administration of fluid into the upper GI tract may be required during sedated endoscopy. For example, during endosonography for gastroduodenal lesions, (temporary) water instillation into the stomach or duodenum is often necessary for accurate characterization of mural-based pathology[16]. This technique is considered standard practice for upper endosonography, a procedure with an excellent safety record spanning approximately three decades. EGD-assisted administration of a purgative solution for bowel preparation involves administration of a greater fluid volume, which is delivered distal to the pylorus. However, in contrast to EUS, the endoscopist actively intervenes to prevent gastric retention of fluid during the procedure to reduce the risk of pulmonary aspiration.
This study had certain limitations. As it was performed at a single center in a relatively small number of subjects, it would be premature to assume the results apply to a wider population of patients and practice settings. In particular, given that this study tested a highly unconventional approach to bowel preparation, which could pose significant risks if it were widely adopted without additional evidence of its safety, the findings should be considered provocative rather than definitive. Since pulmonary aspiration can be a life-threatening adverse event, one would want to confirm in a larger patient population a low - ideally, zero - risk of aspiration with this approach. The fact that most of the EGD-assisted procedures were performed by a single endoscopist limits generalizability regarding safety of the procedure. In terms of study design, it was impractical for this study to be double-blinded, since the scheduling of hospital-based physicians precluded blinding endoscopists to patients’ prep assignments. However, the risk of physician bias in grading the prep was mitigated by adherence to a validated prep scoring system and by the fact that the majority of colonoscopies that followed EGD-assisted preparation were performed by endoscopists who had not conducted the prior EGD. Finally, it is conceivable that a split-dose small-volume bowel prep may have achieved superior colon cleansing and patient tolerance compared to large-volume PEG solution. However, currently in the United States, options for small-volume prep solutions are limited. In addition, as observed in the current study, many hospitalized patients have co-existing conditions such as cardiac or renal failure, which may make the safety profile of a small-volume hyperosmotic prep less than ideal.
In conclusion, among selected hospitalized patients, when compared to a conventional split-dose regimen, use of EGD to administer the majority of PEG solution for bowel cleansing improves patient tolerance and quality of bowel preparation for colonoscopy.
The author is grateful for the participation and cooperation of the physicians of Rockford Gastroenterology Associates and the GI nursing staff of OSF St. Anthony’s and SwedishAmerican Hospitals in Rockford, IL.
Adequate bowel preparation is required for colonoscopy. Poor patient tolerance to large-volume purgatives contributes to suboptimal bowel preparation.
Adequate bowel preparation is of critical importance for colonoscopy. Insufficient colon cleansing may compromise the safety, accuracy and therapeutic potential of the procedure. Particularly among hospitalized patients, inadequate bowel preparation for colonoscopy may arise due to patient intolerance to the prescribed laxative regimen. Bowel preparation for colonoscopy can be challenging under certain circumstances. Experience in other clinical situations has demonstrated the utility and an acceptable safety profile with rapid administration of large-volume polyethylene glycol (PEG) solution.
One purported advantage of this approach is the resultant high quality of colonic mucosal visualization, which may improve the diagnostic or therapeutic yield of colonoscopy. The current study sought to extend this experience by evaluating a novel method of bowel preparation for colonoscopy: Rapid luminal infusion of PEG solution into the duodenum during esophagogastroduodenoscopy.
This is an interesting and methodologically well made paper. Their findings may be applicable to certain patients requiring both a diagnostic colonoscopy and upper gastrointestinal endoscopy.
P- Reviewers Roig JV, Dmochowski R S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 560] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 2. | Chorev N, Chadad B, Segal N, Shemesh I, Mor M, Plaut S, Fraser G, Geller A, Gal E, Niv Y. Preparation for colonoscopy in hospitalized patients. Dig Dis Sci. 2007;52:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96:1797-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 343] [Article Influence: 14.3] [Reference Citation Analysis (2)] |
| 4. | Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single centre study of 10,571 colonoscopies. Colorectal Dis. 2007;9:745-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Marschall HU, Bartels F. Life-threatening complications of nasogastric administration of polyethylene glycol-electrolyte solutions (Golytely) for bowel cleansing. Gastrointest Endosc. 1998;47:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Position paper: whole bowel irrigation. J Toxicol Clin Toxicol. 2004;42:843-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 424] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Juluri R, Eckert G, Imperiale TF. Polyethylene glycol vs. sodium phosphate for bowel preparation: a treatment arm meta-analysis of randomized controlled trials. BMC Gastroenterol. 2011;11:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 343] [Article Influence: 16.3] [Reference Citation Analysis (2)] |
| 11. | Barclay RL. Safety, efficacy, and patient tolerance of a three-dose regimen of orally administered aqueous sodium phosphate for colonic cleansing before colonoscopy. Gastrointest Endosc. 2004;60:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Barclay RL, Depew WT, Vanner SJ. Carbohydrate-electrolyte rehydration protects against intravascular volume contraction during colonic cleansing with orally administered sodium phosphate. Gastrointest Endosc. 2002;56:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1997;92:419-424. [PubMed] |
| 14. | Clarke GA, Jacobson BC, Hammett RJ, Carr-Locke DL. The indications, utilization and safety of gastrointestinal endoscopy in an extremely elderly patient cohort. Endoscopy. 2001;33:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004-1017. [PubMed] |
| 16. | Faigel DO. Endoscopic ultrasound of the stomach and duodenum. Endoscopic ultrasonography. Oxford: Wiley-Blackwell 2009; 79-91. |