Published online Nov 16, 2013. doi: 10.4253/wjge.v5.i11.559
Revised: September 5, 2013
Accepted: October 11, 2013
Published online: November 16, 2013
Processing time: 142 Days and 12.5 Hours
AIM: To assess the feasibility, accuracy and acceptability of PillCam Colon 2 in detection of significant lesions in colorectal cancer risk patients, unable or unwilling to perform colonoscopy.
METHODS: This is a prospective, single center study using the second generation of PillCam Colon capsule. In all patients the readers were instructed to review the entire colon capsule endoscopy (CCE) examination using Rapid 7 software and additionally to note significant extra-colonic findings. Colonic significant findings were described according to European Society of Gastrointestinal Endoscopy guidelines. CCE procedure completion rate, level of bowel preparation and rate of adverse events were assessed.
RESULTS: A total of 70 patients at risk of colorectal cancer were enrolled in the study. In three patients the procedure failed because the capsule was not functioning when entered the colon. PillCam Colon 2 showed positive findings in 23 (34%, 95%CI: 21.6%-44.1%) of the remaining 67 patients. Six patients were diagnosed with tumors: 4 with colon cancers, 1 with gastric cancer and 1 with a small bowel cancer. The capsule findings were confirmed after surgery in all these patients. The capsule excretion rate in twelve hours was 77% with 54 patients having a complete examination. The rectum was not explored during CCE procedure, in 16 patients (23%, 95%CI: 13.7%-34.1%). Every patient accepted CCE as an alternative exploration tool and 65/70 (93%) agreed to have another future control by CCE. No complications were reported during or after CCE examination.
CONCLUSION: PillCam Colon 2 capsule was effective in detecting significant lesions and might be considered an adequate alternative diagnostic tool in patients unable or unwilling to undergo colonoscopy.
Core tip: This is an important article on the second generation colon capsule endoscopy. It shows that it has a high diagnostic yield in an enriched population that have had incomplete colonoscopy or refused colonoscopy. We also diagnosed significant extracolonic lesions. The method had a high acceptability among patients and we did not encounter any complications.
- Citation: Negreanu L, Babiuc R, Bengus A, Sadagurschi R. PillCam Colon 2 capsule in patients unable or unwilling to undergo colonoscopy. World J Gastrointest Endosc 2013; 5(11): 559-567
- URL: https://www.wjgnet.com/1948-5190/full/v5/i11/559.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i11.559
Colorectal cancer (CRC) is the second most common cancer and second most common cause of cancer-related deaths in Europe. CRC screening has been proven to reduce disease-specific mortality[1]. The choice of a screening test takes into consideration parameters such as patient age and the presence of different risk factors for the development of CRC. Several European countries employ national screening programs. They rely almost exclusively on stool tests, with colonoscopy used as an adjunct in some countries. Colonoscopy has been shown to reduce colorectal cancer risk. Its increased use in the population aged 50 years and older in the United States since the 1980s is the reason for decreasing CRC incidence rates, particularly in the sigmoid, colon although some environmental factors may also have contributed to the decreasing risk[2].
A prediction for 2012 expects a decline in mortality from colorectal cancer of 7% in men and 11% in women in the European Union compared with 2007 mainly due to the screening programs[3].
Nevertheless the uptake of patients in the screening programs is disappointingly low. The degree of acceptance of colonoscopy is low because it is perceived by some patients/physicians as invasive and painful and with a degree of complications/risks. Another drawback is the rate of failed colonoscopic examinations. The caecal intubation failure rate is up to 20% of colonoscopies in clinical practice[4]. No guideline exists for these patients but several options are being used with different success rates. Computed tomographic colonography (CTC) is a useful option and seems supported by recent studies[5].
Colon capsule endoscopy (CCE) PillCam Colon was developed by Given Imaging especially for increasing the acceptability and safety of a colorectal examination. Although a bowel preparation similar to colonoscopy is necessary, this technique requires no intubation, insufflation or sedation and has minimal complication rates/risks[6,7].
A second-generation, improved, CCE system (PillCam Colon 2) was developed to increase sensitivity for colorectal polyp detection compared with the first-generation system. A recent study using a second-generation colon capsule showed a higher sensitivity than the first generation, of almost 90% for detection of patients with significant colonic lesions[8]. Recently the European Society of Gastrointestinal Endoscopy published an updated and extensive guideline regarding the current status of capsule endoscopy. It gives a clear perspective about the indications, bowel preparation, reporting and level of evidence[9].
According to these guidelines, CCE is feasible and safe and appears to be an accurate screening tool when used in average-risk individuals. A CCE based screening may be cost-effective if it increases uptake compared with colonoscopy. In high risk patients (alarm symptoms or signs, family or personal history of CRC), which are at increased risk of advanced colorectal neoplasia or cancer, colonoscopy should be the first choice. However, in patients for whom colonoscopy is inappropriate or not possible, the use of CCE could be discussed with the patient[9].
We conducted a pilot trial to asses the feasibility, accuracy and acceptability of PillCam Colon 2 in detection of significant lesions in patients at risk of CRC which were unable or unwilling to perform colonoscopy. Following recent European Society of Gastrointestinal Endoscopy (ESGE) capsule endoscopy guideline, a significant colorectal lesion that requires colonoscopy follow-up was considered to be a colorectal polyp > 6 mm or presence of at least 3 colonic polyps[9].
Since we could not compare colon capsule endoscopy CCE to the gold standard (colonoscopy) we introduced a new end point of “positive” examination: the diagnostic utility index (findings directly explaining symptoms or requiring specific treatment in asymptomatic patients). Although using this end point even a normal examination can be considered successful for a certain patient if it is important for the clinical decision and follow up, we decided to consider significant the capsule findings that required medical or surgical treatment. Also a patient follow up of one year was mandatory. CCE procedure completion rate level of bowel preparation and rate of adverse events were also assessed.
A total of 70 patients of mean age 58.3 years (range 29 to 87) were enrolled in this prospective, single center study.
Inclusion criteria were as follows: (1) patients at risk for CRC unable to undergo the colonoscopic examination because of the anesthetic risk and co-morbidities; (2) patients at risk for CRC who refused colonoscopy.
We considered as patients at risk for CRC, patients with personal or family history of adenomas or colorectal cancer, but also with digestive symptoms such as bleeding, recent bowel habits change, weight loss, anemia, abdominal pain, positive fecal occult blood test and suspect imaging-abdominal ultrasound, computed tomography (CT)/positron emission CT scan were included in the study.
Majority of patients unwilling to undergo the colonoscopic examination have had a negative experience with a prior colonoscopy (either an incomplete or failed colonoscopy because of the abdominal discomfort). The PillCam Colon 2 examination was proposed as an alternative tool to explore the colon to these patients. Exclusion criteria comprised: (1) patients with pacemakers; (2) patients with suspected digestive stenosis or intestinal occlusion; and (3) patients with dysphagia or swallowing disorders.
The study was approved by the Ethics Committee of the University Hospital of Bucharest and patients signed an informed consent for the investigation. Enrollment started in February 2011.
The second generation PillCam Colon 2 capsule and Rapid reader 7 software were used in this study. The Pillcam Colon 2 capsule is slightly longer than the previous generation with 11.6 mm × 31.5 mm in size. It has been designed to work for at least 10 h and it has a variable frame rate (from 4 to 35 frames/second in order to correctly visualize the mucosa when accelerated peristalsis). The angle of view was increased to 172 degrees in both capsule lenses, thus covering almost 360 degrees of the colonic surface. A new smaller and more ergonomic data recorder with a liquid crystal display allowing real time viewing was developed. It permits a bidirectional communication with the capsule and also is friendlier and easier to use by the patient providing automatic visual and audio signals for procedure activities (boost administration).
All the investigators reading the capsule videos had extensive experience in digestive endoscopy and they had previous experience using the small-bowel capsule. Before the study start a training session was organized by Given Imaging. This 2-d training session included several hours of sessions addressing different issues as preparation, procedure and software utilization. It was followed by a self-assessment test consisting of reading ten colon capsule videos. The first three examinations in the study were performed under supervision from Given Imaging.
Participating patients received written and oral explanations of colonic preparation details. The preparation consisted in a low-residue diet starting 48 h before investigation and a clear liquid diet 24 h before ingestion. A 4 L of split-dose polyethylene glycol (PEG) Fortrans® (Macrogol 4000, Ibsen, France) prep was administered in the evening and 2 h prior to capsule ingestion. Since in Romania oral sodium phosphate is not available, PEG was used as booster. Upon capsule exit from the stomach a first liter of PEG was administered and a second boost of one liter of PEG was administered if the capsule was not excreted 3 h after the first one.
Colon cleanliness was graded using a two point scale. This scale was a development of the original 4-point scale used in previous studies and grades preparation as inadequate (poor or fair on the 4-point scale) or adequate (good or excellent on the 4-point scale)[10]. The cleanliness was assessed in each of the five colon segments (cecum, right colon, transverse, left colon and rectum) and then a general estimate of the entire colon was made.
In all patients the readers were instructed to review the entire CCE examination and additionally significant extra-colonic findings (Figure 1).
The main indication for initial colonoscopy or for the otherwise contraindicated/refused colonoscopy had been: 35 symptomatic patients (abnormal transit 8, abdominal pain 4, anemia or overt bleeding 22, weight loss 1), 29 average and high risk colorectal cancer screening patients (familial 21 or personal history of polyps or cancer 5, acromegaly 1, long standing inflammatory bowel disease 1, screening 1) and 6 patients with abnormal imaging or tumor markers. The indications for referral of the patients are detailed in the Table 1.
| Patient | Sex | Age | Reason | Indication for CCE | Findings | Completion | Preparation |
| 1 | Female | 85 | Suspect CT | Refuse | 3 pedunculated polyps in the descending colon 7-9 mm, voluminous diverticula in the sigmoid | c | a |
| 2 | Female | 45 | Transit troubles (diarrhea), family history | Failure | Diverticula | c | a |
| 3 | Male | 76 | Anemia | Failure | 3 polyps 3-8 mm left colon | c | a |
| 4 | Male | 39 | Family history | Refuse | 4 polyps 3-8 mm left colon | c | a |
| 5 | Male | 52 | Personal history of colorectal polyps | Refuse | 4 polyps 4-8 mm left colon | c | a |
| 6 | Male | 60 | Abdominal pain weight loss | Failure | 6 mm polyp cecum | c | a |
| 7 | Female | 69 | Transit troubles | Refuse | 6 mm polyp right colon, diverticula | c | a |
| 8 | Female | 57 | Personal history of polyps | Failure | 6 polyps 3-5 mm 2 transverse 4 left colon, diverticula | c | a |
| 9 | Male | 80 | Anemia severe, weight loss | Failure | Angiomas | c | a |
| 10 | Male | 53 | Transit troubles | Refuse | Diverticula | c | a |
| 11 | Female | 61 | Family history | Failure | Diverticula | c | a |
| 12 | Female | 58 | Transit troubles (diarrhea) | Refuse | Diverticula | c | a |
| 13 | Male | 54 | Family history (mother, aunt and uncle with CRC) | Refuse | Diverticula | c | a |
| 14 | Female | 65 | Abdominal pain history of resected transverse cancer history of urinary bladder cancer | Failure | Diverticula | c | a |
| 15 | Male | 39 | Family history | Refuse | Diverticula | c | a |
| 16 | Female | 56 | Family history (father with CC at 82) polyps | Refuse | Diverticula | c | a |
| 17 | Male | 58 | Personal history of cancer,colectomy | Refuse | Diverticula | c | a |
| 18 | Male | 31 | Family history( father CRC at 46) | Refuse | Diverticula | c | na |
| 19 | Male | 62 | Screening | Failure | Diverticula peridiverticular inflammation small erosion on the IC valve 3 mm polyp in the cecum | c | a |
| 20 | Male | 69 | Anemia weight loss | Refuse | Diverticula polyp 5 mm in the descedent colon internal hemorrhoids | c | a |
| 21 | Female | 49 | Transit troubles | Refuse | Diverticula small polyp 3 mm left colon some petechiae on the descendent colon | c | na |
| 22 | Male | 75 | Transit troubles | Failure | Diverticula,16 mm ulcerated submucosal mass in the sigmoid | c | a |
| 23 | Male | 59 | Family history | Refuse | Diverticula, 4 mm polyp sessile left colon | c | na |
| 24 | Male | 64 | Family history CRCresection of polyps | Failure | Normal | c | a |
| 25 | Female | 60 | Family history (mother with rectal cancer) | Refuse | Normal | c | a |
| 26 | Female | 55 | Suspect mass on CT | Refuse | Normal | c | a |
| 27 | Female | 77 | Anemia | Failure | Normal | c | a |
| 28 | Male | 64 | Anemia weight loss | Failure | Normal | c | a |
| 29 | Female | 60 | Family history | Refuse | Normal | c | a |
| 30 | Female | 56 | Transit troubles | Refuse | Normal | c | a |
| 31 | male | 36 | Family history, transit troubles | Refuse | Normal | c | a |
| 32 | Female | 39 | Family history | Failure | Normal | c | a |
| 33 | Female | 29 | Anemia, grandmother with colon cancer constipation | Refuse | Normal | c | a |
| 34 | Female | 44 | Anemia | Refuse | Normal | c | a |
| 35 | Male | 59 | Family history (colorectal cancer in the mother at early age) abdominal pain | Failure | Normal | c | a |
| 36 | Female | 39 | Acromegaly | Refuse | Normal | c | a |
| 37 | Female | 42 | Tumoral markers | Failure | Normal | c | a |
| 38 | Female | 59 | Anemia weight loss diarrhea suspect CT | Cardiologist choice | Normal | c | a |
| 39 | Female | 49 | Abdominal pain | Refuse | Normal | c | a |
| 40 | Male | 59 | Transit troubles (diarrhea), family history | Refuse | Normal | c | na |
| 41 | Male | 42 | Family history | Refuse | Normal | c | na |
| 42 | Male | 51 | Family history | Failure | Normal | c | na |
| 43 | Female | 43 | suspect pet scan, ovarian cancer | Failure | Normal | c | na |
| 44 | Male | 34 | Family history (mother and father operated with ccr) | Refuse | Normal | c | na |
| 45 | Female | 66 | Tumoral markers | Failure | Normal | c | na |
| 46 | Female | 65 | Family history | Failure | Normal | c | na |
| 47 | Male | 68 | Bleeding, personal history of polyps | Refuse | Normal | c | na |
| 48 | Female | 65 | Personal history (colon resection) | Refuse | Normal resected colon | c | a |
| 49 | Female | 41 | Anemia, fh | Refuse | Polip cecum < 5 mm | c | a |
| 50 | Male | 65 | Long standing uc, renal transplantation | Failure | Ulcerative colitis, pseudopolyps | c | a |
| 51 | Female | 75 | Anemia, suspect ultrasound exam | Refuse | Small bowel tumor 22 × 22 mm, 6 mm polyp descending | c | a |
| 52 | Female | 56 | Anemia weight loss | Failure | Ulcerated tumor in the cecum | c | a |
| 53 | Female | 65 | Anemia | Failure | Ulcerated tumor in the cecum | c | a |
| 54 | Male | 45 | Abdominal pain | Refuse | Ulceration on the ileon and ileal valve, Crohn's? diverticula | c | a |
| 55 | Female | 78 | Anemia | Failure | 10 right transverse polyps 4-9 mm, angiomas, left side not seen, diverticula | i | na |
| 56 | Female | 45 | Family history | Failure | 13 mm pedunculated polyp transverse colon, diverticula | i | na |
| 57 | Male | 77 | Anemia weight loss | cardiologist Choice | 3 polyps 10 mm and 5 and 4 mm left colon | i | na |
| 58 | Female | 68 | Family history | Failure | 3 polyps 3-4 mm left colon,diverticula | i | na |
| 59 | Female | 84 | Personal history (hemicolectomy for right sided cancer) | Failure | 4 polyps 5-7 mm left colon | i | a |
| 60 | Female | 76 | Family history of CRC ( mother and brother ) | Refuse | 7 mm polyp on the ileo-caecal valve; caecal angiodysplasia; multiple diverticula in the right and left colon | i | a |
| 61 | Female | 87 | Suspect CT and barium enema | Failure | Angiomatosis | i | a |
| 62 | Male | 52 | Bleeding, hematochezia | Refuse | Diverticula | i | na |
| 63 | Male | 58 | Anemia, suspect ct, personal and family history | Failure | gastric cancer, 5 polyps 3-4 mm left side, diverticula | i | a |
| 64 | Male | 75 | Weight loss | Refuse | Normal but cancer discovered after 3 mo | i | na |
| 65 | Male | 73 | Anemia weight loss | Refuse | Diverticula battery depleted | I battery | na |
| 66 | Female | 61 | Anemia | Refuse | Cancer | Impaction on cancer | a |
| 67 | Male | 38 | Anemia | Failure | Cancer two tumors | Impaction on cancer | na |
| 68 | Female | 61 | Anemia, weight loss, diarrhea | Failure | Impaction on radiation enteritis stenosis | Impaction on radiation enteritis | |
| 69 | Male | 60 | Family history | Refuse | Impaction | Retention gastric | |
| 70 | Male | 65 | Anemia melena, Normal endoscopy | Cardiologist choice | Impaction | Retention small bowel |
The indication of capsule examination was: refusal of a colonoscopy in 37 patients, previous incomplete colonoscopy (mostly technical failures of initial colonoscopy) in 30 patients or unable to perform colonoscopy (the examination risks-cardiovascular or anesthetic were considered excessive by their own physicians) in 3 patients.
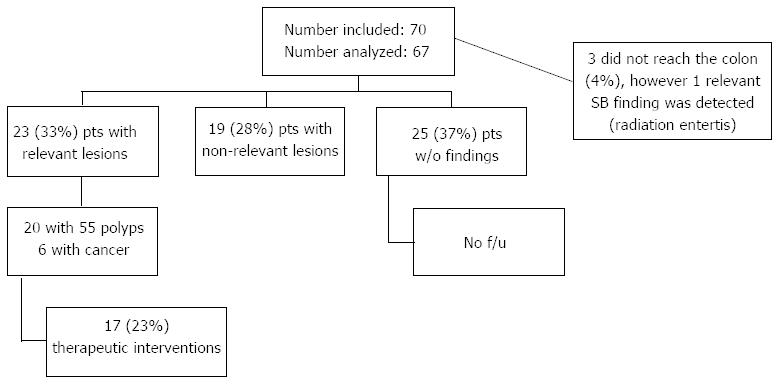
In three patients the procedure failed because the capsule was not functioning when it entered the colon. In the remaining 67 patients a significant diagnosis was made in 23 (34%, 95%CI: 21.6%-44.1%). The significant lesions reported were: polyps > 6 mm in five patients, ≥ 3 polyps in 10 patients, multiple colonic angiomas in 2 patients, colon cancer in 4 patients, other digestive cancers in 2 patients, a newly discovered Crohn’s disease in 1 patient and radiation enteritis in another. A total of 19 patients had insignificant lesions (17 with diverticulosis, 1 with ulcerative colitis and inflammatory pseudopolyps and 1 with a < 6 mm polyp).
Twenty five patients had no findings with normal colonic examinations. Fifty-five colonic polyps were identified by CCE in twenty patients. In the 15 patients with polyps over 6 mm or more than 3 polyps we identified 50 polyps with a median size of 5.8 mm (range 3 to 13 mm) and a median number of 3.5 polyp/patient (range 1 to 10), with locations in the right colon (3), transverse colon (13), left colon and rectum (34). We found 5 polyps < 6 mm in five patients (2 polyps located in the right colon and 3 in the left colon).
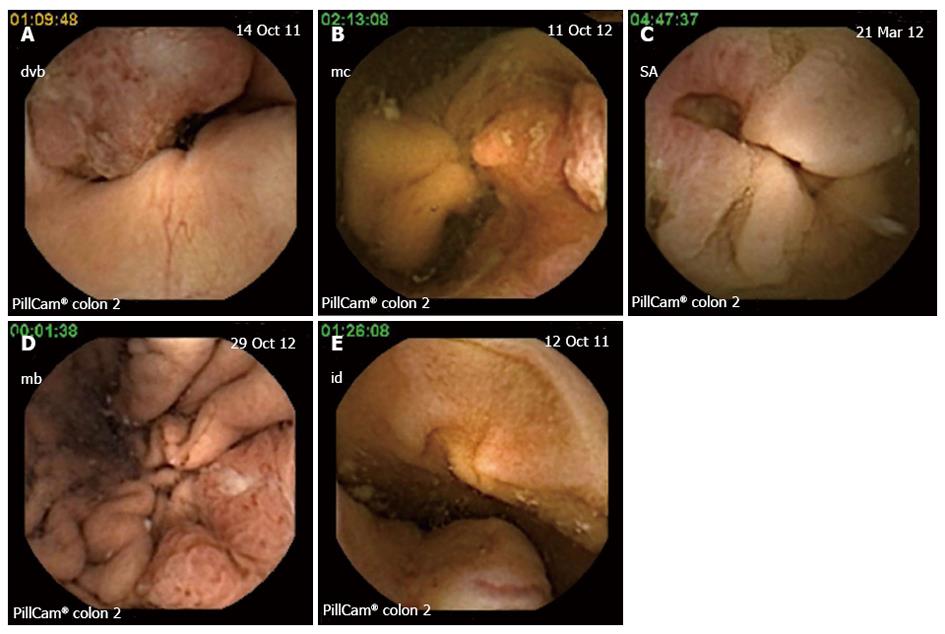
Four patients had colon tumors detected by CCE: (1) patient with two synchronous lesions in the cecum and ascending colon, (2) patients with ulcerated cecal tumors (Figure 2A and B) and 1 patient with a left angle stenotic tumor (Figure 2C). Two other digestive tumors were discovered by the CCE examination. In one patient with iron deficiency anemia, suspect CT scan (abdominal mass) and failure of colonoscopy an ulcerated lesion was discovered by capsule in the stomach. An upper endoscopy with biopsies established the diagnosis of undifferentiated gastric cancer (Figure 2D). In another patient with anemia and suspect imaging (mass seen on ultrasound) and refusing a colonoscopy an ulcerated tumor in the small bowel was visualized at capsule (Figure 2E).
In one of the patients with capsule impaction in the small bowel, we made the diagnosis of radiation enteritis which was considered significant. For the other two patients where capsule did not reach the colon while functioning, no significant lesions were described in the examined segments.
Bowel cleanliness was reported as adequate (good or excellent) in 48 of cases (72%, 95%CI: 60.8%-82.4%) and inadequate (fair or poor) in 19 cases (28%, 95%CI: 17.6%-39.1%). In the three cases where capsule did not reach the colon we could not analyze the preparation.
The capsule excretion rate in 12 h was 77% with 54 patients having a complete examination. The median colonic transit time CTT was 189 min (range 3 to 665 min) with important differences between patients. The rectum was not explored during CCE procedure, in 16 patients (23%, 95%CI: 13.7%-34.1%). Of these 16 patients who did not have a complete capsule procedure, in 3 patients (4%) the capsule did not reach the colon at all. In 11 cases recording ceased in the left colon and in 2 it impacted above tumors of the right and left colon angle, respectively. In 9 of these 11 patients the capsule indication was a failure of a previous colonoscopy so we considered having a complete colonic examination.
All patients but two eliminated the capsule in the following 48 h. A true capsule retention (capsules remaining in the digestive tract more than 14 d and extracted during surgical treatment of the lesions) was seen only in 2 patients due to digestive stenosis. First impaction occurred in an ileal stenosis related to radiation enteritis. This patient was referred from another hospital for suspicion of colon cancer in the descending colon after a failed colonoscopy with impossibility to pass the sigmoid. She had no symptoms suggestive of a digestive stenosis or occlusion but a history of irradiation 24 years ago for uterine cancer. The other case was an impaction in a stenotic tumor of the left colonic angle in a patient referred for anemia and transit troubles and refusing colonoscopy. In both patients surgery was decided based on capsule findings and was successful and without complications and realized in the following month.
We encountered another capsule transient impaction above a tumoral colonic stenosis in a young patient referred for iron deficiency anemia where two lesions of the cecum and right colonic angle were visualized during the examination. The patient eliminated the capsule in the following day. He had surgery after a complete pre operative check up including colonoscopy and CT scan which confirmed the two synchronous lesions. Besides the patient with radic ileal stenosis, the other two where the capsules did not reach the colon while working, excreted the after 48 h without complications. In one patient with a history of colon cancer in both parents and refusing colonoscopy the capsule remained in the stomach during the entire battery lifetime. He refused an upper endoscopy to push the capsule. He remained asymptomatic during and after capsule passage. The other patient was morbidly obese and confined to bed and the capsule remained in the small bowel until battery depletion.
Seventeen patients (74%) out of the 23 with relevant lesions diagnosed by CCE agreed to have a therapeutic intervention. The 4 patients detected with colon tumors had successful surgery. Only 2 of them had colonoscopies before surgery, for the other 2 patients the surgical indication being decided based single on CCE results. The capsule findings were confirmed after surgery. Diagnosis of adenocarcinoma was established in all cases and the tumor location was similar to the capsule findings. One patient detected with small bowel tumor had surgery after the CCE and an ulcerated gist was removed. For the gastric ulcerated lesion visualized by capsule, an upper endoscopy with biopsies was realized. After histological confirmation of undifferentiated gastric cancer, the patient had a subtotal gastric resection.
In the two patients with severe iron deficiency anemia and multiple hospitalizations for transfusions and where previous colonoscopies failed, the CCE made the diagnosis of multiple angiomas. Before CCE both patients had extensive check ups including upper endoscopies, failed colonoscopies, CT scans and barium contrast enemas and they have at least three hospitalizations only in our institution. After CCE repeated séances of argon plasma coagulation were realized with a great deal of improvement of their anemia. In order to reach the cecum a single balloon enteroscope was used for one patient and a variable stiffness colonoscope was used for the other. Six patients with relevant lesions which previously denied colonoscopy accepted the examination after a discussion of the CCE results. Colonoscopy confirmed the findings of the CCE and polypectomy was performed in all cases.
In a patient with a suspicion of locally invading cecal tumor on CT scan, the CCE ruled out this diagnosis and showed only three colonic polyps one in the cecum and two in the descending colon. In this case the CCE had an important role in the clinical decision since it ruled out a colonic cancer. After careful examination of the imaging; exploratory laparotomy established a diagnosis of abdominal wall sarcoma was established. She had surgery soon afterwards. No colonoscopy for the three left side polyps was realized. The newly diagnosed Crohn’s disease patient had a complete check up and he is currently under immune modulator therapy.
We had one clinical failure revealed by the follow up, 4 mo after CCE. A 76-year-old patient with family history and abnormal transit who refused colonoscopy had an incomplete colon examination by CCE caused by poor visualization due to low compliance to the preparation and the booster regimen. He refused a rectoscopy after CCE. Since he remained symptomatic he agreed to have a rectoscopy which revealed a small ulcerated rectal tumor. This patient had successful surgery after pre operatory radiotherapy. Six patients either refused colonoscopy and polypectomy or decided to postpone the procedure. At the moment they are followed up in our center.
The patients included in the study had the indication of a colonoscopy that either failed or was refused. When offered the alternative of having a CCE examination all the 70 patients accepted it, although they were aware that the preparation regimen was more difficult than for a classic colonoscopy. Moreover the examination was subjectively appreciated by all patients as being non invasive and harmless and 65 of them where willing to have the next surveillance exam by CCE.
Capsule ingestion went smoothly in all patients. Although most patients had to ingest a total of six liters of PEG (preparation and boosters) no electrolyte disturbances or adverse effects related to bowel preparation were recorded. No other side effects related to capsule were encountered.
We had one CCE technical failure due to a recorder dysfunction which required another examination.
The existing national CRC screening programs are far from perfect due to different issues: lack of a universal screening policy despite recommendations, lack of uniform measures in all countries, cost issues. One major problem is the disappointingly low number of patients accepting the current screening tools. Furthermore is not negligible that a variable proportion (4%-20%) of patients will have an incomplete colonoscopy although the rate of completeness is as high as 97% in expert centers[4].
After an incomplete examination with a standard adult colonoscope different approaches are available: variable stiffness colonoscope, use of gastroscope, single or double balloon enteroscopy (available in some centers). Changing the centre or the endoscopist is an alternative. However a first failed colonoscopy is significantly associated with a lower cecal intubation rate at further attempts, particularly when stopped in the sigmoid colon[4].
Radiological procedures have been tested and they are proposed as a potential screening test in the average risk population[11], for high risk patients’ colonoscopy remaining the first option. For patients with colonoscopy failure or contraindication, radiological imaging is an option recommended by current guidelines[11].
The use of double contrast barium enema (DCBE) was disappointing considering the low sensitivity for polypoid lesions and adenomas, when compared to colonoscopy or CTC[12]. In a recent Italian meta-analysis, DCBE showed statistically lower sensitivity and specificity than CTC for detecting colorectal polyps ≥ 6 mm, and its use as an alternative imaging test is appropriate only when CTC is not available[12].
Two studies reported varying results using computed CTC after a failed or an incomplete colonoscopy[13,14], with an estimated sensitivity of 88% for advanced neoplasia ≥ 10 mm. Radiation exposure remains a concern despite the evolution of technique and improvement of examination protocols. The cost effectiveness of a CTC based screening program is debatable as the medical and economic impact of extra colonic findings remains unknown[15]. We could not make a direct comparison in our population of patients, since CTC is not reimbursed by the Romanian health system and its availability is very limited. The current ESGE capsule endoscopy guidelines take into consideration the utilization of CCE after failure or refuse of colonoscopy. According to these guidelines, CCE is feasible and safe and appears to be accurate when used in average-risk individuals and in high risk patients for whom colonoscopy is inappropriate or not possible. For these patients the use of CCE could be an alternative[9].
We report the Pillcam Colon 2 use in high risk patients unwilling or unable to perform colonoscopy. Therefore we lack the comparison with colonoscopy which is the gold standard. The introduction of diagnostic utility index and the careful follow-up of the patients partially solved this issue. Clinical significant lesions were seen by Pillcam Colon 2 in 23 patients out of 67 analyzed (34%) CCE had a high clinical impact as endoscopic or surgical treatment was proposed in all these cases based on capsule results and seventeen patients (74%) of the 23 with relevant lesions agreed to and had a therapeutic intervention (Figure 1).
Complete colorectal examination was realized by CCE in 54 patients (77%, 95%CI: 67.3%-86.94%). The rate of complete examinations observed in our group is lower than in the study of Spada et al[8] of 88% but much like the findings of Eliakim et al[6] who reported a capsule egestion rate of 74% in their first generation capsule study. Several factors may have influenced the progression rate: in the absence of classic sodium phosphate boosters unavailable on the local market, the use of Macrogol as a booster has been a factor affecting the transit times. Also our study population included patients with previous difficult colonoscopies or with various co-morbidities and bed confined patients. The presence of fixed sigmoid loops in patients with previous colonoscopy failure might have contributed to slow transit times. Also in three patients with incomplete CCE examination, this was due to impaction over significant lesions (one post-radic stenosis and two cancers) during the procedure. Compared with CTC, CCE has the intrinsic advantage of directly visualizing the colonic mucosa. This may be very important as clinically relevant lesions like angiectasias or flat adenomas are missed by CTC and are easily visible in capsule endoscopy. This is confirmed in our study where capsule endoscopy established the definitive diagnosis of multiple angiomas in two patients who had previous CT scans and barium enemas in several occasions.
In a recently published multicenter (17 hospitals and private practices) study using first generation Pillcam Colon 1 in patients with failure or contraindications to colonoscopy, the CCE showed positive findings in 36 patients out of 107 analyzed (diagnostic yield 33.6%). The Pillcam Colon 1 was considered as having a high clinical impact as in 21% of patients a medical or surgical treatment was proposed. In this study the colon examination by CCE was complete in 83.2% of cases[16]. Our results are comparable. However it is a single center study with a different study design. Also the classical boosts with sodium phosphate where not available for our population leading to the lower excretion rates.
In our study the acceptability of the examination by CCE was extremely high. All patients with a previous failed colonoscopy proposed to take part in the study accepted the CCE examination. The method was perceived as non invasive and harmless by all patients. Moreover the vast majority of patients with significant findings, either failure or refusal of a colonoscopy, agreed to perform a therapeutic gesture (implying colonoscopy) after the discussion of the CCE findings.
The PillCam Colon 2 appears to be effective for the detection of clinically relevant lesions with great acceptability rate, and it might be considered as a useful tool for colorectal imaging in patients unable or unwilling to undergo colonoscopy. Further studies are necessary to validate the best approach to these patients.
The Given Imaging Research Grant supports innovative, original research in Gastroenterology with substantial involvement of capsule endoscopy and is awarded yearly by the European Society of Gastrointestinal Endoscopy. The project “Role of PillCam Colon 2 capsule in patients at risk of CRC unable or unwilling to perform colonoscopy” was awarded with the 2010 grant. The study design, data analysis, results and conclusions of the article are exclusively the investigators work. Given Imaging supported the study, by donating the capsules and loan of equipment.
There is growing evidence that colon capsule endoscopy is a reliable and well tolerated diagnostic method. A lot of technical improvements were made to the capsule endoscopy, including a second generation, more performant, colon capsule.
Since the introduction of the second generation Pillcam Colon 2 very few studies addressed its use after colonoscopy failure or refusal.
This is a 70 patients’ pilot study using the second generation of PillCam Colon capsule endoscopy to detect colon cancers as well as other tumors in the gastrointestinal (GI) tract. They included a heterogeneous population at risk of colorectal cancer that either failed or refused colonoscopy. This study indicated that PillCam Colon 2 capsule endoscopy is feasible and of high acceptance by patients.
This study suggests that PillCam colon 2 capsule endoscopy may eventually used for population-wide colon cancer screening, although more cost effectiveness studies are needed.
Pillcam Colon 2 capsule has 11.6 mm × 31.5 mm in size and has been designed to work for at least 10 h with a variable frame rate (from 4 to 35 frames/second in order to correctly visualize the mucosa when accelerated peristalsis). The angle of view was increased to 172 degrees in both capsule lenses, thus covering almost 360 degrees of the colonic surface.
This is an interesting manuscript describing a pilot lot study using the second generation of PillCam capsule endoscopy to detect colon cancers as well as other tumors in the GI tract. Although case controlled studies are ultimately needed to demonstrate the sensitivity and specificity of PillCam capsule endoscopy, this pilot study indicated that PillCam capsule endoscopy is feasible and of high acceptance by patients. This study suggests that PillCam capsule endoscopy may eventually used for population-wide colon cancer screening. This is a descriptive paper on a new generation colon capsule. Since no comparison with the gold standard technique (colonoscopy) is made specificity and sensitivity of the method could not be assessed. One important point is that lesions outside the colon were found and this point should be underlined.
P- Reviewers: De Nardi P, Wang ZH S- Editor: Cui XM L- Editor: A E- Editor: Wu HL
| 1. | McClements PL, Madurasinghe V, Thomson CS, Fraser CG, Carey FA, Steele RJ, Lawrence G, Brewster DH. Impact of the UK colorectal cancer screening pilot studies on incidence, stage distribution and mortality trends. Cancer Epidemiol. 2012;36:e232-e242. |
| 2. | Stock C, Pulte D, Haug U, Brenner H. Subsite-specific colorectal cancer risk in the colorectal endoscopy era. Gastrointest Endosc. 2012;75:621-630. |
| 3. | Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2012. Ann Oncol. 2012;23:1044-1052. |
| 4. | Dafnis G, Granath F, Påhlman L, Ekbom A, Blomqvist P. Patient factors influencing the completion rate in colonoscopy. Dig Liver Dis. 2005;37:113-118. |
| 5. | Morini S, Zullo A, Hassan C, Lorenzetti R, Campo SM. Endoscopic management of failed colonoscopy in clinical practice: to change endoscopist, instrument, or both? Int J Colorectal Dis. 2011;26:103-108. |
| 6. | Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963-970. |
| 7. | Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38:971-977. |
| 8. | Spada C, Hassan C, Munoz-Navas M, Neuhaus H, Deviere J, Fockens P, Coron E, Gay G, Toth E, Riccioni ME. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581-589.e1. |
| 9. | Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527-536. |
| 10. | Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam COLON capsule: a reliability study. Endoscopy. 2011;43:123-127. |
| 11. | Yee J, Rosen MP, Blake MA, Baker ME, Cash BD, Fidler JL, Grant TH, Greene FL, Jones B, Katz DS. ACR Appropriateness Criteria on colorectal cancer screening. J Am Coll Radiol. 2010;7:670-678. |
| 12. | Sosna J, Sella T, Sy O, Lavin PT, Eliahou R, Fraifeld S, Libson E. Critical analysis of the performance of double-contrast barium enema for detecting colorectal polyps > or = 6 mm in the era of CT colonography. AJR Am J Roentgenol. 2008;190:374-385. |
| 13. | Yucel C, Lev-Toaff AS, Moussa N, Durrani H. CT colonography for incomplete or contraindicated optical colonoscopy in older patients. AJR Am J Roentgenol. 2008;190:145-150. |
| 14. | Sali L, Falchini M, Bonanomi AG, Castiglione G, Ciatto S, Mantellini P, Mungai F, Menchi I, Villari N, Mascalchi M. CT colonography after incomplete colonoscopy in subjects with positive faecal occult blood test. World J Gastroenterol. 2008;14:4499-4504. |
| 15. | de Haan MC, Halligan S, Stoker J. Does CT colonography have a role for population-based colorectal cancer screening? Eur Radiol. 2012;22:1495-1503. |