Published online Jun 16, 2012. doi: 10.4253/wjge.v4.i6.218
Revised: April 27, 2012
Accepted: May 27, 2012
Published online: June 16, 2012
Endoscopic ultrasound (EUS) devices were first designed and manufactured more than 30 years ago, and since then investigators have reported EUS is effective for determining both the staging and the depth of invasion of esophageal and gastric cancers. We review the present status, the methods, and the findings of EUS when used to diagnose and stage early esophageal and gastric cancer. EUS using high-frequency ultrasound probes is more accurate than conventional EUS for the evaluation of the depth of invasion of superficial esophageal carcinoma. The rates of accurate evaluation of the depth of invasion by EUS using high-frequency ultrasound probes were 70%-88% for intramucosal cancer, and 83%-94% for submucosal invasive cancer. But the sensitivity of EUS using high-frequency ultrasound probes for the diagnosis of submucosal invasive cancer was relatively low, making it difficult to confirm minute submucosal invasion. The accuracy of EUS using high-frequency ultrasound probes for early gastric tumor classification can be up to 80% compared with 63% for conventional EUS, although the accuracy of EUS using high-frequency ultrasound probes relatively decreases for those patients with depressed-type lesions, undifferentiated cancer, concomitant ulceration, expanded indications, type 0-I lesions, and lesions located in the upper-third of the stomach. A 92% overall accuracy rate was achieved when both the endoscopic appearance and the findings from EUS using high-frequency ultrasound probes were considered together for tumor classification. Although EUS using high-frequency ultrasound probes has limitations, it has a high depth of invasion accuracy and is a useful procedure to distinguish lesions in the esophagus and stomach that are indicated for endoscopic resection.
- Citation: Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4(6): 218-226
- URL: https://www.wjgnet.com/1948-5190/full/v4/i6/218.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i6.218
Endoscopic ultrasound (EUS) devices were first designed and manufactured in the early 1980s. Since then, EUS has been adapted not only for pancreatic lesions but also for gastrointestinal and perigastrointestinal lesions, such as gastrointestinal cancers, gastrointestinal stromal tumors, and abdominal and mediastinal lymphadenopathy. Some investigators have reported EUS is effective for the staging of esophageal and gastric cancers[1,2], and EUS is also useful for determining the depth of invasion of early esophageal and gastric cancers. Ever since Gotoda et al[3] described the incidence of lymph node metastasis from early gastric cancer, and with the development of endoscopic submucosal dissection (ESD), many early gastric cancer lesions have been resected endoscopically. In addition, ESD has recently been adapted for excision of esophageal lesions. It is important to accurately estimate the depth of the lesion before endoscopic resection of early esophageal and gastric cancers; a vague estimation of lesion depth may allow residual cancer to remain, leading to recurrences and additional resections.
We review the present status of, the methods used for, and the findings of EUS using high-frequency ultrasound probes for diagnosing and staging early esophageal and gastric cancer.
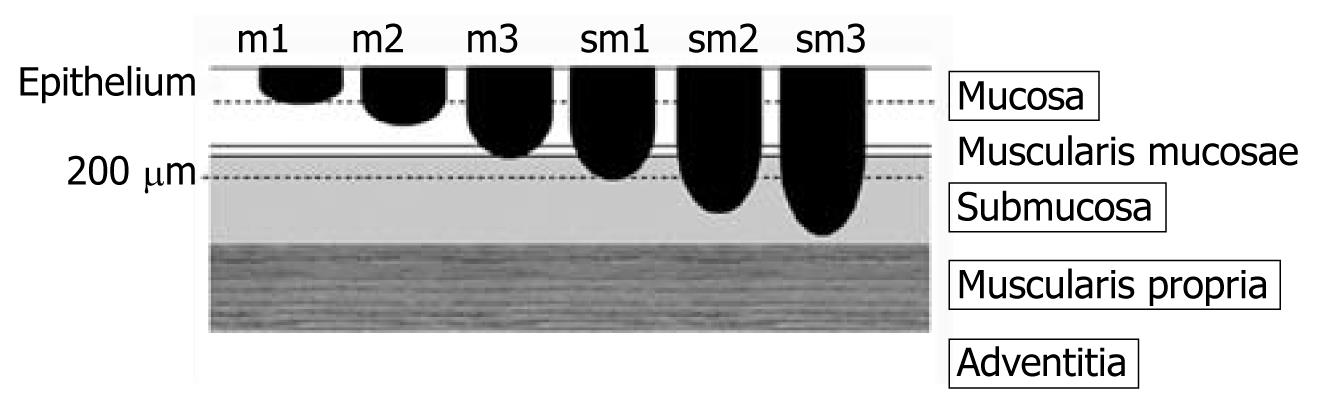
The depth of early esophageal squamous cell cancer invasion is classified according to six categories that range from only penetrating the epithelium to reaching the proper muscle layer: cancer limited to the epithelium is described as m1; cancer limited to the lamina propria is m2; invasion reaching the muscularis mucosa or invading the muscularis mucosa is m3; invasion of the submucosa less than 200 μm in the endoscopically resected specimen or invasion of the first third of submucosa is sm1; invasion of the submucosa by more than 200 μm in the endoscopically resected specimen or invasion of the second third of submucosa is sm2; and that reaching the proper muscle layer is classed as sm3[4] (Figure 1). The rates of lymph node metastasis in m1 and m2 cancers are estimated at less than 5%, while those of m3 and sm1 cancers are 12%-27%, and those of sm2 and sm3 cancers are 36%-46%[5]. This evidence suggests that invasion depth confined to m1 or m2 regions is a good indication for excision using a procedure such as endoscopic mucosal resection (EMR) or ESD. Therefore, an accurate determination of invasion depth will help distinguish indicated lesions from contra-indicated lesions.
Because EUS using high-frequency ultrasound probes is more accurate than conventional EUS in the evaluation of the depth of invasion of early esophageal carcinoma[6], usually EUS using high-frequency ultrasound probes is performed to evaluate tissue penetration. In previous reports, the accuracies of the depth of invasion measurements by ultrasound probes were 70%-88% for intramucosal cancer, and 83%-94% for submucosal invasive cancer[6-8]. Murata et al[4] reported the extent of cancer invasion had been correctly determined in 81% of m1 and m2 lesions, in 60% of m3 and sm1 lesions, and in 87% of sm2 and sm3 lesions. But in another report, the sensitivity for submucosal invasive cancer was only 48%[9], and overall accuracy, sensitivity, and specificity to differentiate submucosal invasive cancers from intramucosal cancers were 74%, 62%, and 77%, respectively[10]. Especially, May et al[9] reported the diagnostic accuracy was not yet satisfactory with submucosal invasive cancers located at the esophagogastric junction (EGJ) or with infiltration of the first third of the submucosa. In addition, it is difficult to distinguish between cancer invasion and inflammatory cell infiltration[4]. Thus, although EUS can distinguish between definite intramucosal cancers and definite submucosal invasive cancers, it is relatively difficult to confirm minute submucosal invasion even when using high-frequency probes.
Although water introduced normally into the esophagus can provide acoustic coupling for EUS, it is difficult to submerge the target lesion because the water flows off easily. To solve this problem, some investigators developed EUS devices utilizing either a water-filled balloon method[11], a device for continuous irrigation of water[12], or a jelly-filled method[13]. However, the balloon interferes with the diagnosis of m1 and m2 cancer, so the water or the jelly-filled methods may be preferred[4,13,14]. In our institute, we use an endoscope with a water-jet system to provide irrigation.
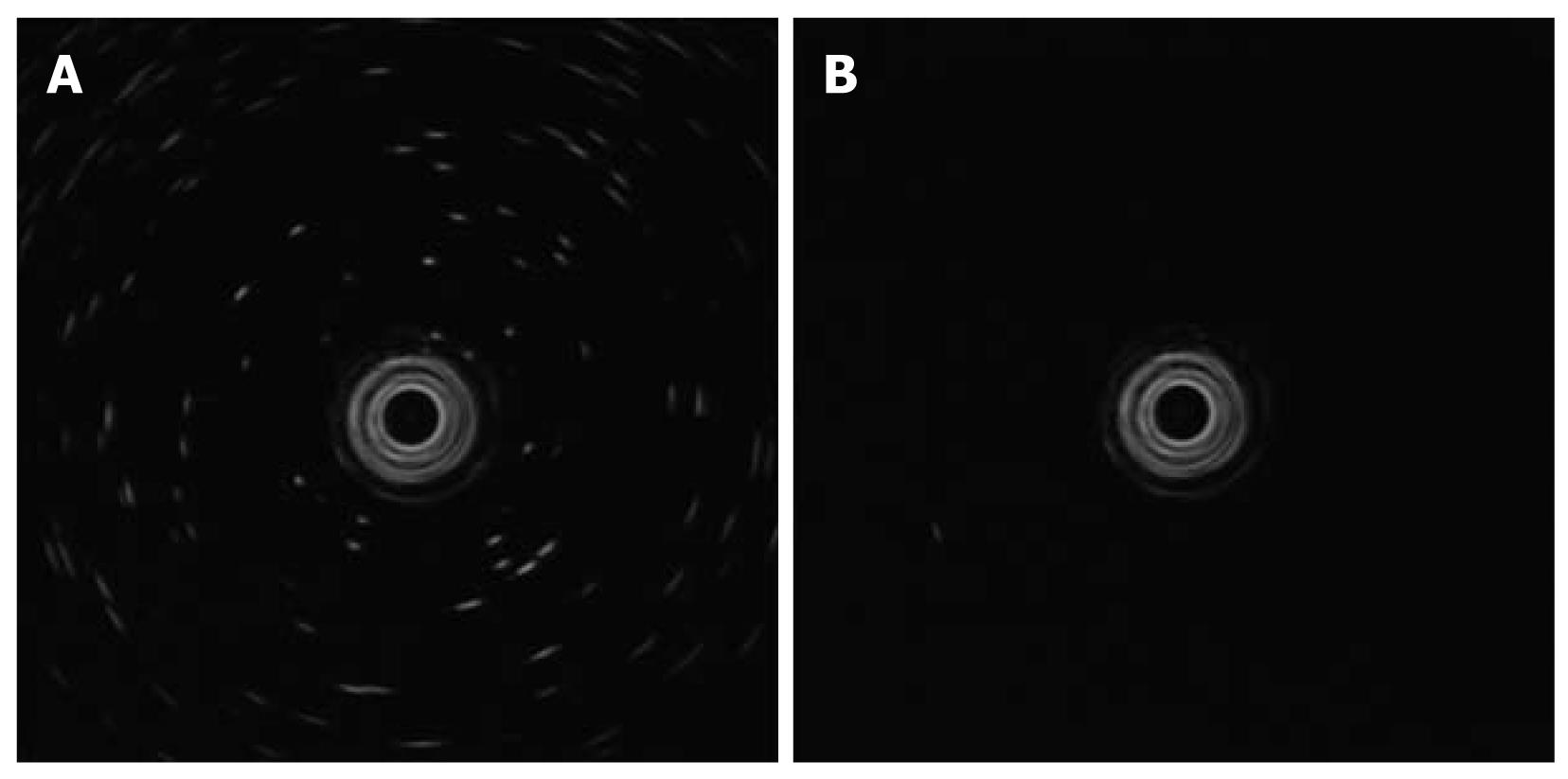
Our EUS procedure is performed using a 20 MHz ultrasound probe (UM-3R; Olympus Optical Co, Ltd, Tokyo, Japan) with an endoscopic ultrasound system (EU-M2000; Olympus) through a forward-viewing endoscope with a water-jet system (GIF-Q260J; Olympus). Deaerated water is boiled at least one day before the procedure and then allowed to rest to remove any bubbles. This preparation is necessary to achieve accurate data from the EUS procedure (Figure 2A and B).
For premedication, scopolamine butylbromide as an antispasmodic and midazolam as a sedative, and, occasionally, pethidine hydrochloride as an analgesic, are administered to the patients. After the patients have received the premedication, their blood pressure, heart rate, and arterial oxygen saturations are monitored until an hour after the procedure is finished.
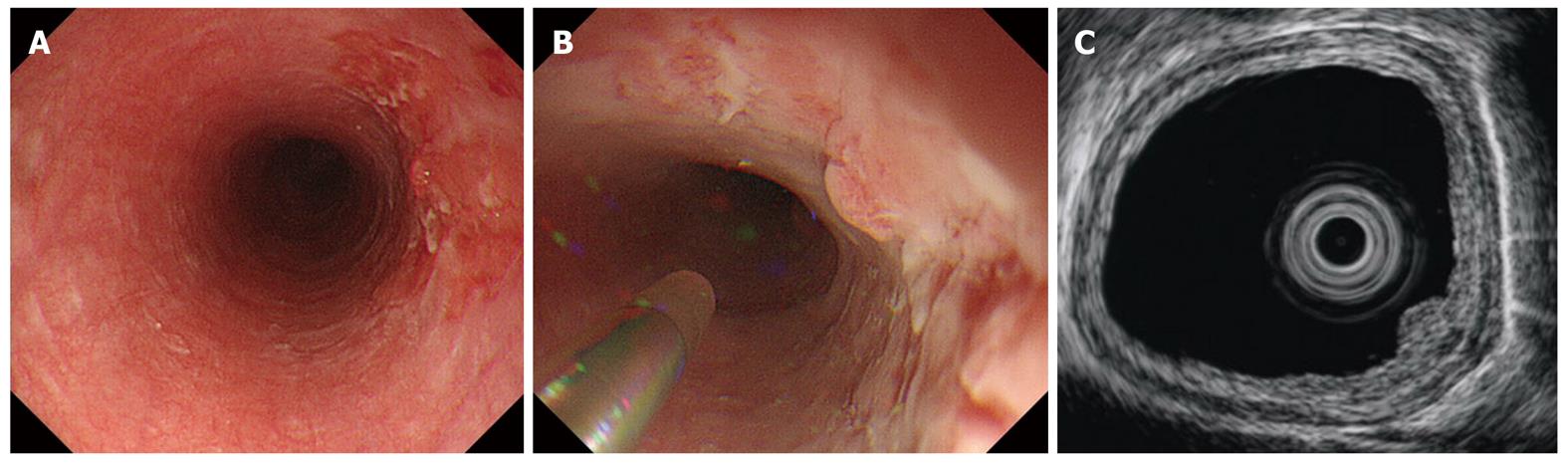
With patients lying in a left lateral decubitus position, we insert an endoscope into the esophagus and attempt to visualize a lesion; if one is discovered, mucus and saliva on the lesion are washed away gently (Figure 3A). An ultrasound probe is inserted through an instrument channel and we begin irrigating with deaerated water through a water jet channel operated by an assistant, while we watch the lesion directly. After sufficient deaerated water is present to act as an acoustic coupling medium, ultrasound scanning is begun (Figure 3B and C). Technically, it is difficult to scan lesions which are located near EGJ precisely because the lower esophagus is sometimes spastic or not distended.
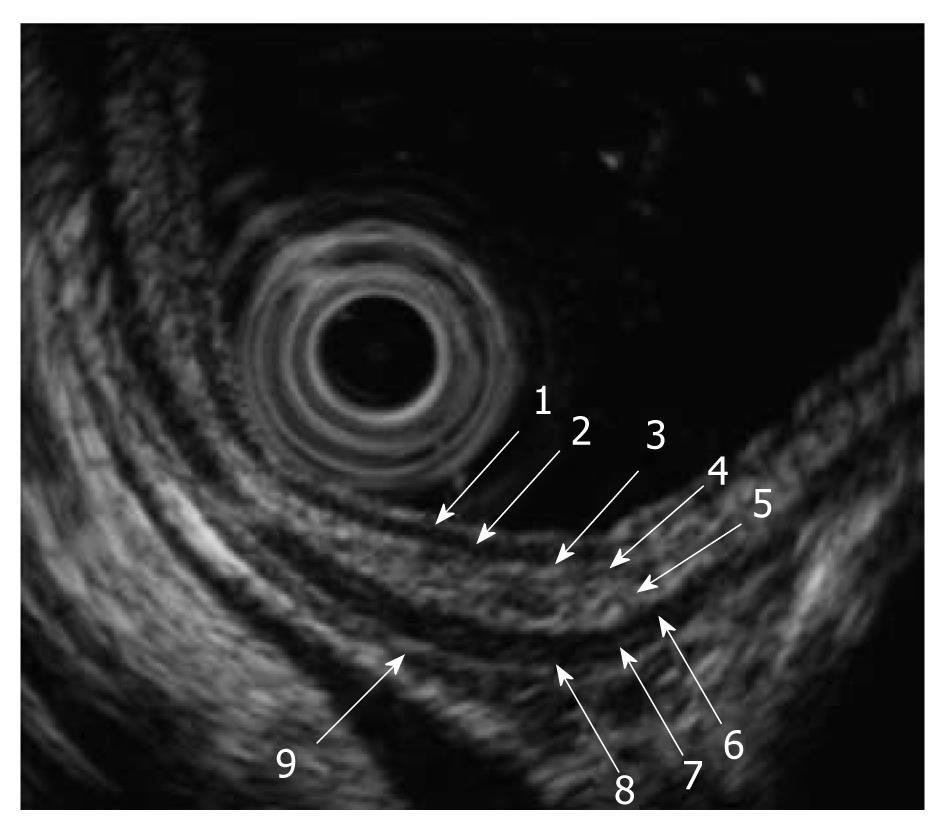
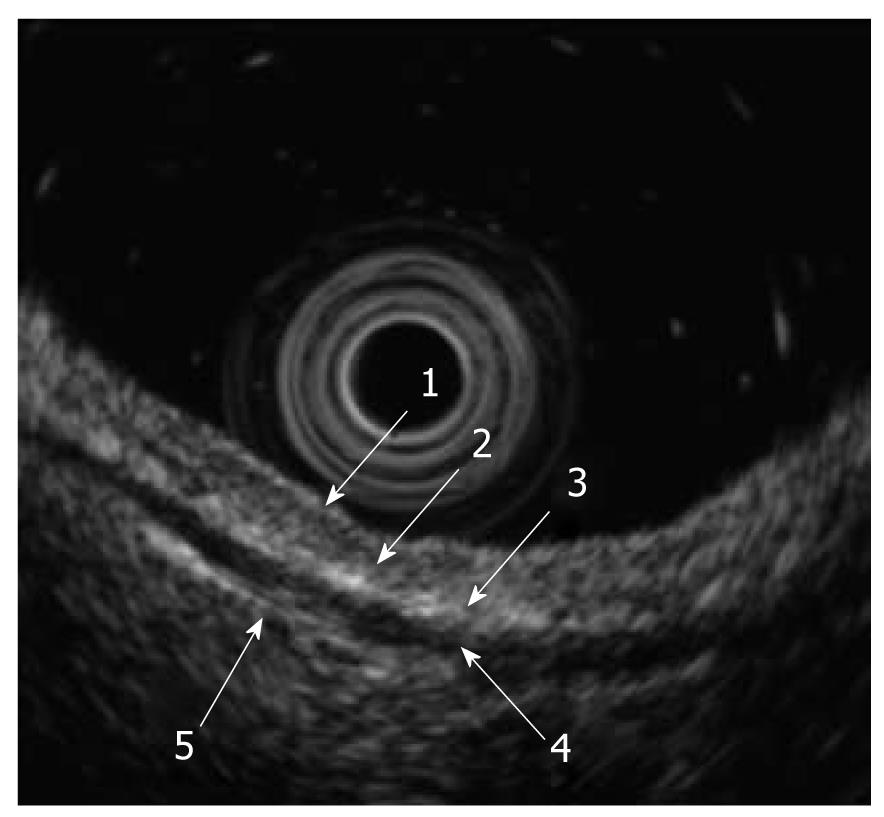
When we use high-frequency ultrasound probes, the esophageal wall is delineated as nine alternating high- and low-echo layers[4]. The first to the fourth layers represent the mucosa, with the first and second layers corresponding to the epithelium, the third layer to the lamina propria, and the fourth layer to the muscularis mucosa. The fifth layer is the submucosa. The sixth to eighth layers are the proper muscle layers, with the sixth layer corresponding to the circular muscle, the seventh to the connective tissue and interface, and the eighth layer to the longitudinal muscle. The ninth layer is the adventitia (Figure 4).
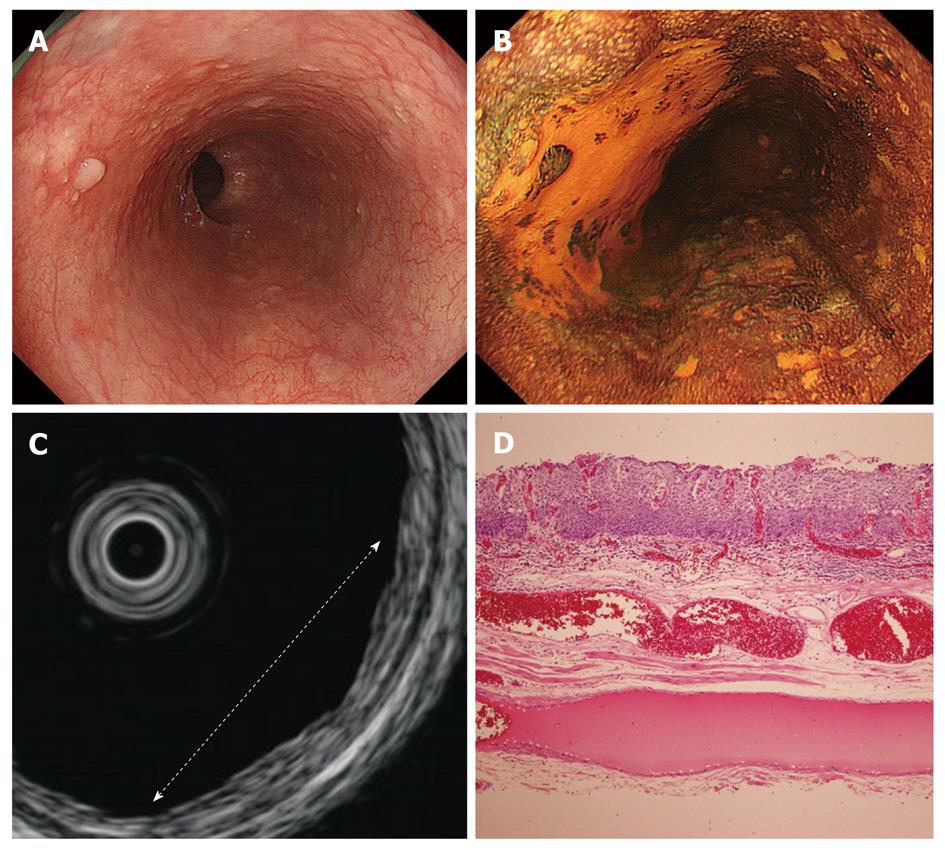
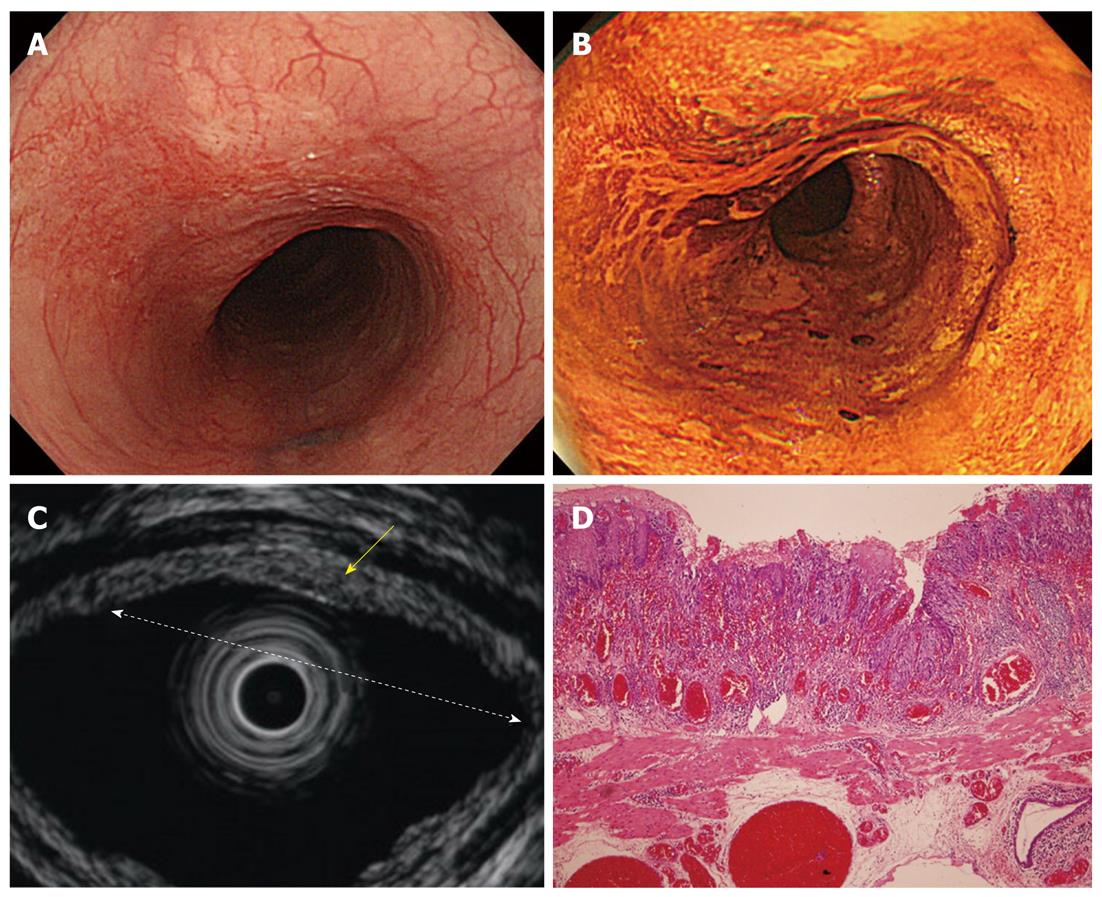
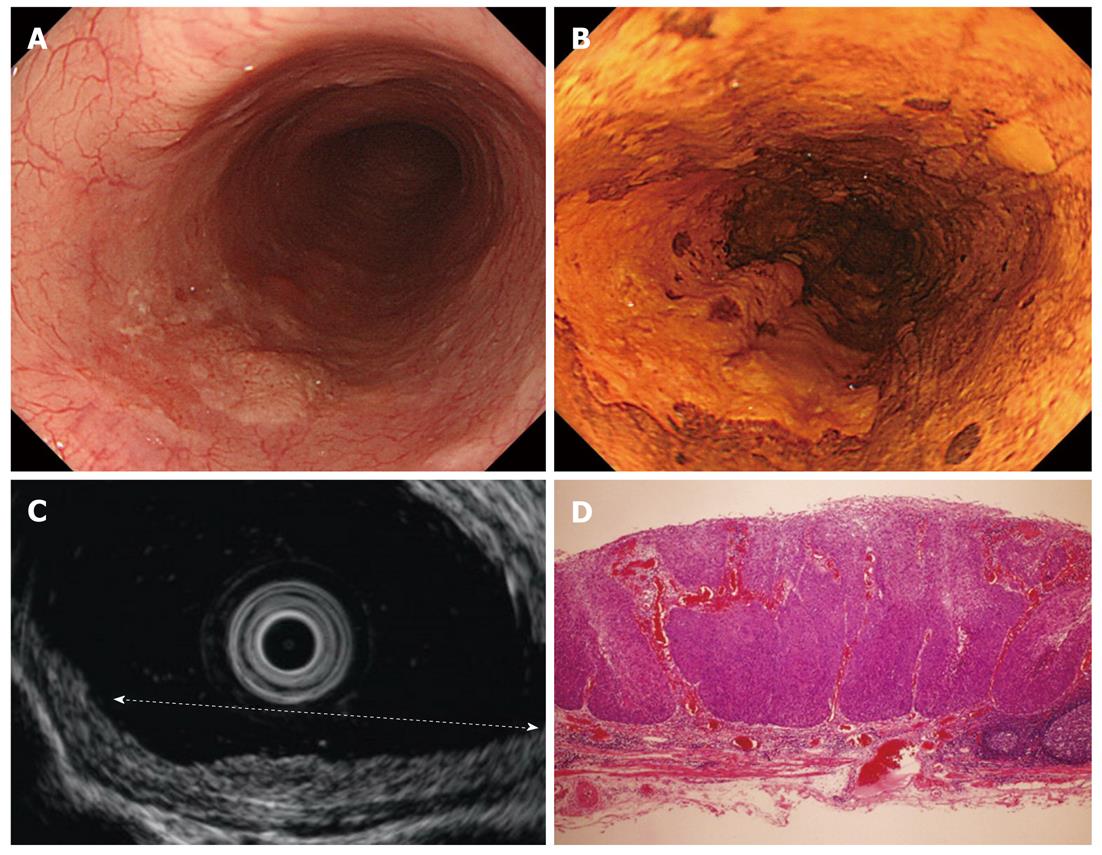
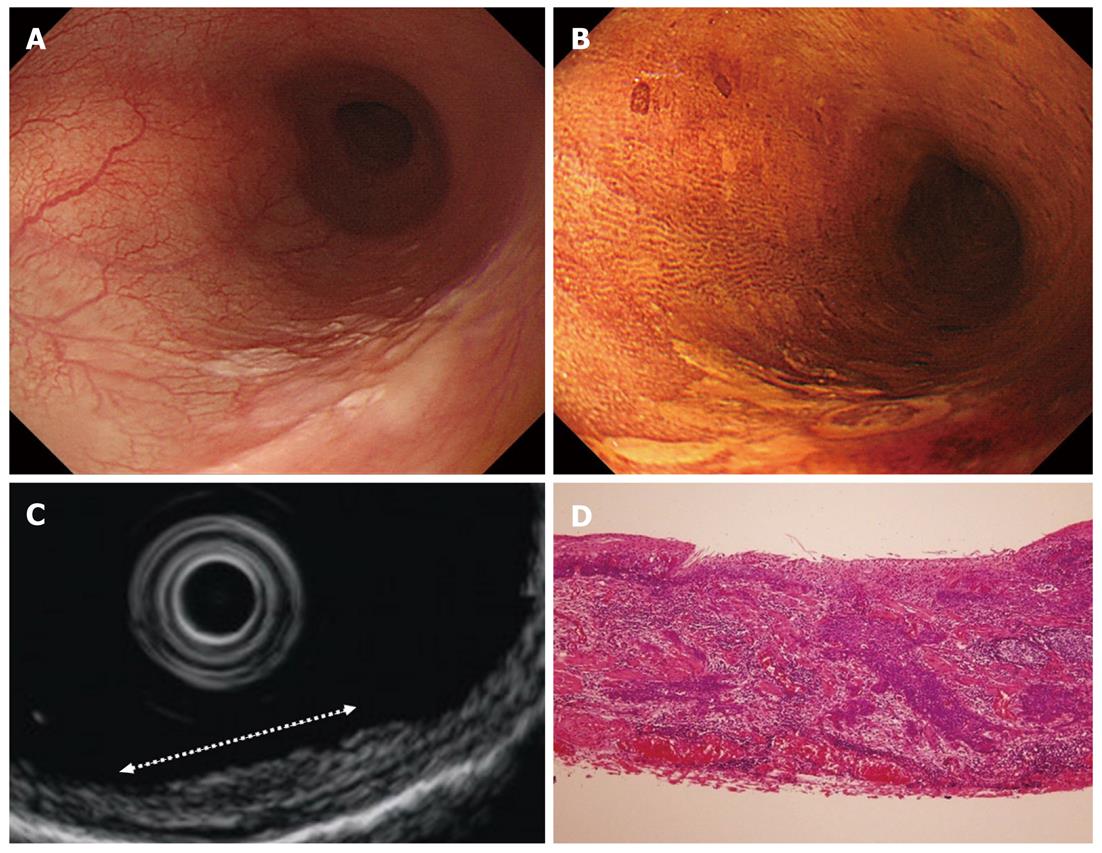
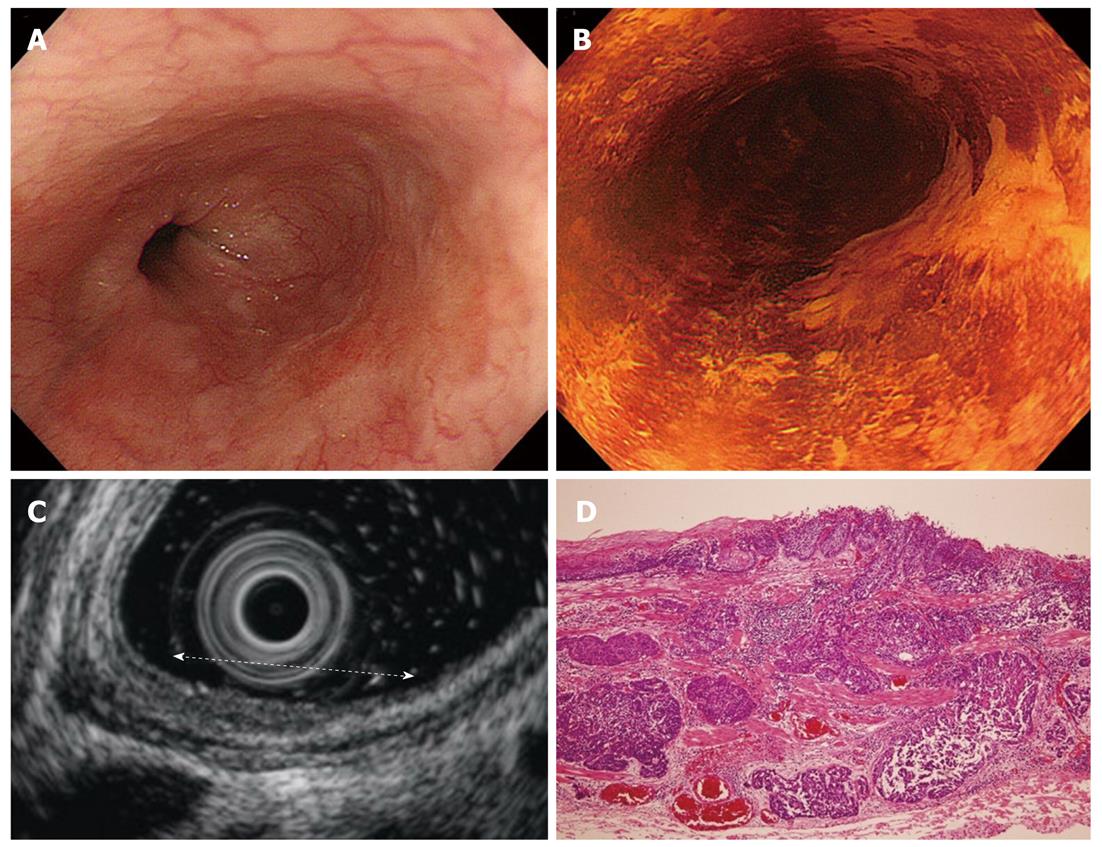
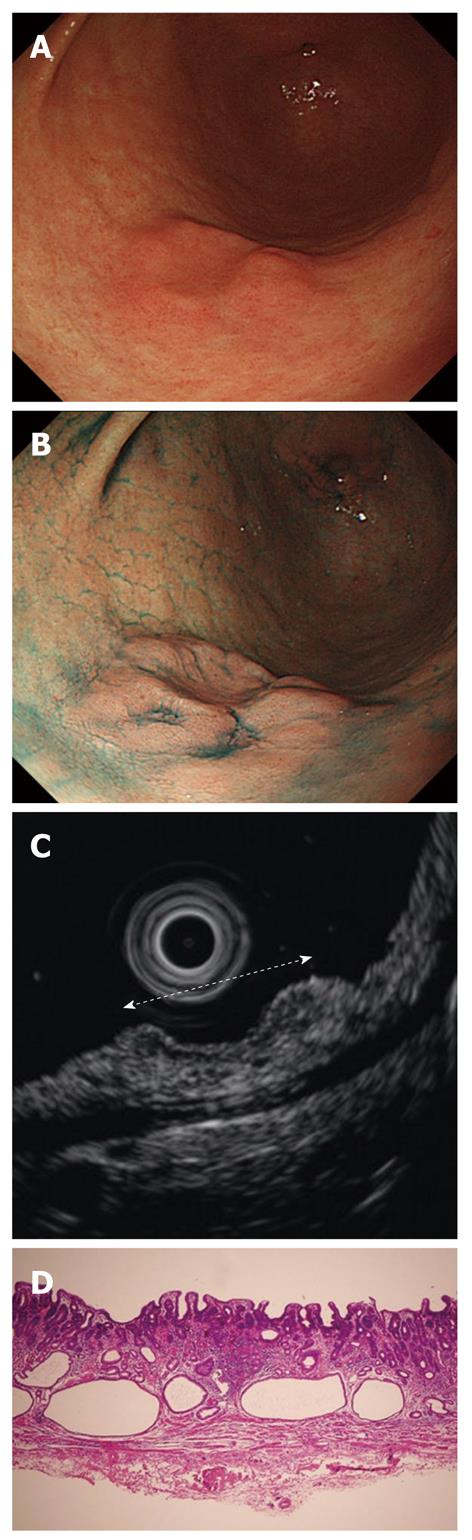
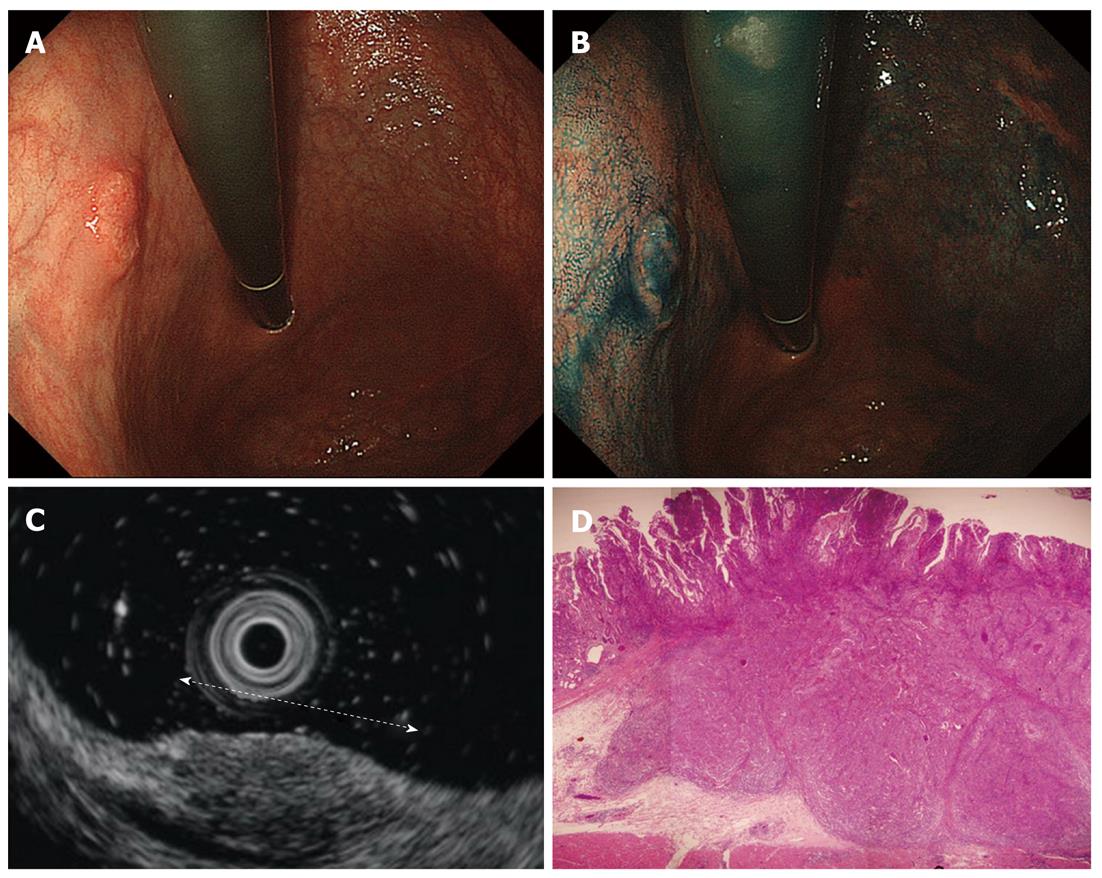
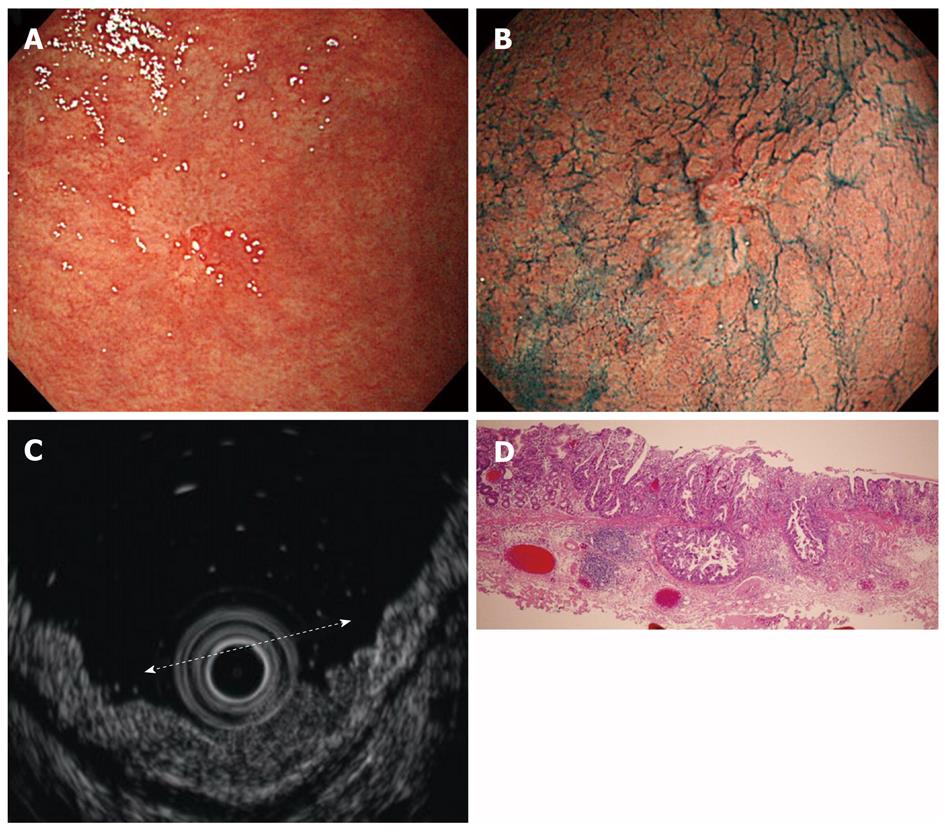
Cancers are visualized as hypoechoic lesions, and it should be recognized which layers are destroyed and which layers are normal. An m1 cancer is located in the first and second layers[4], and sometimes it is difficult to recognize the lesion (Figure 5A-D). An m2 cancer invades the third layer, but the fourth layer under the lesion is preserved (Figure 6A-D). Cancers with m3 to sm1 invasion penetrate the fourth layer, but the fifth layer is intact[4] (Figure 7A-D). In some cases of sm1 cancer, the fifth layer under the lesions appears slightly irregular (Figure 8A-D). An sm2 cancer invades the fifth layer, but there is a hyperechoic layer between the cancer and the sixth layer[4] (Figure 9A-D).
According to the report by Gotoda et al[3], the expanded indications of endoscopic resection for gastric cancer are defined as follows: (1) differentiated type, no lymphatic or venous invasion, intramucosal cancer without ulceration, regardless of tumor size; (2) intramucosal cancer with ulceration, less than 3 cm diameter; (3) minute submucosal cancer that invades less than 500 μm in the submucosa, less than 3 cm diameter; and (4) undifferentiated type, no lymphatic or venous invasion, intramucosal cancer without ulceration, less than 2 cm diameter. Therefore, we should distinguish intramucosal (m) cancer, minute submucosal invasive (sm1) cancer, and massive submucosal invasive (sm2) cancer.
The accuracies of EUS using high-frequency ultrasound probes for the staging of early gastric cancer have been described as up to 80% compared with 63% for conventional EUS[15]. Rodriguez et al[16] mentioned that many endosonographers now feel that catheter-based miniprobes scanning at 20 MHz may be better suited to staging early gastric cancers. In previous reports, the overall accuracies of the depth of invasion by the ultrasound probes were 65%-86%[17-20]. In those reports, the accuracy of EUS relatively decreased for those patients with lesions of depressed type, undifferentiated cancer[18,19], concomitant ulceration, the expanded indications that we described[19], type 0-I lesions, and lesions located in the upper-third of the stomach[20]. Also, Akahoshi et al[18]
mentioned that the accuracy decreased as tumor size increased. In addition, over staging of early gastric cancers with the 20 MHz probe occurs in 19%-24% of patients due to peritumoral fibrosis mimicking deeper invasion[16,17,21]. But when both the endoscopic appearance and EUS findings were applied together for tumor classification, a 92% overall accuracy rate was achieved[17]. Though Mouri et al[22] reported both high-frequency ultrasound probes and conventional EUS are useful for accurately determining the depth of invasion of gastric cancer without ulcerous change, they did not distinguish between intramucosal cancers and minute submucosal invasive cancers in terms of the expanded indication of endoscopic resection, and they also excluded the lesions that EUS could not sufficiently evaluate. In other words, it is still difficult to distinguish those cancers, especially with ulcerous change, and EUS cannot evaluate all gastric lesions.
Our preparations and patient premedications are the same for both gastric and esophageal EUS procedures. Usually, we use a conventional endoscope that can be bent more than 180 degrees, both because we don’t need to use a water jet system for gastric EUS and because sometimes we need to scan at the retroflex position.
After washing the lesion and removing water collected in the stomach, we start irrigating with deaerated water introduced through an instrument channel. After the area to be imaged is filled with deaerated water, an ultrasound probe is inserted through an instrument channel and ultrasound scanning is begun. When it is difficult to approach lesions horizontally (Figure 10A) it is sometimes impossible to scan. In such cases we use a multi-bending endoscope (GIF-2TQ260M; Olympus) to approach lesions horizontally (Figure 10B and C). Technically, it is sometimes difficult to scan lesions which are located in the angle and the antrum because lesions are located on the curve or not submerged under water.
When we use high-frequency ultrasound probes, the normal gastric wall is visualized as the mucosa (combination of the first hyperechoic and second hypoechoic layers) and the submucosa (the third hyperechoic layer). The muscularis propria is visualized as the fourth hypoechoic layer, and the fifth hyperechoic layer is the serosa including the subserosa (Figure 11)[17]. According to the report by Yanai et al[23] the fine hypoechoic layer between the second and third layers is considered to correspond to the muscularis mucosae.
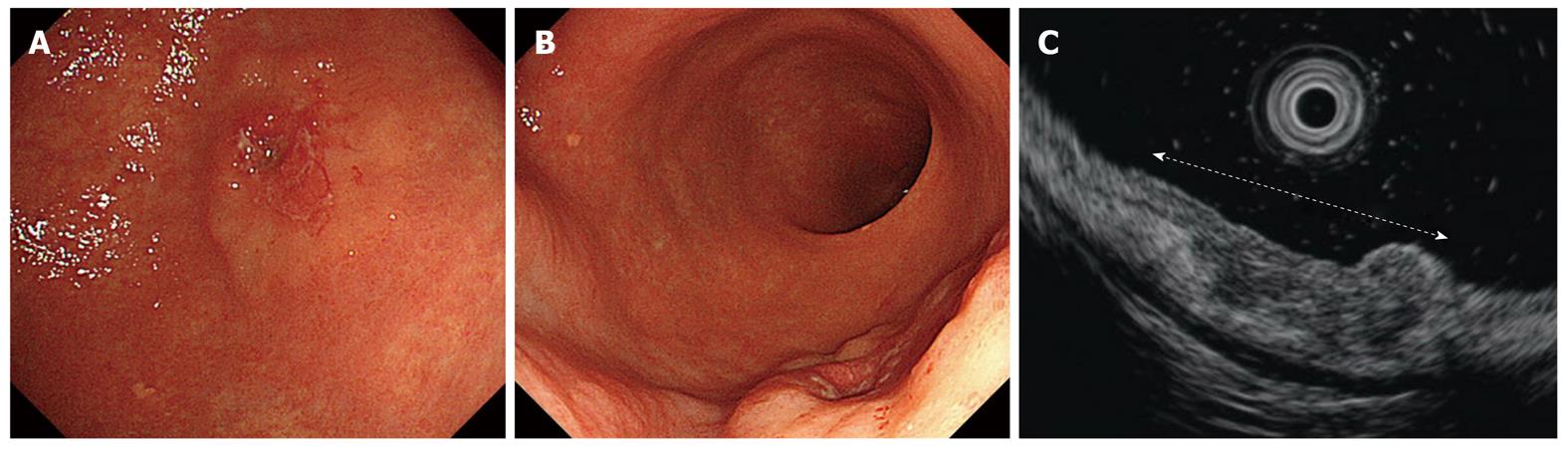
The EUS images were interpreted with regard to tumor invasion according to the five layer architecture of the gastric wall, and lesions were classified as m cancers (Figure 12A-D) and submucosal invasive (sm) cancers[17] (Figure 13A-D). Although the fifth layer under the lesions seems to be slightly irregular in some cases of sm1 cancer (Figure 14A-D), it is difficult to distinguish m and sm1 definitively.
Fortunately, no severe complications of EUS have been reported so far, but aspiration of water occurs occasionally. There is a larger risk for this when patients have a hiatal hernia, as water collected in the stomach runs back easily. Therefore, conscious sedation, rather than deep sedation, is more suitable for EUS. If possible, a balloon should be fixed oral to the tips of an endoscope to prevent water reflex[4] for esophageal lesion procedures.
We reviewed the present status of, the methods used for, and the findings of, EUS using high-frequency ultrasound probes to diagnose and stage early esophageal and gastric cancer. Although EUS using high-frequency ultrasound probes still has some limitations, such as low accuracy for minute submucosal invasion cancers and lesions with ulcerous change, it still has good accuracy for determining the depth of invasion of early esophageal and gastric cancers. Because determining the depth of malignant invasion is essential to distinguish lesions indicated for endoscopic resection, EUS is a useful clinical procedure. When both the endoscopic and EUS diagnoses are considered, clinicians can achieve a high accuracy of staging of early esophageal and gastric cancers.
Peer reviewer: István Rácz, MD, PhD, Professor, Head of Internal Medicine Department and Gastroenterology, Petz Aladár County and Teaching Hospital, Győr, Hungary
S- Editor Yang XC L- Editor A E- Editor Zhang DN
| 1. | Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 243] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (2)] |
| 2. | Tsendsuren T, Jun SM, Mian XH. Usefulness of endoscopic ultrasonography in preoperative TNM staging of gastric cancer. World J Gastroenterol. 2006;12:43-47. [PubMed] |
| 3. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1326] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 4. | Murata Y, Napoleon B, Odegaard S. High-frequency endoscopic ultrasonography in the evaluation of superficial esophageal cancer. Endoscopy. 2003;35:429-35; discussion 436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 253] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Hasegawa N, Niwa Y, Arisawa T, Hase S, Goto H, Hayakawa T. Preoperative staging of superficial esophageal carcinoma: comparison of an ultrasound probe and standard endoscopic ultrasonography. Gastrointest Endosc. 1996;44:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 95] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Murata Y, Suzuki S, Ohta M, Mitsunaga A, Hayashi K, Yoshida K, Ide H. Small ultrasonic probes for determination of the depth of superficial esophageal cancer. Gastrointest Endosc. 1996;44:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Fukuda M, Hirata K, Natori H. Endoscopic ultrasonography of the esophagus. World J Surg. 2000;24:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | May A, Günter E, Roth F, Gossner L, Stolte M, Vieth M, Ell C. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut. 2004;53:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Chemaly M, Scalone O, Durivage G, Napoleon B, Pujol B, Lefort C, Hervieux V, Scoazec JY, Souquet JC, Ponchon T. Miniprobe EUS in the pretherapeutic assessment of early esophageal neoplasia. Endoscopy. 2008;40:2-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Inoue H, Kawano T, Takeshita K, Iwai T. Modified soft-balloon methods during ultrasonic probe examination for superficial esophageal cancer. Endoscopy. 1998;30 Suppl 1:A41-A43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Chak A, Canto M, Stevens PD, Lightdale CJ, Van de Mierop F, Cooper G, Pollack BJ, Sivak MV. Clinical applications of a new through-the-scope ultrasound probe: prospective comparison with an ultrasound endoscope. Gastrointest Endosc. 1997;45:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Esaki M, Matsumoto T, Moriyama T, Hizawa K, Ohji Y, Nakamura S, Hirakawa K, Hirahashi M, Yao T, Iida M. Probe EUS for the diagnosis of invasion depth in superficial esophageal cancer: a comparison between a jelly-filled method and a water-filled balloon method. Gastrointest Endosc. 2006;63:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Odegaard S, Kimmey MB, Martin RW, Yee HC, Cheung AH, Silverstein FE. The effects of applied pressure on the thickness, layers, and echogenicity of gastrointestinal wall ultrasound images. Gastrointest Endosc. 1992;38:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Singh N, Herreros-Tejada A, Waxman I. High-frequency ultrasound probes. Endoscopic Ultrasonography. 2nd ed. Hoboken: Wiley-Blackwell 2009; 63-69. [DOI] [Full Text] |
| 16. | Rodriguez SA, Faigel DO. EUS of the stomach and duodenum. Endoscopic Ultrasonography. 2nd ed. Hoboken: Wiley-Blackwell 2009; 83-97. [DOI] [Full Text] |
| 17. | Yanai H, Matsumoto Y, Harada T, Nishiaki M, Tokiyama H, Shigemitsu T, Tada M, Okita K. Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study. Gastrointest Endosc. 1997;46:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 111] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Akahoshi K, Chijiwa Y, Hamada S, Sasaki I, Nawata H, Kabemura T, Yasuda D, Okabe H. Pretreatment staging of endoscopically early gastric cancer with a 15 MHz ultrasound catheter probe. Gastrointest Endosc. 1998;48:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Kim GH, Park do Y, Kida M, Kim DH, Jeon TY, Kang HJ, Kim DU, Choi CW, Lee BE, Heo J. Accuracy of high-frequency catheter-based endoscopic ultrasonography according to the indications for endoscopic treatment of early gastric cancer. J Gastroenterol Hepatol. 2010;25:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Tsuzuki T, Okada H, Kawahara Y, Nasu J, Takenaka R, Inoue M, Kawano S, Kita M, Hori K, Yamamoto K. Usefulness and problems of endoscopic ultrasonography in prediction of the depth of tumor invasion in early gastric cancer. Acta Med Okayama. 2011;65:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Yanai H, Tada M, Karita M, Okita K. Diagnostic utility of 20-megahertz linear endoscopic ultrasonography in early gastric cancer. Gastrointest Endosc. 1996;44:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009;43:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Yanai H, Fujimura H, Suzumi M, Matsuura S, Awaya N, Noguchi T, Karita M, Tada M, Okita K, Aibe T. Delineation of the gastric muscularis mucosae and assessment of depth of invasion of early gastric cancer using a 20-megahertz endoscopic ultrasound probe. Gastrointest Endosc. 1993;39:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |