Published online Dec 16, 2012. doi: 10.4253/wjge.v4.i12.571
Revised: November 7, 2011
Accepted: April 27, 2012
Published online: December 16, 2012
Portal hypertension, which is a common finding in children awaiting liver transplantation, is also found after transplantation. It’s reported the case of a 6-year-old girl, transplanted for biliary atresia, who had a severe obscure-overt bleeding presenting with melena. An esophagogastroduodenoscopy showed several duodenal small, bulging lesions, with some red signs. Near the lesions, a depressed area of 2 cm, covered with mixed hyperemic and white mucosa, was observed. To better evaluate these lesions, we performed an endoscopic ultrasonography (EUS) that showed multiple, round hypoechoic areas 0.5-5 mm in diameter, compatible with duodenal varices, and several periduodenal anechoic lesions compatible with collaterals. A consecutive computed tomography scan showed a stenosis of the portal vein anastomosis confirmed with a transhepatic portography, which was successfully treated with balloon angioplasty. No further episodes of bleeding were observed during the follow-up. This case report suggests that EUS is safe and feasible in young children when using echoendoscopes designed for use in adults. However further studies are needed to validate the employment of this technique in the management and follow-up of pediatric portal hypertension.
- Citation: Curcio G, Pisa MD, Miraglia R, Catalano P, Barresi L, Tarantino I, Granata A, Spada M, Traina M. Case of obscure-overt gastrointestinal bleeding after pediatric liver transplantation explained by endoscopic ultrasound. World J Gastrointest Endosc 2012; 4(12): 571-574
- URL: https://www.wjgnet.com/1948-5190/full/v4/i12/571.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i12.571
Biliary atresia (BA) is a common cause of neonatal cholestasis, with an approximate incidence of 1:10 000-15 000 live births[1]. Liver transplantation (LT) has become an important option for providing excellent long-term survival for patients with BA[2]. However, after LT, vascular[3] and biliary complications[4,5] can occur, and these are associated with increased morbidity and mortality. Portal hypertension (PH)[6], which is a common finding in children awaiting liver transplantation, is also found after transplantation[7]. A diagnosis of PH can routinely be made by upper gastrointestinal endoscopy. Moreover, in recent years, endoscopic ultrasonography (EUS) has become a complementary modality for the diagnosis and treatment of this condition.
Very little data on PH in infants are available, especially for routine EUS applications in these young patients[8].
We report the case of a 6-year-old girl, transplanted for BA, who had a severe obscure-overt bleeding, in which EUS allowed us to diagnosis severe portal hypertension, with ectopic varices, and to plan the successive diagnostic steps and the adequate treatment.
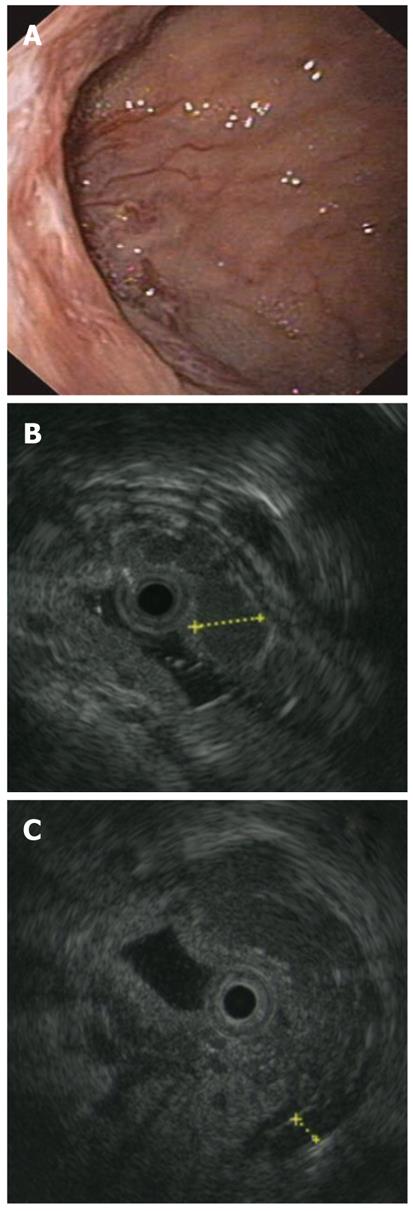
In June 2010, a 6-year-old girl was admitted to our institute because of an episode of melena with severe anemia. She had been affected with BA at the age of 1 mo. Although she underwent a Kasai procedure at the age of 2 mo, she developed cirrhosis, and at 8 mo underwent a cadaveric liver transplant, using an ABO-compatible, split-liver graft (segment II-III). The post transplant clinical course was complicated by an episode of acute rejection, successfully treated with steroids. An anastomotic biliary stricture occurred in February 2010. It was successfully treated with percutaneous biliary catheter placement, and subsequent sessions of cholangioplasty. The patient had been free of the biliary catheter since June 2010. Doppler ultrasound (US) performed at follow-up found no abnormalities in the hepatic artery, hepatic veins or portal vein. No other adverse events occurred until her episode of melena. On admission, she was in stable general condition, and asymptomatic. Her physical examination was unremarkable, with an arterial pressure of 110/60 mmHg. Laboratory data showed hemoglobin 6 gr/dL (NV: 12-16 gr/dL); PLT 193.000 × 103/μL (150-400 × 103/μL); MCV 76.70 (78-102 fL); total bilirubin 0.31 (0.10-1.10 mg/dL); direct bilirubin 0.11 (0-0.30 mg/dL); aspartate aminotransferase 133 (15-37 U/L); alanine aminotransaminase 203 (30-65 U/L); alkaline phosphatase 362 (50-136 U/L); and glutamyltransferase 48 (5-85 U/L). Two units of blood were transfused and, in order to check the cause of the anthemization, an esophagogastroduodenoscopy (EGD) was planned. Under deep sedation, the patient was monitored continuously with electrocardiography, pulse oximetry and automatic recording of blood pressure and pulse. After introducing the scope into the esophagus, small varices, with no red signs, were seen in the lower third. The stomach was normal. There were no signs of active bleeding. In the duodenum, at the bulb apex, several small, bulging lesions, with some red signs, were seen. Near the lesions, a depressed area of 2 cm, covered with mixed hyperemic and white mucosa, was observed (Figure 1A). To evaluate the nature of these lesions, and using the same sedation, a 20 MHz miniprobe (Olympus Medical System Corp.) was inserted into the operative channel of the endoscope. After filling the duodenal lumen with water, multiple, round hypoechoic areas 0.5-5 mm in diameter, compatible with varices, were seen in correspondence to the endoscopically viewed lesions (Figure 1B).
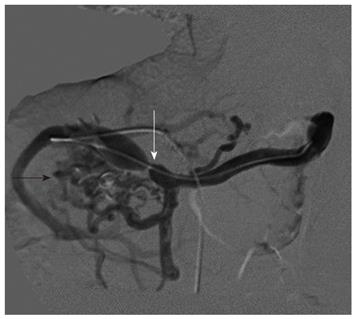
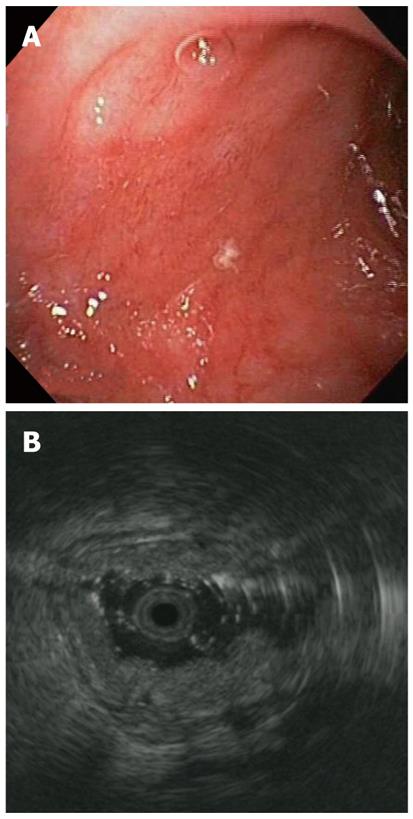
In addition, the EUS showed several periduodenal anechoic lesions (Figure 1C), compatible with collaterals, and confirmed small varices in the esophagus. Because the EUS showed a picture of portal hypertension with ectopic duodenal varices, despite the normal Doppler US findings, a computed tomography scan was done, and showed a stenosis of the portal vein anastomosis. The false-negative finding of the Doppler US was likely related to the deep position of the anastomosis, which was covered by bowel loops. A transhepatic portography was done the day after, showing multiple periduodenal varices and confirming the anastomotic stricture, which was successfully treated with balloon angioplasty (Figure 2). Over the following days, the patient remained hemodynamically stable, with no further episodes of bleeding. Two weeks later, at EGD and EUS control, no duodenal varices were found (Figure 3A and B).
Endoscopic ultrasonography has recently emerged as an accurate, non-invasive and reproducible technique, and is more sensitive than endoscopy in the diagnosis of gastric varices[9]. Dilated venous abnormalities outside the gastroesophageal lumen, which cannot be diagnosed by endoscopy, are readily visible with endoscopic ultrasonography or miniature probes. However, this method has yet to become a routine examination among the diagnostic and therapeutic examinations in the setting of portal hypertension[10]. As for the application of EUS in pediatric patients[11], several reports have been published, though with few indications regarding the use of radial endoscopic probes in pancreatobiliary disorders, gastrointestinal tumors and portal hypertension[12]. As a result, there are few data on the use of ultrasonographic miniprobes for studying vascular abnormalities in pediatric portal hypertension. In our case, the patient developed varices 4 years after liver transplant, and we were able to confirm the presence of ectopic duodenal varices, which can account for up to 5% of variceal hemorrhages[8]. We used a 20 MHz miniprobe, which was introduced into the operative channel of the endoscope. The ultrasonography procedure with miniprobes is safe, non-invasive and does not require preparation. In addition, we can do both gastroscopy and endoscopic ultrasonography using the same sedation and without changing the instrument. The easiness and the quickness of this combined approach allowed us to identify severe portal hypertension despite the endoscopic appearance, and also to plan the successive diagnostic and therapeutic steps. Unfortunately, despite the advantages of EUS, its use in pediatric patients is very limited because of the presumptive limitations inherent in the size of EUS equipment and accessories, the need for an anesthesiologist in the endoscopic room, and the lack of highly trained and experienced endosonographers for pediatric patients[13]. This case report suggests that EUS is safe and feasible in young children when using echoendoscopes designed for use in adults. Further studies are needed to validate the employment of this technique in the management and follow-up of pediatric portal hypertension.
We thank Warren Blumberg for editorial assistance.
Peer reviewer: John A Karagiannis, Professor, Department of Gastroenterology, Konstantopoulio Hospital, 6, Iordanidi Str. P. Psychiko, 15452 Athens, Greece
S- Editor Yang XC L- Editor A E- Editor Zhang DN
| 1. | Bassett MD, Murray KF. Biliary atresia: recent progress. J Clin Gastroenterol. 2008;42:720-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Zheng SS, Huang DS, Wang WL, Liang TB, Zhang M, Shen Y, Lu AW, Liao SY, Xu X. Living related liver transplantation for an infant with biliary atresia. Hepatobiliary Pancreat Dis Int. 2002;1:172-175. [PubMed] |
| 3. | Pareja E, Cortes M, Navarro R, Sanjuan F, López R, Mir J. Vascular complications after orthotopic liver transplantation: hepatic artery thrombosis. Transplant Proc. 2010;42:2970-2972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Egawa H, Uemoto S, Inomata Y, Shapiro AM, Asonuma K, Kiuchi T, Okajima H, Itou K, Tanaka K. Biliary complications in pediatric living related liver transplantation. Surgery. 1998;124:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Chen HL, Concejero AM, Huang TL, Chen TY, Tsang LL, Wang CC, Wang SH, Chen CL, Cheng YF. Diagnosis and interventional radiological treatment of vascular and biliary complications after liver transplantation in children with biliary atresia. Transplant Proc. 2008;40:2534-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Mileti E, Rosenthal P. Management of portal hypertension in children. Curr Gastroenterol Rep. 2011;13:10-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Chu J, Kerkar N, Miloh TA, Rodriguez-Laiz G, Lewis B, Stangl A, Newton KP, Iyer K, Arnon R. Roux-en-Y loop varices in children with portal hypertension after liver transplantation: an unusual cause of "obscure" gastrointestinal bleeding. Pediatr Transplant. 2011;15:E156-E161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Norton ID, Andrews JC, Kamath PS. Management of ectopic varices. Hepatology. 1998;28:1154-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 253] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | El-Saadany M, Jalil S, Irisawa A, Shibukawa G, Ohira H, Bhutani MS. EUS for portal hypertension: a comprehensive and critical appraisal of clinical and experimental indications. Endoscopy. 2008;40:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Bocus P, Ceolin M, Battaglia G. Endoscopic ultrasonography (EUS) in portal hypertension. Minerva Med. 2007;98:431-436. [PubMed] |
| 11. | Cohen S, Kalinin M, Yaron A, Givony S, Reif S, Santo E. Endoscopic ultrasonography in pediatric patients with gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2008;46:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Varadarajulu S, Wilcox CM, Eloubeidi MA. Impact of EUS in the evaluation of pancreaticobiliary disorders in children. Gastrointest Endosc. 2005;62:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Attila T, Adler DG, Hilden K, Faigel DO. EUS in pediatric patients. Gastrointest Endosc. 2009;70:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |