Published online Dec 16, 2012. doi: 10.4253/wjge.v4.i12.532
Revised: November 9, 2012
Accepted: December 1, 2012
Published online: December 16, 2012
Since its initial report in 1992, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) has now been incorporated into the diagnostic and staging algorithm for the evaluation of benign and malignant diseases of the gastrointestinal tract and of adjacent organs. Its introduction constitutes a major breakthrough in the endoscopic field and has gradually transformed EUS from a pure imaging modality into a more interventional. In addition, the possibility of collecting samples, providing a definitive cytological and/or histological evidence of the presence of malignancy, has strongly contributed to changing EUS from a subjective, highly operator dependant procedure into a more objective one. This article will review the instrumentation, technique and the most important clinical applications of EUS-FNA.
- Citation: Tharian B, Tsiopoulos F, George N, Pietro SD, Attili F, Larghi A. Endoscopic ultrasound fine needle aspiration: Technique and applications in clinical practice. World J Gastrointest Endosc 2012; 4(12): 532-544
- URL: https://www.wjgnet.com/1948-5190/full/v4/i12/532.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i12.532
Since its initial report by Henriksen et al[1], endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) has now been incorporated into the diagnostic and staging algorithm for the evaluation of benign and malignant diseases of the gastrointestinal (GI) tract and of adjacent organs[2]. Its introduction constitutes a major breakthrough in the endoscopic field and has gradually transformed EUS from a pure imaging modality into a more interventional and lately therapeutic procedure. In addition, the possibility of collecting samples, providing a definitive cytological and/or histological evidence of the presence of malignancy, has strongly contributed to changing EUS from a subjective, highly operator dependant procedure into a more objective one.
In this article, we have done a critical appraisal of recent published literature and reviewed the instrumentation, technique and the most important clinical applications of EUS-FNA. The role of EUS in diagnosis and staging of esophageal, gastric, rectal cancers, subepithelial tumors, pancreatobiliary cancers, mediastinal cancers, lung and miscellaneous tumors has been dealt with. Despite the availability of advanced cross sectional imaging like computed tomography (CT) and magnetic resonance imaging, EUS is still the gold standard for diagnosis and staging of various gastrointestinal cancers in particular of the pancreas, due to its high sensitivity and specificity and safe technique of acquiring tissue. We have not covered topics like general role of EUS in diagnosis of pancreatobiliary stone disease, chronic pancreatitis, autoimmune pancreatitis or in screening patients with a family history. We have also not dealt with the most challenging and exciting therapeutic aspect of EUS in stone disease, pseudocysts, celiac blocks and fine needle injection of tumors. We have tried to focus on the issues that have strongest evidence currently and also just mention the controversial topics like use of EUS post neoadjuvant chemotherapy.
EUS-FNA is performed using curvilinear array echoendoscopes (CLA-EUS) that are produced by three leading manufacturers: Olympus (Olympus Medical Systems Inc., Tokyo, Japan), Pentax (Pentax, Tokyo, Japan) and Fujinon (Fujifilm Corp., Tokyo, Japan). CLA-EUS provide a plane of imaging parallel to the long axis of the endoscope, with the transducer placed on the tip of the echoendoscope and directed to provide ultrasound images along one aspect of the instrument. These features allow for tracing the needle from its exit from the tip of the echoendoscope to its entrance into the target lesion under real-time ultrasound guidance. The working channel must be at least 2.8 mm to accept the FNA needle and presents at its end located on the side of the scope an elevator that is able to make changes in the exit angle of the FNA needle to facilitate the targeting process. Curvilinear echoendoscopes with a larger working channel diameter (from 3.7 to 4.2 mm) to allow for the passage of larger devices, such as stent, are available and are used for interventions that are beyond the present discussion. Recently, a forward viewing EUS scope has become available, with the working channel placed on the tip of the scope that allows for accessories to exit with the axis parallel to the longitudinal axis of the scope[3,4]. As compared to the oblique view, the perpendicular approach would theoretically facilitate access, optimize precision, and maximize transfer of force to the target site.
Needles for EUS-FNA are currently available in 3 sizes (19, 22 and 25 gauge) (Table 1). All the needles have a removable stylet and the more recently developed are equipped with an adjustable length sleeve or sheath to fit precisely to the length of the working channel of the EUS scope, which varies between the three different brands. The stylet could have either a sharp tip or a smooth one, the latter requiring the stylet to be pulled back few millimeters before performing the FNA. Finer needles are used to gather cytological specimens, while larger needle can be utilized when acquisition of a tissue specimen for histological examination can be more useful to reach the definitive diagnosis. For this purpose, specifically designed biopsy needles have been developed (Quick-core biopsy needle and ProCore needle, Cook Medical Inc., Bloomington, IN).
When available, EUS-FNA should be done under deep sedation with the assistance of an anesthesiologist. The FNA technique includes several phases: (1) Targeting of the lesion: The best position to perform EUS-FNA is with the target lesion in the center of the US image, adjacent to the transducer. Minimization of the distance between the target lesion and the tip of the EUS scope is made by using the up-down wheel of the scope. Color Doppler imaging should be then used to exclude the presence of interposing vessels; (2) Preparation of the needle: The needle-catheter assembly is passed through the working channel of the echoendoscope until the system handle locks in at the end of the biopsy channel. When necessary, the sheath is put forward in the working channel until its tip becomes visible on the ultrasound screen. The needle stop on the system handle is then unlocked to be ready to perform FNA; (3) Puncturing: Before advancing the needle, for some of the commercially available 19- and 25- gauge needles the stylet needs to be pulled out. When targeting lymph nodes important for staging of esophageal, gastric, and rectal cancers, attention should also be paid not to pass through an affected area of the wall of the GI tract under evaluation to avoid false positive results. The needle is then advanced under real-time EUS guidance into the middle part of the target lesion by using a quick, strong thrust of the handle. If the stylet has been pulled out as described above, it is necessary to push it all the way back to remove debris eventually collected. The stylet is then completely withdrawn and a 10-20 mL syringe attached to the end of the needle device; (4) Sample collection: Once inside the lesion, after applying negative pressure suction by opening the lock device of the syringe, the needle is moved back and forth 10 to 20 times under EUS guidance. The suction syringe is then released, the needle withdrawn into the catheter, and the whole system removed from the echoendoscope; and (5) Specimen handling: The material in the FNA needle is obtained by pushing air from a syringe and it is placed on pre-labeled glass slides or into a container with CytolitTM (Hologic-Cytyc Co, Marlborough, United States) to perform liquid-based cytology or a container with formalin for histological analysis or cell-block preparation. The use of one or the other technique varies from center to center.
The choice of the needle to be used depends on the type and site of the lesion to be sampled, whether the lesion is solid or cystic, and whether the access to the lesion can be difficult because of the angle or bend of the needle required to reach the target. Studies comparing 22- and 25-gauge needles have mainly been performed in patients with pancreatic masses with controversial results[5-8]. Most of these studies, however, were based on a small sample size, while the only one with a prospective, randomized design on a large patient population has found no significant difference between the two needles with respect to cellular yield and ability to obtain a diagnosis[7].
The use of the tru cut biopsy needle (TCB) may overcome some of the limitations of EUS-FNA permitting histological analysis and performance of immunostaining[9]. Studies with this device conducted on different patient populations have reported a diagnostic accuracy ranging from 61% to 84%[10-17]. No clear advantages of EUS-TCB over EUS-FNA have been demonstrated, even in patients with possible lymphomas and GI subepithelial tumors that have been considered a class IIa indication for the use of EUS-TCB[9], without definitive evidence of superiority[10,15,16,18-22]. Overall, EUS TCB may be useful as adjunctive technique used in tandem or as a rescue procedure after negative FNA. A new needle (ProCore needle, Cook Medical Inc., Bloomington, IN) to acquire histological samples has recently become available. Results from the first feasibility multicentric study have shown a very promising diagnostic accuracy with only one single needle pass performed.
Alternatively to EUS-TCB and the ProCore needle, a standard 19-gauge needle can be used to acquire tissue samples for histological examination as described by Yasuda et al[23], who investigated this technique in 104 patients with mediastinal and/or intra-abdominal lymphadenopathy of unknown origin. They found an overall diagnostic accuracy of 98% with an 88% chance of correct subtyping of lymphomas based on the World Health Organization classification. In a more recent study including patients in whom histology was deemed to be more appropriate than cytology to reach a definitive diagnosis, Larghi et al[24], reported a diagnostic accuracy of 93.2% by using a modification of the technique first described by Yasuda et al[23]. The technique was referred to as EUS-guided fine needle tissue acquisition (EUS-FNTA) to distinguish it from EUS-FNA.
The usefulness of suctioning while doing needle passes has been evaluated by Wallace et al[25] during EUS-FNA of lymph nodes and by Puri et al[26], during EUS-FNA of solid masses. In lymph nodes the use of suction was associated with an increase in the cellularity of the specimen with, however, worse quality because of excessive bloodiness, without improvement in the likelihood of obtaining a correct diagnosis[25]. On the contrary in solid masses, suction yielded a significantly higher sensitivity and negative predictive values despite the proportion of target cells was relatively similar between the suction and non-suction sampling techniques[26].
Traditionally, the stylet is reinserted into the needle before each pass to prevent sample contamination while traversing the digestive wall. Recent studies, however, have found no differences in sample quality, contamination rate, and in sensitivity for cancer detection regardless of whether the stylet was reinserted or not[27,28].
Higher accuracy rates are achieved with on-site cytopathology examination to assess specimen adequacy that, however, is not available in all centers and may increase the cost of the procedure[29,30]. In case the procedure is performed without the assistance of on-site cytopathology examination, the minimum number of needle passes that are recommended to achieve a good diagnostic accuracy are 5 to 7 for pancreatic solid masses and 3 for lymph nodes, liver and miscellaneous lesions[25,31-33].
The potential clinical role of EUS-FNA is evolving with various medical advances in oncology and molecular genetics. These help us not only in staging of tumors but also in the treatment and prognostication of the same, taking us to newer frontiers.
The foremost indications of EUS-FNA are in taking biopsies from N1/M1 nodes in esophageal malignancy, mediastinal lymphnodes (suspected lung tumor N2/3) and masses, pancreatic tumor, pancreatic cyst assessment, perirectal and retroperitoneal nodes/masses, left adrenal, left lobe of the liver and subepithelial lesions, just to elaborate a few instances[1,2]. The main ones are discussed at length in this review.
EUS-FNA is not done in situations when it is unlikely to alter the management of a cancer. In addition to the usual contraindications for any endoscopic procedure including severe bleeding diathesis and thrombocytopenia, EUS-FNA is not advocated when good views of the lesion are not obtained or when there is a blood vessel or tumor on the way to the target and or high risk of tumor seeding[1,2,9]. The various pitfalls of EUS-FNA include underdiagnosis of pancreatic malignancy in a background of chronic pancreatitis or in cystic lesions, misinterpretation of bowel wall smooth muscle cells as gastrointestinal stromal tumor (GIST) and overinterpretation of metastasis in contamination by normal gastrointestinal epithelium.
Overall complication rate of EUS-FNA is 0.5% to 3%[2,4]. Most of these can be avoided and rectified by careful sampling by the endoscopist with an on-site cytopathologist if available and immunocytochemistry in addition to close clinical correlation and follow up.
In esophageal cancer, once distant metastases to other organs have been ruled out by CT and/or FDG-pancreatic neuroendocrine tumor (PET), the choice between immediate surgery, neoadjuvant therapy followed by surgery or palliative treatment is mainly based on loco-regional staging. EUS-FNA may affect patient management by providing cytopathological confirmation of metastasis to regional and non-regional lymph nodes (mostly celiac) or to distant sites (Figure 1). The adjunct of EUS-FNA significantly improves EUS accuracy for lymph nodes staging as reported by a landmark study from the Mayo Clinic, in which a total of 125 patients with esophageal carcinoma were evaluated[34]. EUS-FNA proved to be more accurate than CT (87% vs 51%, P < 0.001) or EUS alone (87% vs 74%, P = 0.012) and to be able to significantly modify tumor stage determined by helical CT in 38% of patients (mostly towards a worse stage)[34]. This more accurate staging resulted in an increased rate of neoadjuvant treatments rather than direct surgery. This study, however, did not directly assess the impact of EUS-FNA on the overall patient management. The same group has subsequently proposed the possibility that the addition of more criteria to the standard four criteria to define malignant lymph nodes by EUS could result in a more selective use of FNA[35]. In particular, the use of six criteria permitted to avoid EUS-FNA in 42% of evaluated patients, a result that needs further confirmation before becoming standard practice.
Other studies have evaluated the clinical impact of EUS-FNA on patient management. EUS-FNA demonstration of distant lymph node metastases have been found to change the management strategy in 7% and 12% in one prospective and one retrospective study that involved an overall of 307 patients[36,37]. Moreover, small hepatic metastases and small metastatic pleural fluid collections undetected at previously performed CT have been discovered by EUS-FNA in 3% to 5% of patients with esophageal cancer[36,38]. Importantly, EUS-FNA can also be used to select the surgical approach to be used in patients with a resectable distal esophageal carcinoma and mediastinal LN visualized on EUS. EUS-FNA demonstration of positive mediastinal lymph nodes changed the management in 23% of the evaluated patients who underwent transthoracic esophagectomy, while those without proven involvement of the mediastinum underwent transhiatal resection that offers limited capability of lymph nodes removal[39].
The role of EUS-FNA after neoadjuvant chemoradiotherapy appears more limited in view of the significantly lower accuracy than that of integrated FDG-PET/CT (78% vs 93%; P = 0.04), which is also superior in predicting complete pathologic response[40].
Treatment options for gastric cancer strongly depend on tumor staging. It is well established that patients with early localized and those with metastatic disease should undergo surgery and palliation, respectively. On the other hand, in patients with a locally advanced cancer who cannot be resected for cure and in those who are potentially amenable to curative resection, neoadjuvant chemotherapy has proved to significantly improve prognosis[41]. Based on these new treatment paradigms, besides the degree of tumor infiltration, the exclusion of distant metastases and of loco-regional lymph node involvement is of paramount importance.
Data on the impact of EUS-FNA in patients with gastric cancer are limited. In a study by Mortensen et al[36], on 62 patients, EUS-FNA was performed in 12 patients (19.3%) for staging purposes. Overall, EUS-FNA demonstrated the presence of M1 disease in 8 patients and correctly excluded malignant ascites in another one, with an overall clinical impact in 14% of the studied cohort. The same group published two subsequent papers on 134 and 273 patients with gastric cancer, respectively, in whom staging was performed by combining endoscopic and laparoscopic ultrasound[42,43]. EUS-FNA was performed during the procedure if a positive (malignant) biopsy would have changed the patient’s management. Unfortunately, data on the impact of EUS-FNA are not presented and cannot be extrapolated.
Recently, Hassan et al[44] studied the impact of EUS-FNA on the management of gastric cancer in 234 consecutive patients. EUS-FNA was performed in 81 patients (35%) in whom 99 lesions suspected for distant metastases were biopsied (78 were mediastinal lymph nodes). Overall 61 of these lesions in a total of 38 patients, mainly with tumor in the cardia, were found to be malignant. Excluding 4 patients in whom liver metastases were suspected but not verified by CT-guided FNA, in the remaining 34 patients these metastases were not seen or suspected by CT or other imaging modalities performed. As judged by the board of surgeons EUS-FNA changed the management in 34 of the 234 patients (15%) undergoing EUS for staging, avoiding unnecessary surgery[44].
EUS-FNA for rectal cancer has been evaluated for both lymph nodes staging and for detection of extraluminal recurrence. In the preoperative staging of rectal cancer, two studies have evaluated the clinical impact of EUS-FNA, with very similar results[45,46]. Both studies reported that EUS-FNA did not alter significantly the management as compared with EUS alone. In particular, Harewood et al[45] studied 80 patients and found that the addition of EUS-FNA did not change in all but one patient, what the surgeons would have done based on the results of EUS alone. Forty-one patients of the entire cohort underwent EUS-FNA of non-juxtatumoral lymph nodes detected during EUS examination and found that specificity and diagnostic accuracy of N staging by EUS alone or EUS-FNA were similar, with indeed a lower sensitivity of EUS-FNA (52% vs 74%). Shami et al[46] studied 60 patients of whom 48 underwent both CT and EUS. The authors found that EUS changed management in 38% of patients, while 16 patients identified as having nonjuxtatumoral lymph nodes underwent EUS-FNA and the additional information obtained changed therapy in three (19%) of these patients, but only in 6% of the entire cohort[46]. It is possible that the lack of clinical impact of EUS-FNA in rectal cancer staging might be related to the close correlation between the T and N stages and to the fact that most perirectal lymph nodes detected at EUS during rectal cancer staging are malignant.
Differently, in patients evaluated for perirectal lesions suspicious for tumor recurrence (Figure 1), EUS-FNA has a strong clinical impact as demonstrated by the high diagnostic accuracy reported in 2 published studies[47,48]. In both series, EUS-FNA was significantly more accurate than EUS alone to diagnose malignant recurrence. Moreover, in the study with the largest patient population, EUS-FNA had a considerable impact on patient management in 26% of the cases.
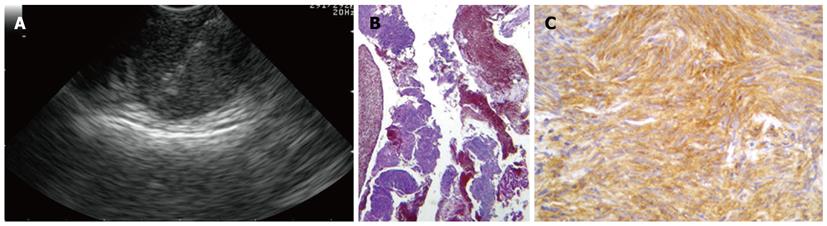
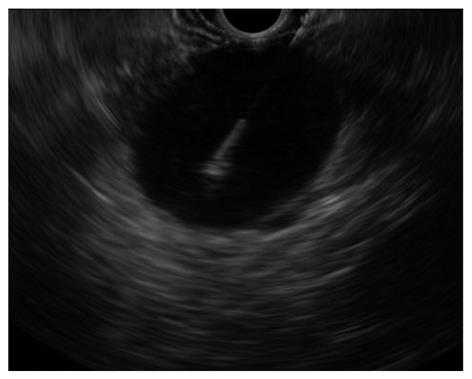
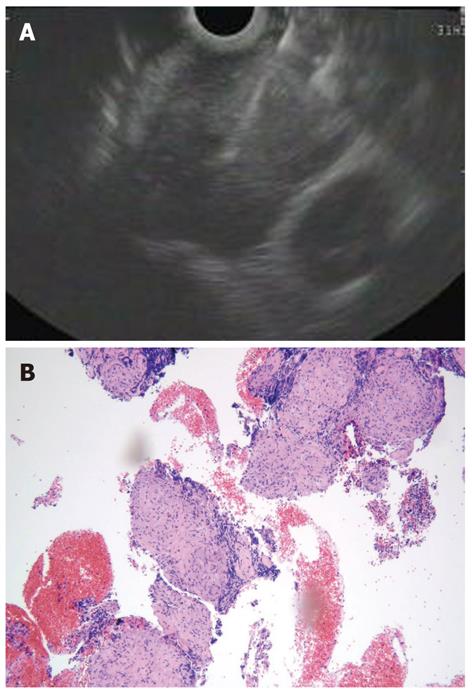
The term subepithelial incorporates a variety of lesions including non-neoplastic lesions, as well as benign, premalignant, and overtly malignant neoplasms that are all located in the digestive wall beneath the epithelial layer. In this clinical setting, EUS-FNA has been used to overcome the limitation of the pure endosonographic inspection of these lesions, that can be useful to identify the layer of origin and formulate a differential diagnosis, but cannot differentiate benign and malignant conditions (Figure 2)[49]. Most of the studies have been performed on gastric lesions, which are represented predominantly by GISTs. EUS-FNA is able to gather representative material for cytopathological analysis in about 70%-84%[50-52], but often the material is insufficient to perform immunostaining, which are needed to distinguish GIST from other mesenchymal tumors. Moreover, the mitotic index that expresses the malignant potential of GISTs, cannot be reliably assessed on cytological specimens and even on tissue specimens acquired with the use of the tru cut needle[20,21].
This limits the usefulness of EUS-FNA and EUS-TCB for lesions in which the knowledge of the mitotic index is needed to guide further management (i.e., incidentally discovered asymptomatic lesions between 2 and 3 cm). On the other hand, in patients with a presumptive diagnosis of unresectable GIST in whom primary treatment with tyrosine kinase inhibitors is considered EUS-FNA or EUS-TCB confirmation of the diagnosis is required and they are likely to impact the management of these patients[53].
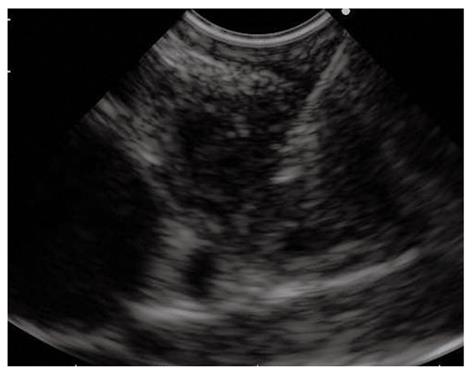
Tissue diagnosis of pancreatic masses is one of the most important indications for EUS-FNA. Overall, EUS-FNA of pancreatic masses is safe[54], has a mean accuracy of about 85%[55], which can be increased with on-site cytopathology examination to assess specimen adequacy[29,30]. Two clinical scenarios need to be distinguished: (1) masses that are clearly unresectable on previous cross-sectional imaging studies from (2) masses that appear resectable. In the first case scenario a definitive diagnosis to guide further treatment is mandatory and should be obtained, when available, by EUS-FNA that is preferred to percutaneous image-guided tissue sampling (Figure 3)[56]. A recent single center, prospective, randomized, cross-over study has demonstrated EUS-FNA to be superior to US or CT-guided biopsy to determine the nature of pancreatic solid lesions[57]. EUS-FNA has also been found to be the most cost effective test for pancreatic adenocarcinoma when compared with CT-guided FNA and surgical diagnosis[58]. In addition, EUS-FNA can provide additional staging information, by sampling: (1) lymph node metastases in the celiac, lumboaortic, retroduodenopancreatic and superior mesenteric regions; (2) small hepatic lesions missed on CT[59]; and (3) small pocket of previously undetected ascites[60], all sites that when positive for malignancy indicate a poor prognosis for the patient[61]. Another important advantage of EUS-FNA over the percutaneous route is the lower risk of tumor seeding[62], and the potential to sample smaller lesions. On the other hand, the percutaneous route may be indicated in patients who are at risk for sedation-related complications and in those with surgically altered upper GI anatomy. When other biopsy techniques have failed, or in cases of previously done negative or inconclusive EUS-FNA, the use or the repetition of EUS-FNA is highly advised[63,64].
In potentially resectable pancreatic lesions, the argument for a definitive diagnosis before undergoing surgery is still debated[65]. Arguments made for EUS-FNA in potentially resectable lesions include an established protocol of preoperative neoadjuvant therapy, a demand by the patient for a conclusive diagnosis of cancer before consenting to surgery, and lastly to exclude unusual histology (lymphoma, acinar cell carcinoma, solid pseudopapillary tumor and pancreatic metastases) that can be found in up to 5% of individuals with pancreatic masses, who would not benefit from surgery[61,66]. The main argument against EUS-FNA in resectable lesions is that the performance of the procedure would not significantly affect further management, because a negative result cannot exclude the presence of malignancy due to its low negative predictive value[65]. Thus, the utility of EUS-FNA in this setting should be balanced with the potential risk of tumor seeding and varies among different centers.
A very important clinical challenge is the differentiation of pancreatic cancer from inflammatory masses due to focal chronic pancreatitis or autoimmune pancreatitis. A review of the performance of EUS-FNA for the differentiation of benign and malignant pancreatic masses, which included 25 studies with an overall of 4224 patients, found a median sensitivity of 83% (range 54%-95%), a median specificity of 100% (range 71%-100%), a median negative predictive value of 72% (range 16%-92%), and a median diagnostic accuracy of 88% (range 65%-96%)[65]. Definitions used to classify diagnostic cytology and the exclusion of non-diagnostic specimens in some studies may account for the wide ranges reported above. New techniques including contrast-enhanced EUS and elastosonoendosonography[67,68], DNA analysis[69], and K-ras mutation determination on FNA aspirates[70,71], have been tested to increase the possibility of differentiating cancer from focal chronic pancreatitis. If there is a high index of suspicion of autoimmune pancreatitis, tru cut biopsy needle may improve the diagnostic yield and can be used as a rescue procedure if conventional EUS-FNA has failed (100% vs 36%, P = 0.0006)[72]. When a pancreatic lymphoma is suspected by the clinical presentation, the use of flow cytometry significantly increases the diagnostic accuracy from 30.8% to 84.6% (P = 0.01)[73].
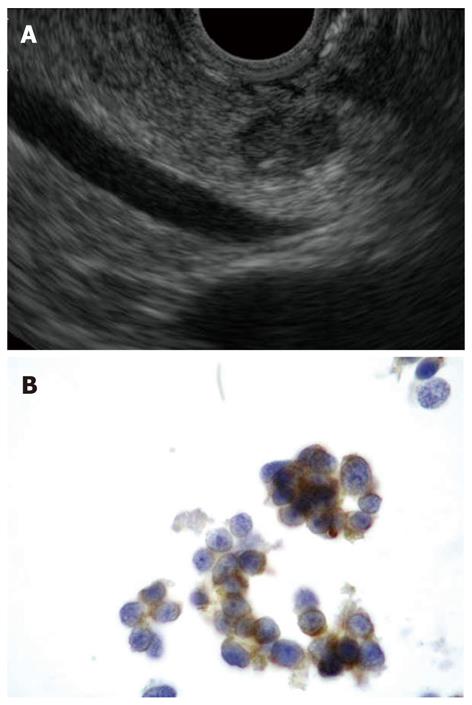
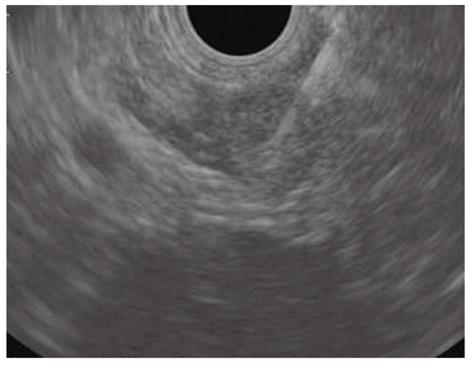
High sensitivity and diagnostic accuracy of EUS-FNA with immunocytochemical studies for PETs have been reported in two recent large retrospective cohorts of patients, including both functional and non-functional PETs (Figure 4)[74,75]. EUS-FNA helped to assess the malignant behavior of PETs and was able to predict 5-year survival after diagnosis[76]. Determination of Ki-67 expression on cytological specimens has still not gained widespread use due to the difficulty to obtain reproducible results and the availability of tissue biopsy specimens has been advocated[77,78].
Besides tissue diagnosis, studies aimed at directly assessing the clinical impact of EUS-FNA by demonstrating metastatic disease that would change the management of patients with pancreatic cancer are lacking. Mortensen et al[36] have performed the only study specifically designed to assess the impact of EUS-FNA in patients with pancreatic cancer. In 12 of 99 (12%) patients evaluated, EUS-FNA disclosed metastatic lymph nodes (6), liver lesions (4), malignant ascites (1), and retroperitoneal infiltration (1) that affected treatment decisions.
In the study by Spier et al[79] in Wisconsin on the predictors of pancreatic malignancy, they recommended close clinical follow up of patients suspected to have pancreatic cancer, with negative EUS-FNA, for at least 6 mo, since the FNA was not 100% reliable in ruling it out. This was more so in those individuals with vascular invasion or lymphadenopathy. Further they went on to say that further surveillance is not needed beyond 6 mo if there was no clinical or radiologic feature suggestive of malignancy[79].
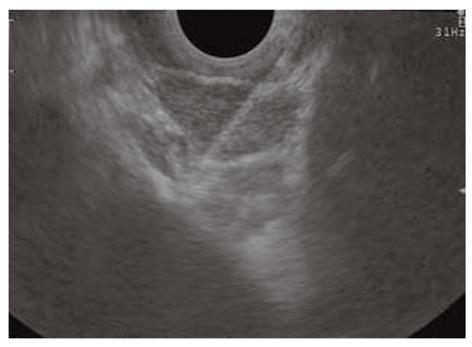
Pancreatic cysts encompass a wide spectrum of histological findings, including inflammatory pseudocysts, benign serous cystadenoma, and premalignant or malignant lesions, such as intraductal papillary mucinous neoplasms (IPMN), mucinous cystadenoma and cystadenocarcinoma. Because EUS imaging features are not sufficiently accurate to differentiate between mucinous and non-mucinous lesions and between benign and malignant IPMN, EUS-FNA has increasingly been used for the evaluation of pancreatic cysts (Figure 5)[80]. Cystic contents collected with EUS-FNA should be analyzed at least for cytology, amylase and carcinoembryonic antigen (CEA) (Table 2). The poor cellularity of the aspirated fluid limits the value of the cytologic examination in the distinction between mucinous and non-mucinous cysts. For this distinction, cystic fluid concentration of CEA has proved to be the most important test to identify mucinous lesions, despite considerable variation and overlapping in values[81]. CEA levels less than 5 ng/mL and greater than 800 ng/mL have been found in a pooled analysis of the published studies to be highly diagnostic for serous cystadenomas and mucinous lesions, respectively[82]. Determination of cyst amylase concentrations may help to further narrow the differential diagnosis, with high levels more frequently found in cysts that communicate with the main pancreatic duct (pseudocyst or IPMN), while values below 250 U/L virtually excludes a pancreatic pseudocyst[82]. The value of all of these analyses is limited by a relatively low sensitivity and by the fact that a minimum of 1 mL of liquid is required to perform the analysis, a task that is not feasible in lesions less than 1 cm in diameter.
| Cytology | Viscosity | Cyst CEA levels | Cyst amylase level | |
| Serous cyst adenoma | Bland PAS+ | Low | Low | Low |
| Mucinous cyst | Mucinous | Increased | High | Low |
| IPMN | Mucinous | High | High | High |
| Pseudocyst | Pigmented histiocytes | Low | Low | High |
A very high sensitivity and specificity of fluid analysis has been reached by using a combination of viscosity measurements, CEA and amylase levels[83]. Moreover, promising data on the use of cystic fluid DNA analysis in combination with CEA levels have been recently published[84,85], as well as the proteomic analysis of cyst fluid that could provide reliable candidates for developing new biomarkers for the preoperative management of pancreatic cysts[86]. Finally, the utilization of the tru cut needle and the echo brush to respectively acquire histological and cytological sample directly from the cystic wall appeared appealing, but their used is not widely accepted because of the potential risk of complications[87-89].
Malignant bile duct tumors, cholangiocarcinomas, present as biliary strictures that need to be differentiated from strictures of benign origin. EUS FNA has been used in some centers as the second diagnostic modality in case of encoscopic retrograde cholangio-pancreatography failure[90-92]. In this clinical setting, EUS FNA has been found to have a sensitivity ranging from 43% to 86% for all biliary strictures and from 25% to 83% for those limited to the hilum[92]. Interestingly, preliminary results from an ongoing experience have used EUS FNA, performed with the newly developed forward viewing scope that seems to have some advantages over the conventional linear EUS scope for visualization of the hilar region, as a first diagnostic modality to guide further management decision in patients with hilar strictures (Figure 6)[93]. Finally, in patients with hilar tumors who are suitable for a multidisciplinary therapeutic approach recently developed at the Mayo Clinic including chemo-and radiation-therapy in association with liver transplantation[94], EUS-FNA of regional lymph nodes has a tremendous clinical impact in the selection of patients, avoiding unnecessary transplantation in about 20% of them[95].
In patients with or suspected lung cancer established indications of EUS-FNA, which is able to access the posterior mediastinum (Figure 7), the paraesophageal lymph nodes, the left adrenal gland (Figure 8), and the liver, are either to obtain a definitive diagnosis by sampling centrally located lesions or mediastinal lymph nodes or to sample tissue from mediastinal lymph nodes and other locations in order to stage non-small cell lung cancer (NSCLC)[96]. Correct staging of NSCLC is important for rational allocation of patients to surgery, neoadjuvant or palliation therapy, whereas the current recommended treatment of small cell lung cancer involves chemotherapy and radiotherapy[97].
In patients with centrally located lesions after a previously non-diagnostic bronchoscopy, EUS-FNA has been found to have an extremely high diagnostic accuracy (97%) and may replace CT-guided biopsies and reduce the number of exploratory thoracotomies[98]. In view of the high sensitivity and specificity of EUS-FNA in the diagnosis of mediastinal lymph node metastases, as shown in two recently published large meta-analyses, the possible role of this technique as the first diagnostic test to be performed in patients with suspected lung cancer has also been evaluated[99]. Among 93 patients with a chest CT suspicious of lung cancer, EUS-FNA was able to establish tissue diagnosis in 70% of cases. Moreover, EUS-FNA was significantly better than CT at detecting distant metastases (accuracies of 97% and 89%, respectively; P = 0.02) and was able to detect small lymph node metastases (less than 1 cm) often missed by CT[99].
Several studies have clearly demonstrated the impact of EUS-FNA for staging of NSCLC. In patients with suspected or proven lung cancer and enlarged (> 1 cm) mediastinal lymph nodes on chest CT, EUS-FNA significantly reduces futile thoracotomies and prevents 66% of scheduled surgical procedures in these patients[100,101]. Regarding CT negative patients[102], EUS-FNA is able to detect advanced disease in 11% to 25% of cases, thus suggesting that it should be performed in all patients with NSCLC, irrespectively of the size of lymph nodes demonstrated by CT. EUS-FNA has also an important role in patients with a positive PET/CT to confirm or exclude the presence of mediastinal involvement[103,104], and the two methods should be seen as complementary[105]. On the other hand, patients without enlarged lymph nodes and a PET-negative mediastinum should proceed directly to surgery[106]. In addition, EUS-FNA should also be performed to provide tissue proof of left adrenal metastases in patients with enlarged or PET positive left adrenal gland[107].
EUS-FNA, however, cannot investigate the anterior mediastinum and cannot completely replace mediastinoscopy. Despite the fact that EUS-FNA can reduce the need for surgical staging[108], the best results are obtained when the two procedures are used together in a complimentary fashion with a significant reduction in the number of futile thoracotomies than when used alone[109,110]. Moreover, the recent introduction of the endobronchial linear EUS, able to perform transbronchial FNA (EBUS-TBNA) of the anterior mediastinum and to perform, in combination with EUS-FNA, a complete endoscopic mediastinoscopy has revolutionized the approach to the mediastinal staging of NSCLC, which will become more and more an endoscopic than a surgical procedure[111,112].
Posterior mediastinal lesions representing enlarged lymph nodes or masses can be caused by a variety of benign and malignant diseases other than lung or esophageal cancer. Compared with alternative techniques available for sampling the mediastinum, EUS-FNA is safer and less invasive than CT-guided biopsies or surgical procedures. In cases of suspected lymphoma or sarcoidosis, a core biopsy sample can be more useful than a cytological specimen and can be obtained using the tru cut biopsy needle or a standard 19 gauge needle (Figure 9)[16,23,113]. On the other hand, studies have demonstrated that EUS-FNA with cytological examination has a very high yield to diagnose both lymph node metastases derived from cancers located outside the mediastinum and extra-pulmonary tuberculosis[114,115]. Overall, EUS-FNA has a major impact in the management of 73%-94% of the patients with mediastinal lesions of unknown origin, most frequently by guiding therapy and avoiding surgery[116-118].
EUS-FNA has been reported to be able to sample lesions of the left and right adrenal glands[112,113,119,120], solid liver lesions[114,121] and more recently, lesions of the left and right kidneys[115,122]. For all these indications, the diagnostic yield of EUS-FNA has been reported to be quite high, with no evidence of procedure related complications apart from one patient with an occluded biliary stent at the time of EUS, who died of cholangitis after EUS-FNA of a liver lesion[114,121].
EUS-FNA has become an indispensable tool for the diagnosis and staging of gastrointestinal and thoracic malignancies. In the era of personalized medicine where cancer therapy is more frequently directed by molecular profiling[123], future efforts should be directed towards the development of safe and very accurate methods for acquiring tissue samples that will allow for genetic analysis to identify patients who can benefit from targeted therapies. The potential role of EUS-FNA in molecular diagnostics coupled with the emerging potential of EUS-guided antitumor injection and tumor ablation procedures, will further expand the diagnostic and therapeutic applications of EUS-FNA in the future.
Peer reviewer: Stefanos Karagiannis, MD, PhD, Gastrointestinal and Liver Unit, General and Oncology Kifissia Hospital Agioi Anargiri’, Kaliftaki 14564, Greece
S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Henriksen FW, Hancke S. Percutaneous cystogastrostomy for chronic pancreatic pseudocyst. Br J Surg. 1994;81:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 410] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 2. | Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Voermans RP, Eisendrath P, Bruno MJ, Le Moine O, Devière J, Fockens P. Initial evaluation of a novel prototype forward-viewing US endoscope in transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2007;66:1013-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Binmoeller KF. Optimizing interventional EUS: the echoendoscope in evolution. Gastrointest Endosc. 2007;66:917-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Imazu H, Uchiyama Y, Kakutani H, Ikeda K, Sumiyama K, Kaise M, Omar S, Ang TL, Tajiri H. A prospective comparison of EUS-guided FNA using 25-gauge and 22-gauge needles. Gastroenterol Res Pract. 2009;2009:546390. [PubMed] |
| 6. | Lee JH, Stewart J, Ross WA, Anandasabapathy S, Xiao L, Staerkel G. Blinded prospective comparison of the performance of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of the pancreas and peri-pancreatic lesions. Dig Dis Sci. 2009;54:2274-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Siddiqui UD, Rossi F, Rosenthal LS, Padda MS, Murali-Dharan V, Aslanian HR. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Yusuf TE, Ho S, Pavey DA, Michael H, Gress FG. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: a multicenter experience. Endoscopy. 2009;41:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Levy MJ, Wiersema MJ. EUS-guided Trucut biopsy. Gastrointest Endosc. 2005;62:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Larghi A, Verna EC, Stavropoulos SN, Rotterdam H, Lightdale CJ, Stevens PD. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc. 2004;59:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Varadarajulu S, Fraig M, Schmulewitz N, Roberts S, Wildi S, Hawes RH, Hoffman BJ, Wallace MB. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Ginès A, Wiersema MJ, Clain JE, Pochron NL, Rajan E, Levy MJ. Prospective study of a Trucut needle for performing EUS-guided biopsy with EUS-guided FNA rescue. Gastrointest Endosc. 2005;62:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Wittmann J, Kocjan G, Sgouros SN, Deheragoda M, Pereira SP. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: a prospective study. Cytopathology. 2006;17:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Săftoiu A, Vilmann P, Guldhammer Skov B, Georgescu CV. Endoscopic ultrasound (EUS)-guided Trucut biopsy adds significant information to EUS-guided fine-needle aspiration in selected patients: a prospective study. Scand J Gastroenterol. 2007;42:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Storch I, Shah M, Thurer R, Donna E, Ribeiro A. Endoscopic ultrasound-guided fine-needle aspiration and Trucut biopsy in thoracic lesions: when tissue is the issue. Surg Endosc. 2008;22:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Thomas T, Kaye PV, Ragunath K, Aithal G. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: a large tertiary referral center experience. Am J Gastroenterol. 2009;104:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Berger LP, Scheffer RC, Weusten BL, Seldenrijk CA, de Bruin PC, Timmer R, Stolk MF. The additional value of EUS-guided Tru-cut biopsy to EUS-guided FNA in patients with mediastinal lesions. Gastrointest Endosc. 2009;69:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Ribeiro A, Vernon S, Quintela P. EUS-guided trucut biopsy with immunohistochemical analysis of a gastric stromal tumor. Gastrointest Endosc. 2004;60:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Polkowski M, Gerke W, Jarosz D, Nasierowska-Guttmejer A, Rutkowski P, Nowecki ZI, Ruka W, Regula J, Butruk E. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: a prospective study. Endoscopy. 2009;41:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Fernández-Esparrach G, Sendino O, Solé M, Pellisé M, Colomo L, Pardo A, Martínez-Pallí G, Argüello L, Bordas JM, Llach J. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: a randomized crossover study. Endoscopy. 2010;42:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 23. | Yasuda I, Tsurumi H, Omar S, Iwashita T, Kojima Y, Yamada T, Sawada M, Takami T, Moriwaki H, Soehendra N. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919-924. [PubMed] |
| 24. | Larghi A, Verna EC, Ricci R, Seerden TC, Galasso D, Carnuccio A, Uchida N, Rindi G, Costamagna G. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: a prospective study. Gastrointest Endosc. 2011;74:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Wallace MB, Kennedy T, Durkalski V, Eloubeidi MA, Etamad R, Matsuda K, Lewin D, Van Velse A, Hennesey W, Hawes RH. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Puri R, Vilmann P, Săftoiu A, Skov BG, Linnemann D, Hassan H, Garcia ES, Gorunescu F. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Sahai AV, Paquin SC, Gariépy G. A prospective comparison of endoscopic ultrasound-guided fine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2010;42:900-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Rastogi A, Wani S, Gupta N, Singh V, Gaddam S, Reddymasu S, Ulusarac O, Fan F, Romanas M, Dennis KL. A prospective, single-blind, randomized, controlled trial of EUS-guided FNA with and without a stylet. Gastrointest Endosc. 2011;74:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 30. | Alsohaibani F, Girgis S, Sandha GS. Does onsite cytotechnology evaluation improve the accuracy of endoscopic ultrasound-guided fine-needle aspiration biopsy? Can J Gastroenterol. 2009;23:26-30. [PubMed] |
| 31. | Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 299] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S, Collins E. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475-481. [PubMed] |
| 33. | Pellisé Urquiza M, Fernández-Esparrach G, Solé M, Colomo L, Castells A, Llach J, Mata A, Bordas JM, Piqué JM, Ginès A. Endoscopic ultrasound-guided fine needle aspiration: predictive factors of accurate diagnosis and cost-minimization analysis of on-site pathologist. Gastroenterol Hepatol. 2007;30:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Vazquez-Sequeiros E, Wiersema MJ, Clain JE, Norton ID, Levy MJ, Romero Y, Salomao D, Dierkhising R, Zinsmeister AR. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology. 2003;125:1626-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Vazquez-Sequeiros E, Levy MJ, Clain JE, Schwartz DA, Harewood GC, Salomao D, Wiersema MJ. Routine vs. selective EUS-guided FNA approach for preoperative nodal staging of esophageal carcinoma. Gastrointest Endosc. 2006;63:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Mortensen MB, Pless T, Durup J, Ainsworth AP, Plagborg GJ, Hovendal C. Clinical impact of endoscopic ultrasound-guided fine needle aspiration biopsy in patients with upper gastrointestinal tract malignancies. A prospective study. Endoscopy. 2001;33:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Giovannini M, Monges G, Seitz JF, Moutardier V, Bernardini D, Thomas P, Houvenaeghel G, Delpero JR, Giudicelli R, Fuentes P. Distant lymph node metastases in esophageal cancer: impact of endoscopic ultrasound-guided biopsy. Endoscopy. 1999;31:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | McGrath K, Brody D, Luketich J, Khalid A. Detection of unsuspected left hepatic lobe metastases during EUS staging of cancer of the esophagus and cardia. Am J Gastroenterol. 2006;101:1742-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Marsman WA, Brink MA, Bergman JJ, Tytgat GN, ten Kate FJ, van Lanschot JJ, Fockens P. Potential impact of EUS-FNA staging of proximal lymph nodes in patients with distal esophageal carcinoma. Endoscopy. 2006;38:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA, Eloubeidi MA. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. 2005;129:1232-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4611] [Article Influence: 242.7] [Reference Citation Analysis (0)] |
| 42. | Mortensen MB, Fristrup CW, Ainsworth AP, Pless T, Nielsen HO, Hovendal C. Combined preoperative endoscopic and laparoscopic ultrasonography for prediction of R0 resection in upper gastrointestinal tract cancer. Br J Surg. 2006;93:720-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Mortensen MB, Fristrup C, Ainsworth A, Nielsen HO, Pless T, Hovendal C. Combined pretherapeutic endoscopic and laparoscopic ultrasonography may predict survival of patients with upper gastrointestinal tract cancer. Surg Endosc. 2011;25:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Hassan H, Vilmann P, Sharma V. Impact of EUS-guided FNA on management of gastric carcinoma. Gastrointest Endosc. 2010;71:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Harewood GC, Wiersema MJ, Nelson H, Maccarty RL, Olson JE, Clain JE, Ahlquist DA, Jondal ML. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Shami VM, Parmar KS, Waxman I. Clinical impact of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in the management of rectal carcinoma. Dis Colon Rectum. 2004;47:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Sasaki Y, Niwa Y, Hirooka Y, Ohmiya N, Itoh A, Ando N, Miyahara R, Furuta S, Goto H. The use of endoscopic ultrasound-guided fine-needle aspiration for investigation of submucosal and extrinsic masses of the colon and rectum. Endoscopy. 2005;37:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Hünerbein M, Totkas S, Moesta KT, Ulmer C, Handke T, Schlag PM. The role of transrectal ultrasound-guided biopsy in the postoperative follow-up of patients with rectal cancer. Surgery. 2001;129:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Landi B, Palazzo L. The role of endosonography in submucosal tumours. Best Pract Res Clin Gastroenterol. 2009;23:679-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 51. | Mekky MA, Yamao K, Sawaki A, Mizuno N, Hara K, Nafeh MA, Osman AM, Koshikawa T, Yatabe Y, Bhatia V. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 52. | Philipper M, Hollerbach S, Gabbert HE, Heikaus S, Böcking A, Pomjanski N, Neuhaus H, Frieling T, Schumacher B. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 53. | Casali PG, Blay JY. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 54. | Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc. 2006;63:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 55. | Chang KJ. State of the art lecture: endoscopic ultrasound (EUS) and FNA in pancreatico-biliary tumors. Endoscopy. 2006;38 Suppl 1:S56-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Boujaoude J. Role of endoscopic ultrasound in diagnosis and therapy of pancreatic adenocarcinoma. World J Gastroenterol. 2007;13:3662-3666. [PubMed] |
| 57. | Horwhat JD, Paulson EK, McGrath K, Branch MS, Baillie J, Tyler D, Pappas T, Enns R, Robuck G, Stiffler H. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006;63:966-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 58. | Eisen GM, Dominitz JA, Faigel DO, Goldstein JA, Petersen BT, Raddawi HM, Ryan ME, Vargo JJ, Young HS, Wheeler-Harbaugh J. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 59. | DeWitt J, LeBlanc J, McHenry L, Ciaccia D, Imperiale T, Chappo J, Cramer H, McGreevy K, Chriswell M, Sherman S. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: a large single-center experience. Am J Gastroenterol. 2003;98:1976-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 60. | DeWitt J, LeBlanc J, McHenry L, McGreevy K, Sherman S. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007;5:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | DeWitt J, Yu M, Al-Haddad MA, Sherman S, McHenry L, Leblanc JK. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by EUS-FNA. Gastrointest Endosc. 2010;71:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 63. | Eloubeidi MA, Varadarajulu S, Desai S, Wilcox CM. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol. 2008;23:567-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Tadic M, Kujundzic M, Stoos-Veic T, Kaic G, Vukelic-Markovic M. Role of repeated endoscopic ultrasound-guided fine needle aspiration in small solid pancreatic masses with previous indeterminate and negative cytological findings. Dig Dis. 2008;26:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Hartwig W, Schneider L, Diener MK, Bergmann F, Büchler MW, Werner J. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 66. | Mortenson MM, Katz MH, Tamm EP, Bhutani MS, Wang H, Evans DB, Fleming JB. Current diagnosis and management of unusual pancreatic tumors. Am J Surg. 2008;196:100-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet JC, Queneau PE, Scoazec JY. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 69. | Khalid A, Nodit L, Zahid M, Bauer K, Brody D, Finkelstein SD, McGrath KM. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am J Gastroenterol. 2006;101:2493-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Bournet B, Souque A, Senesse P, Assenat E, Barthet M, Lesavre N, Aubert A, O'Toole D, Hammel P, Levy P. Endoscopic ultrasound-guided fine-needle aspiration biopsy coupled with KRAS mutation assay to distinguish pancreatic cancer from pseudotumoral chronic pancreatitis. Endoscopy. 2009;41:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Maluf-Filho F, Kumar A, Gerhardt R, Kubrusly M, Sakai P, Hondo F, Matuguma SE, Artifon E, Monteiro da Cunha JE, César Machado MC. Kras mutation analysis of fine needle aspirate under EUS guidance facilitates risk stratification of patients with pancreatic mass. J Clin Gastroenterol. 2007;41:906-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Mizuno N, Bhatia V, Hosoda W, Sawaki A, Hoki N, Hara K, Takagi T, Ko SB, Yatabe Y, Goto H. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol. 2009;44:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 73. | Al-Haddad M, Savabi MS, Sherman S, McHenry L, Leblanc J, Cramer H, Emerson R, O'Neil J, Khashab M, Dewitt J. Role of endoscopic ultrasound-guided fine-needle aspiration with flow cytometry to diagnose lymphoma: a single center experience. J Gastroenterol Hepatol. 2009;24:1826-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Figueiredo FA, Giovannini M, Monges G, Charfi S, Bories E, Pesenti C, Caillol F, Delpero JR. Pancreatic endocrine tumors: a large single-center experience. Pancreas. 2009;38:936-940. [PubMed] |
| 75. | Pais SA, Al-Haddad M, Mohamadnejad M, Leblanc JK, Sherman S, McHenry L, DeWitt JM. EUS for pancreatic neuroendocrine tumors: a single-center, 11-year experience. Gastrointest Endosc. 2010;71:1185-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | Figueiredo FA, Giovannini M, Monges G, Bories E, Pesenti C, Caillol F, Delpero JR. EUS-FNA predicts 5-year survival in pancreatic endocrine tumors. Gastrointest Endosc. 2009;70:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Piani C, Franchi GM, Cappelletti C, Scavini M, Albarello L, Zerbi A, Giorgio Arcidiacono P, Bosi E, Manzoni MF. Cytological Ki-67 in pancreatic endocrine tumours: an opportunity for pre-operative grading. Endocr Relat Cancer. 2008;15:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 78. | Klöppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, Scarpa A, Scoazec JY, Wiedenmann B, Papotti M. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 79. | Spier BJ, Johnson EA, Gopal DV, Frick T, Einstein MM, Byrne S, Koscik RL, Liou JI, Broxmeyer T, Selvaggi SM. Predictors of malignancy and recommended follow-up in patients with negative endoscopic ultrasound-guided fine-needle aspiration of suspected pancreatic lesions. Can J Gastroenterol. 2009;23:279-286. [PubMed] |
| 80. | Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 490] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 81. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 901] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 82. | van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 83. | Linder JD, Geenen JE, Catalano MF. Cyst fluid analysis obtained by EUS-guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: a prospective single-center experience. Gastrointest Endosc. 2006;64:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 84. | Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, Brugge WR, Edmundowicz SA, Hawes RH, McGrath KM. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 85. | Sawhney MS, Devarajan S, O'Farrel P, Cury MS, Kundu R, Vollmer CM, Brown A, Chuttani R, Pleskow DK. Comparison of carcinoembryonic antigen and molecular analysis in pancreatic cyst fluid. Gastrointest Endosc. 2009;69:1106-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 86. | Cuoghi A, Farina A, Z'graggen K, Dumonceau JM, Tomasi A, Hochstrasser DF, Genevay M, Lescuyer P, Frossard JL. Role of proteomics to differentiate between benign and potentially malignant pancreatic cysts. J Proteome Res. 2011;10:2664-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Levy MJ, Smyrk TC, Reddy RP, Clain JE, Harewood GC, Kendrick ML, Pearson RK, Petersen BT, Rajan E, Topazian MD. Endoscopic ultrasound-guided trucut biopsy of the cyst wall for diagnosing cystic pancreatic tumors. Clin Gastroenterol Hepatol. 2005;3:974-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Al-Haddad M, Gill KR, Raimondo M, Woodward TA, Krishna M, Crook JE, Skarvinko LN, Jamil LH, Hasan M, Wallace MB. Safety and efficacy of cytology brushings versus standard fine-needle aspiration in evaluating cystic pancreatic lesions: a controlled study. Endoscopy. 2010;42:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 89. | Sendino O, Fernández-Esparrach G, Solé M, Colomo L, Pellisé M, Llach J, Navarro S, Bordas JM, Ginès A. Endoscopic ultrasonography-guided brushing increases cellular diagnosis of pancreatic cysts: A prospective study. Dig Liver Dis. 2010;42:877-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 90. | Fritscher-Ravens A, Broering DC, Sriram PV, Topalidis T, Jaeckle S, Thonke F, Soehendra N. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a case series. Gastrointest Endosc. 2000;52:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 91. | DeWitt J, Misra VL, Leblanc JK, McHenry L, Sherman S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 92. | Pavey DA, Gress FG. The role of EUS-guided FNA for the evaluation of biliary strictures. Gastrointest Endosc. 2006;64:334-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Larghi A, Lecca PG, Ardito F, Rossi ED, Fadda G, Nuzzo G, Costamagna G. Evaluation of hilar biliary strictures by using a newly developed forward-viewing therapeutic echoendoscope: preliminary results of an ongoing experience. Gastrointest Endosc. 2009;69:356-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, Gores GJ, Nagorney DM. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451-48; discussion 451-48;. [PubMed] |
| 95. | Gleeson FC, Rajan E, Levy MJ, Clain JE, Topazian MD, Harewood GC, Papachristou GI, Takahashi N, Rosen CB, Gores GJ. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 96. | Vilmann P, Annema J, Clementsen P. Endosonography in bronchopulmonary disease. Best Pract Res Clin Gastroenterol. 2009;23:711-728. [PubMed] |
| 97. | Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1876] [Cited by in RCA: 1999] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 98. | Annema JT, Veseliç M, Rabe KF. EUS-guided FNA of centrally located lung tumours following a non-diagnostic bronchoscopy. Lung Cancer. 2005;48:357-61; discussion 363-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Singh P, Camazine B, Jadhav Y, Gupta R, Mukhopadhyay P, Khan A, Reddy R, Zheng Q, Smith DD, Khode R. Endoscopic ultrasound as a first test for diagnosis and staging of lung cancer: a prospective study. Am J Respir Crit Care Med. 2007;175:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Larsen SS, Vilmann P, Krasnik M, Dirksen A, Clementsen P, Maltbaek N, Lassen U, Skov BG, Jacobsen GK. Endoscopic ultrasound guided biopsy performed routinely in lung cancer staging spares futile thoracotomies: preliminary results from a randomised clinical trial. Lung Cancer. 2005;49:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Annema JT, Versteegh MI, Veseliç M, Voigt P, Rabe KF. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. J Clin Oncol. 2005;23:8357-8361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 102. | Wallace MB, Ravenel J, Block MI, Fraig M, Silvestri G, Wildi S, Schmulewitz N, Varadarajulu S, Roberts S, Hoffman BJ. Endoscopic ultrasound in lung cancer patients with a normal mediastinum on computed tomography. Ann Thorac Surg. 2004;77:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Annema JT, Hoekstra OS, Smit EF, Veseliç M, Versteegh MI, Rabe KF. Towards a minimally invasive staging strategy in NSCLC: analysis of PET positive mediastinal lesions by EUS-FNA. Lung Cancer. 2004;44:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 104. | Eloubeidi MA, Cerfolio RJ, Chen VK, Desmond R, Syed S, Ojha B. Endoscopic ultrasound-guided fine needle aspiration of mediastinal lymph node in patients with suspected lung cancer after positron emission tomography and computed tomography scans. Ann Thorac Surg. 2005;79:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 105. | Kalade AV, Eddie Lau WF, Conron M, Wright GM, Desmond PV, Hicks RJ, Chen R. Endoscopic ultrasound-guided fine-needle aspiration when combined with positron emission tomography improves specificity and overall diagnostic accuracy in unexplained mediastinal lymphadenopathy and staging of non-small-cell lung cancer. Intern Med J. 2008;38:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Fischer BM, Mortensen J, Hansen H, Vilmann P, Larsen SS, Loft A, Bertelsen AK, Ravn J, Clementsen P, Høegholm A. Multimodality approach to mediastinal staging in non-small cell lung cancer. Faults and benefits of PET-CT: a randomised trial. Thorax. 2011;66:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 107. | Schuurbiers OC, Tournoy KG, Schoppers HJ, Dijkman BG, Timmers HJ, de Geus-Oei LF, Grefte JM, Rabe KF, Dekhuijzen PN, van der Heijden HF. EUS-FNA for the detection of left adrenal metastasis in patients with lung cancer. Lung Cancer. 2011;73:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 108. | Tournoy KG, De Ryck F, Vanwalleghem LR, Vermassen F, Praet M, Aerts JG, Van Maele G, van Meerbeeck JP. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. Am J Respir Crit Care Med. 2008;177:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 109. | Annema JT, Versteegh MI, Veseliç M, Welker L, Mauad T, Sont JK, Willems LN, Rabe KF. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA. 2005;294:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 110. | Witte B, Neumeister W, Huertgen M. Does endoesophageal ultrasound-guided fine-needle aspiration replace mediastinoscopy in mediastinal staging of thoracic malignancies? Eur J Cardiothorac Surg. 2008;33:1124-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Annema JT, van Meerbeeck JP, Rintoul RC, Dooms C, Deschepper E, Dekkers OM, De Leyn P, Braun J, Carroll NR, Praet M. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304:2245-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 378] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 112. | Wallace MB, Pascual JM, Raimondo M, Woodward TA, McComb BL, Crook JE, Johnson MM, Al-Haddad MA, Gross SA, Pungpapong S. Minimally invasive endoscopic staging of suspected lung cancer. JAMA. 2008;299:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 113. | Iwashita T, Yasuda I, Doi S, Kato T, Sano K, Yasuda S, Nakashima M, Hirose Y, Takaimi T, Moriwaki H. The yield of endoscopic ultrasound-guided fine needle aspiration for histological diagnosis in patients suspected of stage I sarcoidosis. Endoscopy. 2008;40:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 114. | Peric R, Schuurbiers OC, Veseliç M, Rabe KF, van der Heijden HF, Annema JT. Transesophageal endoscopic ultrasound-guided fine-needle aspiration for the mediastinal staging of extrathoracic tumors: a new perspective. Ann Oncol. 2010;21:1468-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Puri R, Vilmann P, Sud R, Kumar M, Taneja S, Verma K, Kaushik N. Endoscopic ultrasound-guided fine-needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy. 2010;42:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 116. | Catalano MF, Rosenblatt ML, Chak A, Sivak MV, Scheiman J, Gress F. Endoscopic ultrasound-guided fine needle aspiration in the diagnosis of mediastinal masses of unknown origin. Am J Gastroenterol. 2002;97:2559-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Larsen SS, Krasnik M, Vilmann P, Jacobsen GK, Pedersen JH, Faurschou P, Folke K. Endoscopic ultrasound guided biopsy of mediastinal lesions has a major impact on patient management. Thorax. 2002;57:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 118. | Savides TJ, Perricone A. Impact of EUS-guided FNA of enlarged mediastinal lymph nodes on subsequent thoracic surgery rates. Gastrointest Endosc. 2004;60:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Eloubeidi MA, Black KR, Tamhane A, Eltoum IA, Bryant A, Cerfolio RJ. A large single-center experience of EUS-guided FNA of the left and right adrenal glands: diagnostic utility and impact on patient management. Gastrointest Endosc. 2010;71:745-753. [PubMed] |
| 120. | DeWitt J, Alsatie M, LeBlanc J, McHenry L, Sherman S. Endoscopic ultrasound-guided fine-needle aspiration of left adrenal gland masses. Endoscopy. 2007;39:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 121. | tenBerge J, Hoffman BJ, Hawes RH, Van Enckevort C, Giovannini M, Erickson RA, Catalano MF, Fogel R, Mallery S, Faigel DO. EUS-guided fine needle aspiration of the liver: indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 122. | DeWitt J, Gress FG, Levy MJ, Hernandez LV, Eloubeidi MA, Mishra G, Sherman S, Al-Haddad MA, LeBlanc JK. EUS-guided FNA aspiration of kidney masses: a multicenter U.S. experience. Gastrointest Endosc. 2009;70:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 123. | Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1149] [Article Influence: 76.6] [Reference Citation Analysis (0)] |