Published online May 16, 2011. doi: 10.4253/wjge.v3.i5.105
Revised: December 24, 2010
Accepted: December 31, 2010
Published online: May 16, 2011
Gastric antral vascular ectasia often results in chronic gastrointestinal bleeding with few options for effective treatment. The Halo® 90 system has been newly approved for this indication. A 56 year old male with ETOH cirrhosis and gastrointestinal bleeding from gastric vascular ectasia presented for endoscopy with Halo® 90 radiofrequency ablation. Over the past two years he had undergone multiple bipolar electric coagulation and argon plasma coagulation treatments. Despite this therapy, he con-
tinued to receive monthly blood transfusions. We therefore opted to treat the vascular anomalies with the Halo® 90 system utilizing radiofrequency ablation. Upon withdrawal of the endoscope post procedure, mild resistance and bleeding was noted at the gastroesophageal junction. Repeat endoscopy revealed a submucosal tear at the gastroesophageal junction. This is the first reported complication of the Halo® 90 system when used for gastric antral vascular ectasia.
- Citation: Gutkin E, Schnall A. Gastroesophageal junction tear from HALO 90® System: A case report. World J Gastrointest Endosc 2011; 3(5): 105-106
- URL: https://www.wjgnet.com/1948-5190/full/v3/i5/105.htm
- DOI: https://dx.doi.org/10.4253/wjge.v3.i5.105
Gastric antral vascular ectasia (GAVE) can present in patients with cirrhosis and portal hypertension, as well as patients with autoimmune disease[1]. GAVE is characterized by red patches or spots in either diffuse or linear array in the antrum of the stomach[1]. These vascular ectasias can lead to acute or chronic hemorrhage and iron deficiency anemia[2]. The initial management of these patients includes endoscopic argon plasma coagulation; however, despite repeat APC, some patients require frequent transfusions. Evaluation for liver transplantation should also be performed as vascular ectasias have been noted to improve post transplant[2]. Other therapies include Nd:YAG (neodymium:yttrium-aluminum-garnet) laser coagulation but this carries a higher risk of perforation given the deeper thermal effect. Endoscopic sclerotherapy, heater probe, cryotherapy and banding in the antrum of the stomach have also been described in the literature[2]. When endoscopic therapy is unsuccessful, surgery with antrectomy can be considered but carries a high surgical risk, especially in the cirrhotic patient[1].
The BARRX-Halo® is a radiofrequency ablation system (RFA) used for endoscopic treatment of Barrett’s esophagus[3]. The device can be fitted with a balloon (Halo® 360) or an electrode plate (Halo® 90). The Halo® 90 radiofrequency ablation system has been newly approved for treatment of gastric antral vascular ectasia. Only once case series of its use exists in the literature and no complications of its use have been reported until now.
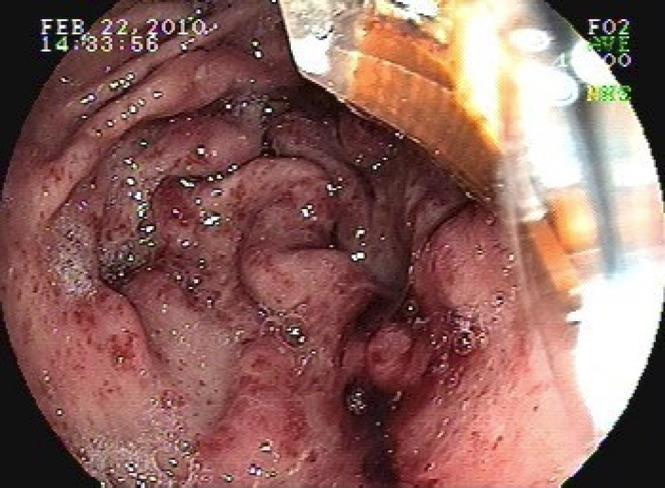
A 56 year old male with ETOH cirrhosis and gastrointestinal bleeding from gastric vascular ectasia (Figure 1) presented for endoscopy with Halo® 90 radiofrequency ablation. He had undergone multiple bipolar electric coagulation and argon plasma coagulation treatments over the past two years. He was maintained on double dose proton pump inhibitors, sucralfate suspension, as well as estrogen for stabilization of vascular endothelial membranes and B-blockers for portal hypertension. Over the past two months his transfusion requirement increased to four units of packed red cells monthly and he had undergone three treatments with the argon plasma coagulator without diminution of bleeding. We therefore opted to treat the vascular anomalies with the Halo® 90 system utilizing radiofrequency ablation.
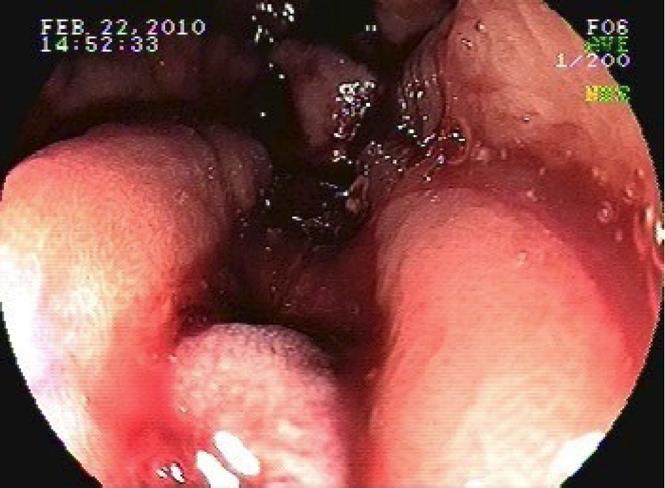
On endoscopy, multiple vascular ectasias were seen throughout the stomach, with an abundance of lesions in the antrum along with fresh blood. The area was treated with Halo® 90 RFA at four sites (48 ablations at 12 joules/40 watts). The gastroesophageal junction (GEJ) was viewed multiple times and was normal other than the presence of vascular anomalies. Upon withdrawal of the endoscope, there was mild resistance felt at the GEJ and immediate bleeding was noted (Figure 2). When the instrument was removed from the patient, the Halo® probe was alongside but no longer attached to the scope. The endoscope was reinserted and a mucosal/submucosal tear was noted at the GE junction which was not amenable to placement of Hemoclips. The bleeding was self-limited and ceased spontaneously. There was no endoscopic evidence of perforation. The exact mechanism of the esophageal tear remains unclear. The patient did not retch during the exam, nor was the withdrawal of the endoscope rapid or forceful, but we surmise that it was a result of the Halo® system as it dislodged from the endoscope.
The patient was subsequently admitted to the hospital for twenty-four hours for monitoring; there was no free air seen on radiological imaging and his blood counts remained stable.
One month later, a follow up endoscopy revealed healing of the GE junction tear and there was dramatic improvement and diminution of the antral vascular anomalies without bleeding. The patient’s hemoglobin has increased to 15 mg/dL without any further transfusion requirement.
Gastric antral vascular ectasia often results in chronic gastrointestinal bleeding with few options for effective treatment. The Halo® 90 system has been newly approved for this indication and appears to have promising results. A recent pilot study of six patients with GAVE using the Halo® system showed a reversal of transfusion requirements in 5/6 patients[4]. No complications were reported in that study.
This is the first reported complication of the Halo® 90 system when used for GAVE. Despite the lack of definitive proof, we believe that the use of a foreign body hood placed above the Halo® 90 device when gastric manipulations are performed would prevent trauma to the GE junction; upon withdrawal of the endoscope the hood would retract over the device and avoid complications similar to ours.
Peer reviewers: Adam Donald Farmer, DR, Wingate Institute of Neurogastroenterology, 26 Ashfield Street, London E1 2AJ, United Kingdom
S- Editor Zhang HN L- Editor Roemmele A E- Editor Zhang L
| 1. | Burak KW, Lee SS, Beck PL. Portal hypertensive gastropathy and gastric antral vascular ectasia (GAVE) syndrome. Gut. 2001;49:866-872. |
| 2. | Ripoll C, Garcia-Tsao G. Management of Gastropathy and Gastric Vascular Ectasia in Portal Hypertension. Clin Liver Dis. 2010;14:281-295. |
| 3. | Fleischer DE, Sharma VK. Endoscopic Ablation of Barrett’s Esophagus Using the Halo® System. Dig Dis. 2008;26:280-284. |
| 4. | Gross SA, Al-Haddad M, Gill KR, Schore AN, Wallace MB. Endoscopic mucosal ablation for the treatment of gastric antral vascular ectasia with the HALO90 system: a pilot study. Gastrointest Endosc. 2008;67:324-327. |