INTRODUCTION
The history of gastrointestinal endoscopy is one of striking technical advances. From the first rigid instrument developed by Kussmaul in Germany, or the semi-flexible instruments designed by Rudolf Schindler in Chicago, to the current video endoscopes, a more accurate visualization of the gastrointestinal tract offers increasing knowledge of gastrointestinal disease and better therapeutic possibilities. However, endoscopic assessment of the small bowel has remained a challenge, because its length and tortuosity determines a major difficulty for its exploration with flexible endoscopes. Sonde and push enteroscopes provided a significant advance in this field[1-3].
Capsule endoscopy (CE) was initially marketed in 2001, and, to date, has been the greatest advance in the field of small bowel exploration. The procedure provides state-of-the-art imaging of the small intestine, and is recommended as the third test to be used in the investigation of obscure gastrointestinal bleeding, after colonoscopy and upper endoscopy[4]. Nevertheless, in small bowel Crohn’s disease, the role of capsule endoscopy is not clear, but despite this, there have been some papers which have addressed this issue[5-16]. A meta-analysis of eleven studies which included 223 patients and compared CE to other imaging modalities for inflammatory bowel disease, established that CE has an incremental diagnostic yield of 25%-40% over other methods, such as barium studies or CT scanning[17]. However, some other well designed papers have limited the role of CE in comparisons with other procedures as CT enterography, ileocolonoscopy or small bowel follow-through[18]. Indeed, in the setting of Crohn’s disease, it is widely recognized that its clinical role remains uncertain[14,19].
When evaluating the importance of this new technology in inflammatory bowel disease, several issues regarding its role present themselves[20]: (1)The suspected Crohn’s disease, when the usual diagnosis workup in negative or non diagnostic; (2) Assessment of severity of small bowel Crohn’s disease; (3) Study of the patient with IBD and a gastrointestinal bleeding of obscure origin; (4) CE as a guide to therapy; (5) Unclassified IBD; and (6) The role of CE in Ulcerative Colitis (UC).
Nowadays, the first item is most frequently encountered, and the one which has given rise to greater contention, given the widespread use of CE for diagnostic purposes. Nevertheless, these indications have already been established, but remain subjects of research, and the number of papers focused on each of these issues is increasing. In the following pages, the evidence will be examined in order to suggest a recommendation for each indication.
ROLE OF CE IN SUSPECTED CROHN’S DISEASE
To date, there is no single gold standard diagnostic test for Crohn’s disease[14]. The diagnosis is based on a composite of findings, including the clinical history and physical examination of the patient, and biochemical, endoscopic, radiologic and pathologic findings[20]. Until the implementation of CE, the diagnosis was made on the basis of small bowel radiology and ileocolonoscopy, and was seldom aided by push enteroscopy. Histological confirmation of the disease is still possible only in a minority of those patients, and an image-based diagnosis is the usual setting[18].
However, the main question, when using CE in this case, is in which position of the diagnostic workup it can be performed in order to optimize the use of what is an expensive and time consuming technology. Previously, suspicion of Crohn’s disease was left to the expertise of the treating physician, and the diagnostic protocol was triggered when a patient had abdominal pain or diarrhea. The diagnostic yield of CE is low when performed in patients with abdominal pain alone, or in patients with abdominal pain and diarrhea[21,22]. When other criteria are present, this yield increases. These criteria are inflammatory serum markers (ESR, C reactive protein, thrombocytosis or leukocytosis). In an interesting paper by Fireman, which enrolled patients with abdominal pain, diarrhea, anemia and weight loss with an average symptom duration of 6.3 years, and all of them having presented with normal colonoscopies, upper endoscopies and small bowel follow-through, Crohn’s disease was found by CE in 12 of the 17 patients[23,24].
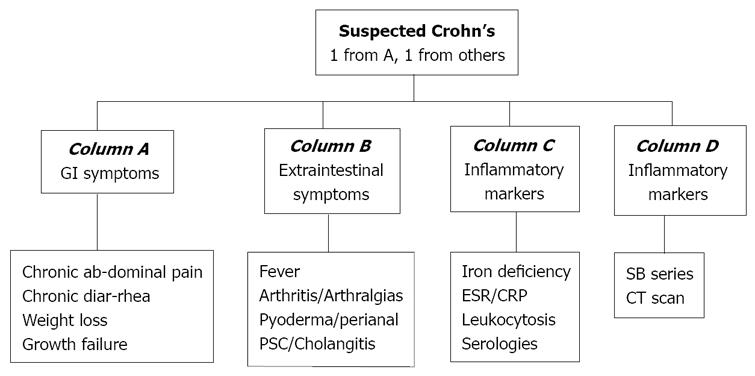
Recently, an international OMED and ECCO consensus confirmed a previous recommendation in this group of patients: ileocolonoscopy and small bowel imaging should generally precede CE. the choice of the radiographic procedure depends on local availability[20]. Furthermore, some of those radiologic methods have shown a similar sensitivity to CE, which supports the initiation of the diagnostic work-up as recommended[18]. A previously established consensus panel, the ICCE, agreed to expand their definition of patients who should be considered as being suspected of having Crohn’s disease. An algorithm was formulated, which included symptoms as well as extraintestinal manifestations, inflammatory markers or abnormal imaging studies[25] (Figure 1). Moreover, a recent follow-up study including 102 patients with suspected Crohn’s disease observed small bowel inflammatory changes in 35% of patients, and a prevalence of Crohn’s disease after 12 mo follow-up of 13%. A poor positive predictive value was observed (31%), although it increased by up to 50% when more stringent criteria were required for the diagnosis of Crohn’s disease by CE, as well as when ICCE clinical criteria (Figure 1) where considered as a guide to patient selection[14]. In summary, it seems obvious that the addition of more than one suggestive clinical symptom increases the adequacy of CE for the diagnosis of Crohn’s disease[9,19,20].
Figure 1 Criteria for suspected Crohn’s disease.
(Mergener et al[18]) (PSC: Primary sclerosing cholangitis; ESR: Erythrocyte sedimentation rate; CRP: C reactive protein; SB series: Small bowel series; CT scan: Computed tomography scan).
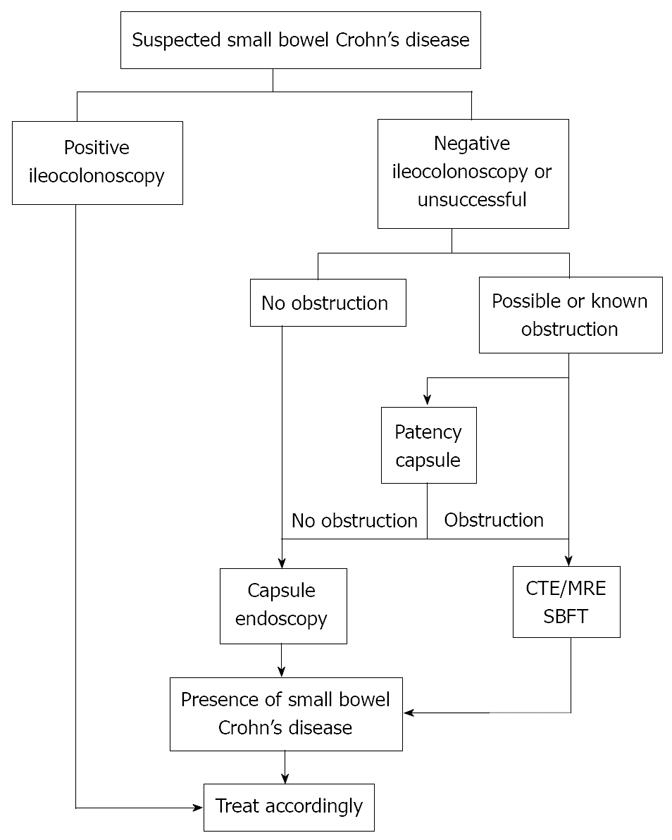
Another controversial issue is the position of CE in the diagnostic algorithm of suspected Crohn’s disease.Although, as mentioned above, it is widely accepted to perform it after ileocolonoscopy and a small bowel radiographic method (as SB series), in view of the results of the Mayo Clinic trial[18], the authors recommend the performance of CT-enterography after ileocolonoscopy but before CE. Indeed, they had a similar sensitivity but a higher specificity rate for CT enterography compared to CE. In the discussion, they finally state that the algorithm ought to be adapted to local availability and expertise[18]. On the other hand, the same group has published a recent paper showing economic benefits when CE is performed after ileocolonoscopy, but before small bowel series[12]. Furthermore, the vast majority of centers still follow the algorithm in which CE is a first line diagnostic tool in Crohn’s disease[26-30] (Figure 2). A meta-analysis has demonstrated that CE is superior to small bowel radiology, ileocolonoscopy, and CT-enterography in the evaluation of suspected Crohn’s disease patients[11]. The main concern with CE as a diagnostic tool is the absence of a confident gold standard test to which it may be compared. This has led to a majority of studies in which the concept of diagnostic yield is the main outcome variable, providing evidence that is not easily applicable to daily clinical practice. The diagnostic yield can be defined as the likelihood of a positive finding. However, it is not the same as sensitivity, that is, the likelihood of a positive test given true disease (based on a criterion standard). The diagnostic yield is the first step in determining what finding a test is capable of producing; nevertheless, the diagnostic yield does not provide the technical accuracy specifications of sensitivity and specificity. Indeed, a lower threshold for positive findings lead to a high diagnostic yield[19]. This tendency is changing, with some new studies which are trying to determine diagnostic accuracy with a gold standard represented by a panel of investigators with expertise in the diagnostic tools compared in the study[18,31]. Moreover, other authors established a follow-up period in which patients were considered as having established Crohn’s disease if they met the current clinical and radiological criteria during this period, despite CE findings[14]. Although somewhat artificial, these research designs mark a promising research field in which the exact accuracy of the procedure, and therefore its position in the diagnostic workup, will eventually be defined.
Figure 2 Algorithm for approaching suspected Crohn’s disease.
(Leighton et al[19]).
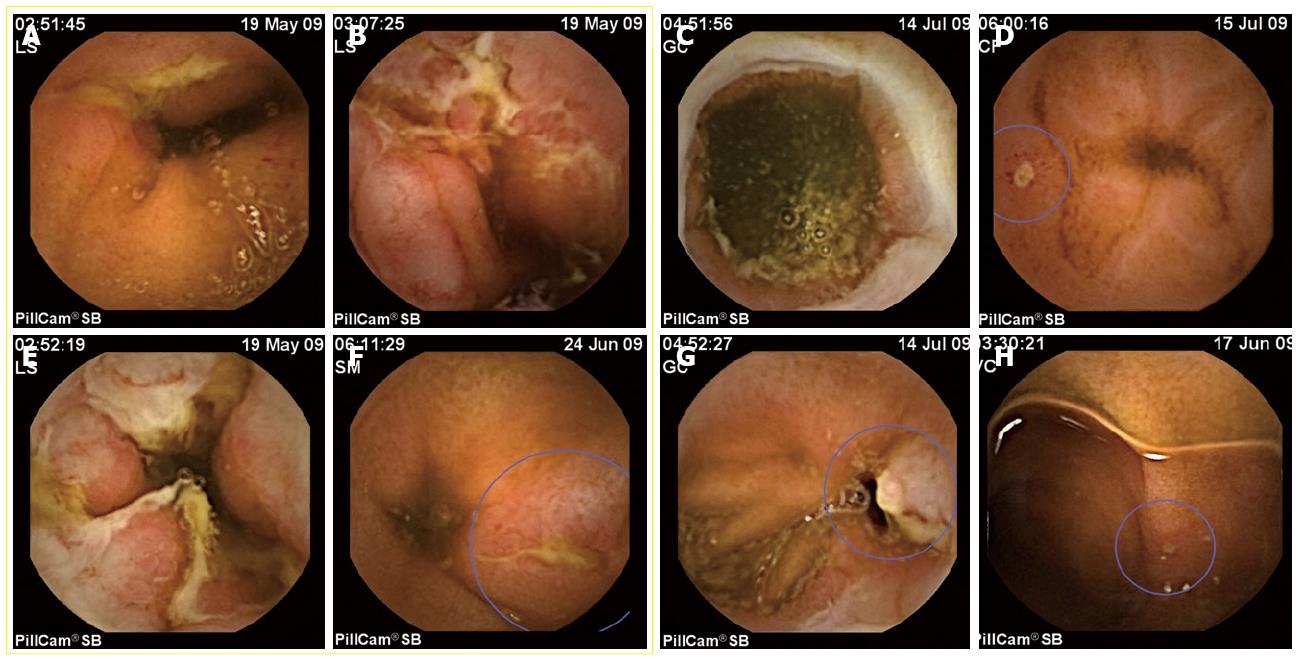
But not only the aforementioned issues are controversial. When the CE yields a finding, there are no validated diagnostic criteria for the diagnosis of Crohn’s disease. Many of the lesions described in studies of suspected Crohn’s disease are unspecific, and this could explain the variability of the diagnostic yield[20] (Figure 3). To date, the presence of more than three ulcerations, in the absence of NSAIDs ingestion, constitutes the most commonly used diagnostic criterion[7,14]. The lesson to be learned from this uncertainty is that clinical history, including the ingestion of NSAIDs, is the basis of the diagnostic workup. Physicians have to take into account that minimal mucosal leaks can be of no significance. Evidence suggests that up to 13% of normal, asymptomatic individuals can have mucosal breaks and other minor lesions of the small bowel detected by CE[27]. Nevertheless, it is obviously urgent to define criteria or scores for the diagnosis and for assessing both the activity and severity of Crohn’s disease by CE.
Figure 3 CE findings for Crohn’s disease are not specific.
In the images, only the ones included inside the yellow square are confirmed cases of Crohn’s disease. The other four were NSAIDs related lesions. A, B: Aftous ulcers, typical of Crohn’s disease; B, E: Geographical ulcers, observed in severe cases of small bowel Crohn’s disease, with strictures associated. D, H: Very small aftae, quite often observed in normal people, but, in these cases, in patients with a recent NSAIDs therapy. C: A typical ring-shaped stricture associated to NSAIDs. G: An ulcer in a patient with anemia and in treatment with high doses of NSAIDs for arthritis.
Despite all these concerns, CE has changed the diagnosis and management of small bowel Crohn’s disease, and it has been recognized as a cost-effective diagnostic tool in these patients[12,30].
ASSESSMENT OF SEVERITY OF SMALL BOWEL CROHN’S DISEASE
Regarding the activity of the disease, valuable results have been demonstrated in two studies proposing and validating a CE score (CECDAI)[27,28]. The CECDAI has been validated but still has to be tested in further prospective trials. The attempt to reach a consensus in the score index has provided other potential benefits: (1) The score, in conjunction with clinical data, is an important tool for the diagnosis in suspected cases; (2) It establishes a more objective method for following up mucosal healing; (3) It could help to individualize the treatment in each subject; and (4) A score tends to unify terminology and improves scientific communication between investigators[24]. While waiting for a definitive validation, its use can improve the procedure’s accuracy when assessing severity.
CE AS A GUIDE TO THERAPY
Endoscopy plays an important role in the evaluation and monitoring of established Crohn’s disease. Ileocolonoscopy and upper endoscopy have well-established roles for this purpose, but the exact position of CE still needs to be defined[32].
Therefore, international consensus states that in established Crohn’s disease, CE should be focused on patients with unexplained symptoms when other investigations are inconclusive, if this option may change management[20]. In this case, it is mandatory to assess the absence of evident strictures in the bowel prior to CE by radiographic methods. Although most patients with Crohn’s disease have lesions accessible to ileocolonoscopy, sometimes there are patients with unexplained symptoms and inconclusive radiographic imaging on ileocolonoscopy, who may well have subtle small bowel lesions. CE allows the detection of these superficial lesions with a relevant influence on therapeutic management[16,20].
A recent paper[9] has also studied whether symptoms represented flares in disease activity. CE yielded negative findings in approximately 48% of symptomatic patients, which, the authors interpreted as symptoms caused by other diseases (bacterial overgrowth, irritable bowel syndrome, etc.) CE yielded negative findings in approximately 48% of symptomatic patients, which the authors interpreted as symptoms caused by other diseases (bacterial overgrowth, irritable bowel syndrome…). Accordingly, they believe that the use of CE avoided unnecessary treatments, and recommended that every patient with Crohn’s disease should undergo CE early in the evolution of the disease, in order to have an accurate evaluation of disease extension. Nevertheless, this study has a retrospective design and does not describe a follow up, so the results must be interpreted with caution[19].
Another major advantage of CE is that being comparable to other radiographic methods in the assessment of activity in patients with established Crohn’s disease[17], it offers of no radiation exposure[33].
Capsule endoscopy may also be useful to determine early postoperative recurrence of Crohn’s disease. In one prospective study, CE was more sensitive in the detection of proximal lesions, but ileocolonoscopy was more sensitive overall[34]. Accordingly, the ICCE consensus recommended to consider CE only if ileocolonoscopy is contraindicated or unsuccessful[20].
INFLAMMATORY BOWEL DISEASE-UNCLASSIFIED
Population-based studies have shown that in 4%-10% of adult patients with inflammatory bowel disease affecting the colon, it is impossible to distinguish between Crohn’s disease and ulcerative colitis using current diagnostic techniques. In children, this group accounts up to 30% of patients. The determination of the definitive diagnosis has implications in terms of medical and surgical therapy, as well as in the clinical outcomes[35,36]. Obviously, when a total colectomy is being considered, this differentiation is essential. Therefore, CE can be helpful in this setting, although a negative study does not exclude a future diagnosis of Crohn’s disease[20].
Nevertheless, the papers published in this field are all retrospective and have enrolled only a small number of patients, with neither control groups nor a systematic exclusion of CMV infection. The authors used the Mow criteria for the diagnosis, which cannot reliably exclude a future diagnosis of Crohn’s disease[37,38]. These concerns make the conclusions established in these papers weak, and firm recommendations can not be offered.
Nonetheless, in the most important of these studies[37], 10% of patients were previously diagnosed with ulcerative colitis with atypical symptoms, 9% of patients with refractory ulcerative colitis, 33% of patients with a previous colectomy and new symptoms, and 17% with Inflammatory bowel disease unclassified (IBDU) who were reclassified as Crohn’s disease patients. In view of these results, a better presurgical diagnosis is critical in this situation.
ROLE OF CE IN UC
The diagnosis of UC does not require a CE. Nevertheless, some experts advocate small bowel imaging in patients with ulcerative colitis prior to elective colectomy for medically refractory cases. CE may also be indicated in UC patients with unexplained anemia or abdominal symptoms[20,38]. Minimal mucosal abnormalities in patients with UC after an ileal pouch-anal anastomosis do not predict the outcome, with no clear clinical significance[20].
Despite the absence of strong evidence, it seems reasonable to perform CE in patients with ulcerative colitis who experience atypical symptoms or have medically refractory disease, if there are no contraindications to the procedure[38,39].
COMPLICATIONS OF CE IN PATIENTS WITH CROHN’S DISEASE
Contraindications for CE include having a known or suspected gastrointestinal tract obstruction and/or known small bowel strictures, because of the increased risk of capsule retention in such patients[40]. The overall rate of retention is variable between the different studies, but it has been estimated in 1.8%-5.8% of the procedures for any indication[20]. The retention rate is low in patients with suspected Crohn’s disease, but it can increase up to 13% in patients with known Crohn’s disease, despite a normal radiographic study[41,42]. For this reason, in cases were there are doubts about the possible presence of a stricture, it is recommended to prevent possible capsule retention with a patency capsule. The patency capsule is a self-dissolving capsule that is the same size as the video capsule. It contains a radiofrequency identification tag that allows it to be detected by a scanning device placed on the abdominal wall. The tag can also be seen easily with a plain abdominal film. When its passage is blocked by a stenosis, the patency capsule dissolves 40-80 h after ingestion. If strictures are indentified, an alternative method, as enteroscopy, should be considered[20].
A retained capsule endoscopy does not usually cause obstruction, and can remain intact for up to 4 years[7,24].However, single cases of acute obstruction have been reported, with perforation in some cases[20]. The usual approach for the removal of the retained CE is surgery, but double balloon enteroscopy can be an alternative. An option in patients with known Crohn’s disease and strictures might be a medical therapy with steroids or infliximab, but nowadays there is no evidence about the appropriateness of this alternative. Retention should be suspected when the capsule does not reach the colon in the recorded study[24]. In this situation, it is advisable to follow up with a self report of CE excretion or a plain abdominal film after 2 wk. Visualization of the cecum might be a reliable measure for excluding retained CE[20].
FUTURE OF CE IN INFLAMMATORY BOWEL DISEASE
It is fairly clear that capsule endoscopy identifies the earliest inflammatory changes in the small bowel. CE therefore offers the opportunity to diagnose Crohn’s disease earlier than ever before, but it remains unclear whether an early diagnosis provides any benefit to the patient. It is currently thought that an earlier diagnosis will bring a better outcome[24]. Another paradigm shift is the change from a symptom-based disease activity estimation to direct monitoring of mucosal healing, with the resulting CE scores activity indexes plus fecal and serum biomarkers, endoscopy and radiology[24].
A better definition of specific lesions in inflammatory bowel disease, indications of the procedure in patients with unspecific symptoms, validation of activity scores, and the technical modifications to allow biopsy sampling are some of the main unresolved questions that will probably be addressed in upcoming papers.
Peer reviewers: Akira Hokama, MD, PhD, First Department of Internal Medicine, University of the Ryukyus, 207 Uehara, Nishihara, Okinawa 903-0215, Japan; Jean-Francois Rey, MD, Hepatogastroenterology Institut Arnault Tzanck, Saint Laurent Du Var Cedex 06721, France; Carlo M Girelli, MD, 1st Department of Internal Medicine, Service of Gastroenterology and Digestive Endoscopy, Hospital of Busto Arsizio, Via Arnaldo da Brescia, Busto Arsizio (VA) 121052, Italy
S- Editor Zhang HN L- Editor Herholdt A E- Editor Liu N