Published online Aug 16, 2025. doi: 10.4253/wjge.v17.i8.109104
Revised: June 3, 2025
Accepted: June 27, 2025
Published online: August 16, 2025
Processing time: 108 Days and 5.6 Hours
Gastrointestinal (GI) tract injuries and defects form a heterogeneous group of con
We present two cases of patients who underwent total gastrectomy for gastric malignancy. A combined therapeutic approach was employed, based on isolating the injury site with local vacuum-assisted closure (VAC) therapy and simulta
The combination of injury site isolation, local VAC therapy, and enteral feeding proved to be safe, effective, and easily adaptable to standard surgical practice. This approach may expand surgical options in the treatment of GI tract injuries and defects.
Core Tip: The treatment of gastrointestinal tract defects and fistulas remains a complex challenge without a standardized approach, due to the highly variable and often unique nature of injuries. Our experience highlights a novel method combining isolation of the affected gastrointestinal segment with local vacuum-assisted therapy while maintaining enteral nutrition. This approach not only facilitates early detection and dynamic monitoring of fistulas but also promotes faster healing by preventing contact with gastrointestinal contents and preserving mucosal integrity through continued enteral feeding. By applying this method in oncologic patients undergoing similar surgeries, we demonstrate its feasibility, safety, and potential to significantly improve clinical outcomes. Further exploration of segmental isolation may expand therapeutic options for various abdominal pathologies.
- Citation: Kashintsev AA, Eselevich RV, Surov DA, Balyura OV, Proutski V. Isolation techniques for gastrointestinal tract defects: Two case reports. World J Gastrointest Endosc 2025; 17(8): 109104
- URL: https://www.wjgnet.com/1948-5190/full/v17/i8/109104.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i8.109104
Defects of the gastrointestinal (GI) tract wall represent a heterogeneous group of conditions that can result in the formation of external or internal fistulas, often complicated by mediastinitis, peritonitis, or sepsis. Mortality associated with these complications varies widely, ranging from 10% to 60% of cases[1]. Treatment effectiveness depends on multiple factors, among which the interval between the onset of symptoms and the identification of the defect plays a critical role. This period can be highly variable: From 1 to 58 months in oncological cases, and from 5 to 30 days in benign conditions[2]. Such variability is largely attributable to the broad spectrum of clinical manifestations, ranging from purulent-septic presentations to bleeding and emphysematous changes in the subcutaneous tissue.
The patient’s immune status is another key determinant of outcomes. However, earlier recognition of complications and the prompt initiation of targeted therapy consistently correlate with better results. Endoscopic investigations, including video esophagoscopy and bronchoscopy, together with radiographic studies, remain the cornerstone of diag
Current guidelines advocate minimally invasive treatments for fistulas, such as clipping, endoscopic suturing, fibrin glue application, and the placement of various types of covered stents (plastic, metallic, or self-expandable). Despite this trend, there is no universally accepted standardized treatment algorithm, reinforcing the necessity for individualized, multifactorial approaches[4].
Among the available modalities, endoluminal vacuum therapy (EndoVAC) is the most extensively studied for the treatment of non-malignant GI fistulas. Its major advantage lies in providing continuous aspiration of pathological exudate, thus accelerating fistula healing under negative pressure conditions. However, EndoVAC therapy is not universally effective. Reported success rates are around 85%, predominantly among patients with postsurgical fistulas (e.g., after esophagectomy, gastrectomy, or proximal gastrectomy) or fresh iatrogenic injuries[5,6]. Several limitations to EndoVAC therapy have been identified, including the lack of a clearly defined optimal range for negative pressure—especially the upper limit—which may contribute to additional esophageal wall trauma[7,8]. Moreover, EndoVAC is associated with high treatment costs, an increased risk of stricture formation, the need for frequent system exchanges, and a necessary shift to parenteral nutrition.
Importantly, with existing treatment strategies, the defect area often remains exposed to external influences from the gastrointestinal environment, potentially delaying healing and increasing infection risk. Additionally, prolonged pa
Considering the advantages and limitations of existing therapies, an ideal approach to managing GI wall defects and fistulas should combine the creation of an isolated environment with controlled negative pressure to enhance healing, while simultaneously preserving the ability to maintain enteral nutrition. Such a strategy may significantly improve tissue repair, minimize complications associated with prolonged parenteral nutrition, and ultimately lead to better clinical outcomes. The principle of gastrointestinal tract isolation and creation of therapeutic conditions has previously been successfully utilized by us for various pathologies[9,10].
Two male patients—aged 74 and 70 years—were admitted in August and September 2024, respectively, with similar symptoms. Both presented with progressive dysphagia, vomiting, generalized weakness, anemia, and unintentional weight loss.
Both patients underwent preoperative endoscopic evaluation, confirming gastric cancer. The first patient was diagnosed with moderately differentiated adenocarcinoma; the second, with well-differentiated adenocarcinoma. Contrast-enhanced computed tomography (CT) showed no regional lymphadenopathy or distant metastases in either case. Following review by a multidisciplinary tumor board, both cases were staged as IIA. In September 2024, the patients underwent video-assisted laparoscopic total gastrectomy with D2 Lymphadenectomy and Roux-en-Y reconstruction, including stapled side-to-end esophagojejunostomy.
Both patients had a known history of arterial hypertension. The second patient had additionally undergone partial gastrectomy 40 years earlier for peptic ulcer disease (Billroth II reconstruction, Hofmeister–Finsterer modification), which introduced significant anatomical and technical challenges for the current surgical procedure.
Neither patient reported a personal or family history of malignancy.
In the first patient, the immediate postoperative course was complicated by acute traumatic pancreatitis, confirmed by an elevated serum amylase level (605 U/L), as well as intestinal failure syndrome and small bowel paresis. A subhepatic drain was maintained to evacuate peripancreatic fluid. On postoperative day 12, the drain output became enteric in nature. Fistulography revealed a leak from the duodenal stump, consistent with a Grade IV fistula according to the Clavien-Dindo classification. An emergency reoperation was performed, which included laparotomy, intra-abdominal decontamination, and placement of a vacuum-assisted closure (VAC) system. Serial abdominal debridement was carried out every 3-4 days.
In the second patient, enteric drainage was noted on postoperative day 8 from a surgically placed drain located adjacent to the esophagojejunal anastomosis.
Both patients exhibited leukocytosis and elevated inflammatory markers, consistent with an ongoing infectious process.
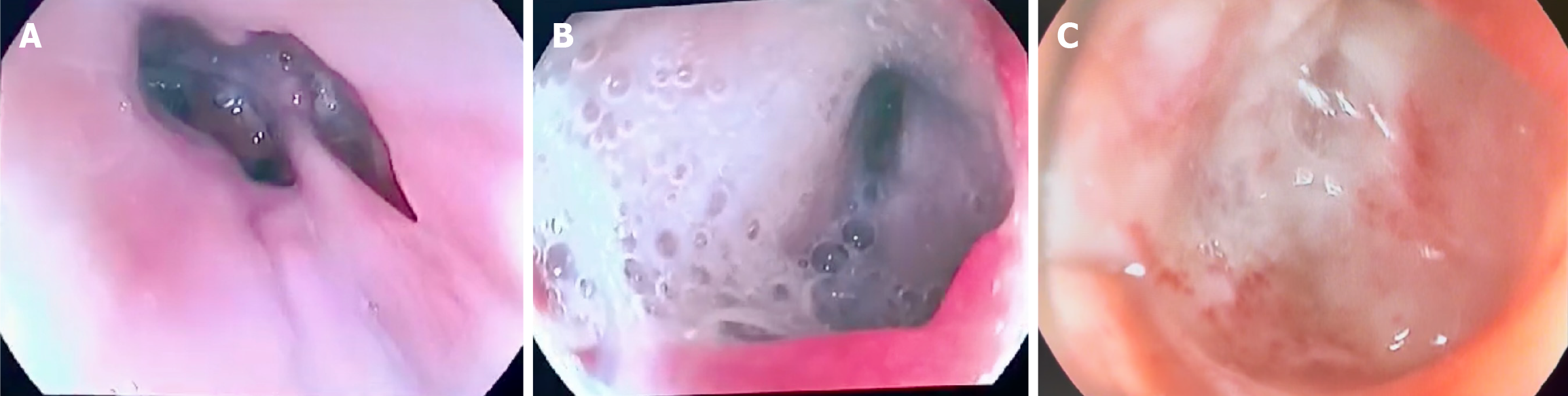
In the first case, oral contrast-enhanced CT revealed contrast extravasation distal to the esophagojejunostomy. Endoscopy identified two distinct fistulous tracts: One located 8 cm distal to the anastomosis at the Roux limb, and another ori
In the second case, oral contrast fluoroscopy demonstrated extravasation from the jejunal stump, which was confirmed endoscopically.
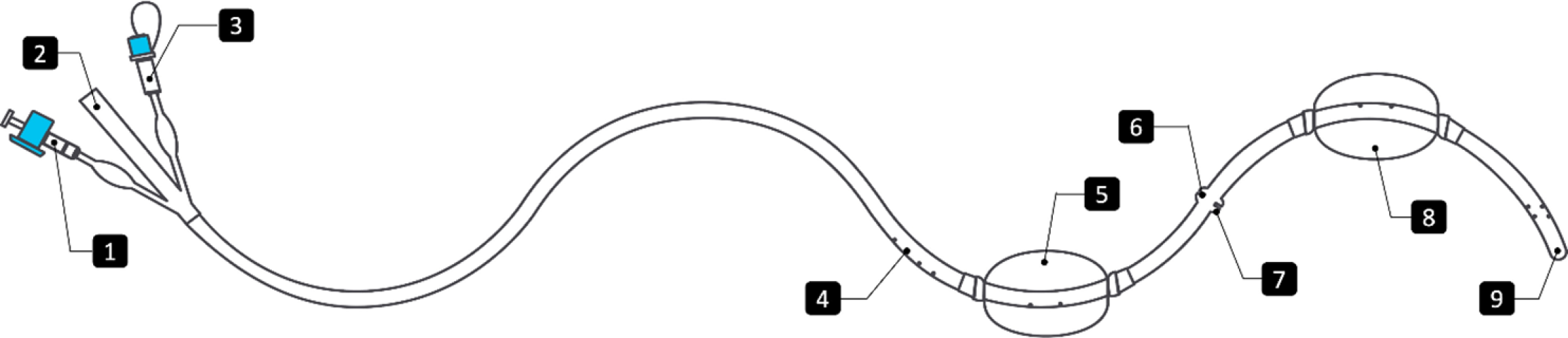
A multidisciplinary team comprising surgeons, endoscopists, and anesthesiologists convened to evaluate treatment options. It was agreed to initiate therapy with the PandiCath® system (Figure 2), which allows isolation of the affected gastrointestinal segment while preserving enteral nutrition.
PandiCath® comprises two low-pressure, water-inflatable balloons spaced 16.5 cm apart along a flexible catheter, creating a sealed intraluminal segment for targeted interventions such as vacuum-assisted therapy and contrast instillation for real-time verification of device positioning and assessment of the fistulous tract. The balloons are connected via a shared inflation channel that equalizes internal pressure, thereby minimizing interference with bowel motility. Placement is guided endoscopically or fluoroscopically to ensure precision and safety. Balloon volumes range from 40-100 mL, adjusted endoscopically based on anatomy to ensure effective sealing without compromising perfusion. To minimize potential pressure injury, balloons are deflated once or twice daily. A continuous negative pressure of -80 mmHg supports drainage and healing. The device typically remains in place for 3-4 days, with routine clinical and endoscopic monitoring. Contraindications include bowel ischemia, uncontrolled coagulopathy, or anatomical limitations. Repositioning or replacement is done as needed.
Gastric adenocarcinoma, stage IIA.
Postoperative acute traumatic pancreatitis, Grade IV duodenal and esophagoenteric fistulas, and severe purulent peritonitis.
Grade III anastomotic fistula involving the jejunal stump.
Under endoscopic guidance, the PandiCath® system was positioned to fully isolate the esophagojejunostomy between the two balloons. Injection of contrast medium into the isolated segment confirmed the presence and location of the fistulas, as well as proper device positioning.
In the first patient, contrast administration additionally revealed a previously undetected proximal fistulous tract located above the anastomosis (Figure 1B). Localized VAC therapy was initiated in both patients through the PandiCath® system.
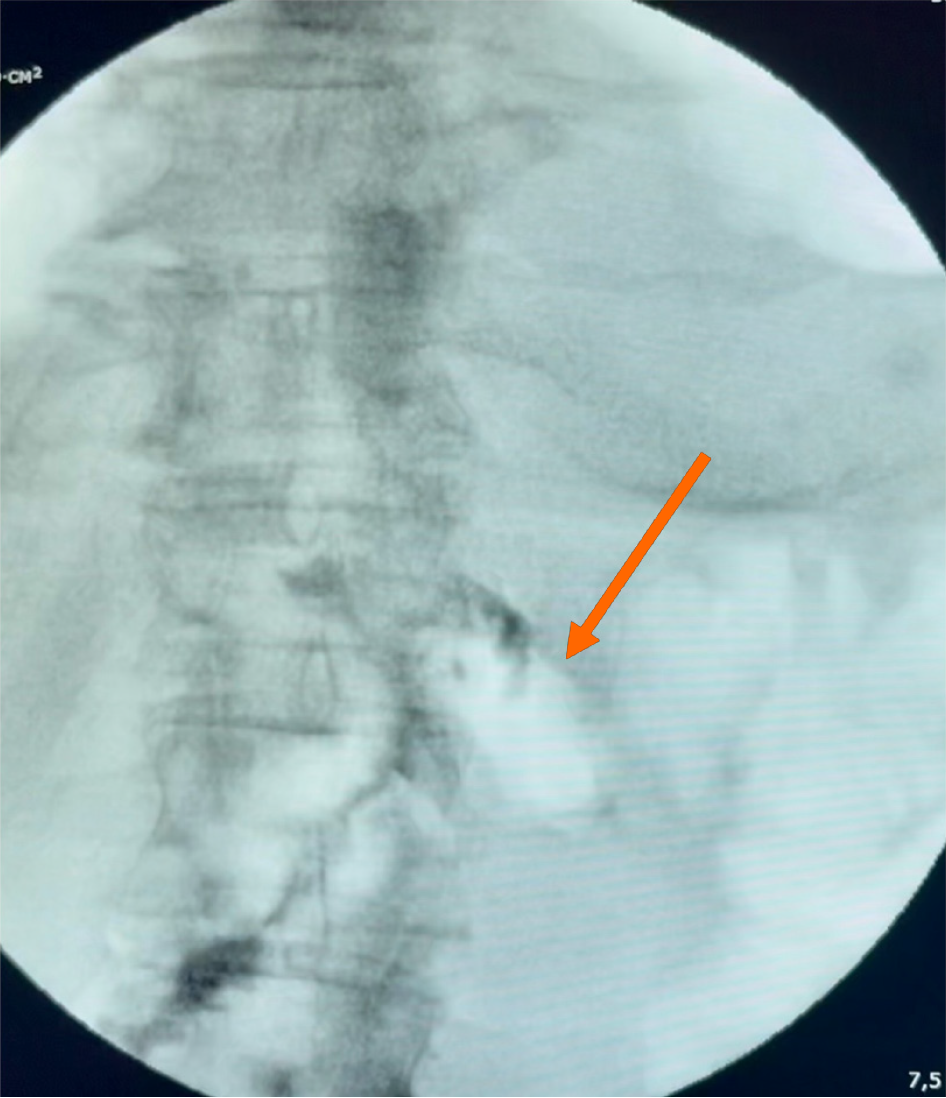
In the first case, additional scheduled debridement procedures were performed to manage the duodenal stump fistula and associated peritonitis. Complete closure of all GI defects was confirmed endoscopically in this patient after 10 days of therapy (Figure 1C). In the second patient, fistula closure was confirmed by control fistulography after three days of treatment (Figure 3). Both patients were discharged in satisfactory condition.
After 8 months of follow-up on May 22, 2025, patient was interviewed and examined. They reamed asymptomatic, with no evidence of dysphagia or cancer recurrence.
The treatment of gastrointestinal tract defects and fistulas remains a significant clinical challenge, for which standardized, one-size-fits-all solutions are rarely feasible. The underlying causes, predisposing factors, and clinical contexts of such injuries are highly variable, and often unique, making it difficult to design and implement large-scale, randomized multi
Global experience indicates that the key to successful treatment of GI defects and injury is a multidisciplinary approach and flexibility in selecting additional therapeutic strategies. However, the proposed method offers several important advantages. First, it allows for both the identification of the defect and dynamic monitoring of its healing process. Second, isolating the damaged area prevents fistula passage from coming into contact with the gastrointestinal contents, pro
The proposed method, which combines isolation of the injury site with localized VAC therapy and simultaneous enteral nutrition, has demonstrated both safety and effectiveness in the diagnosis and treatment of gastrointestinal wall defects. It is also straightforward to implement in routine clinical practice. While it is challenging to fully isolate the method’s effects from other factors influencing outcomes in the presented cases, we believe this multimodal approach holds significant promise in the management of complex abdominal pathologies. In particular, it may offer valuable therapeutic benefit in cases of esophageal burns, gastrointestinal hemorrhage, and trauma resulting from high-kinetic-energy injuries. Further studies involving larger patient cohorts are warranted to validate and optimize the safety and efficacy of this treatment strategy.
| 1. | Biancari F, D'Andrea V, Paone R, Di Marco C, Savino G, Koivukangas V, Saarnio J, Lucenteforte E. Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg. 2013;37:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Diddee R, Shaw IH. Acquired tracheo-oesophageal fistula in adults. CEACCP. 2006;6:105-108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Mathisen DJ, Grillo HC, Wain JC, Hilgenberg AD. Management of acquired nonmalignant tracheoesophageal fistula. Ann Thorac Surg. 1991;52:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Kim HS, Khemasuwan D, Diaz-Mendoza J, Mehta AC. Management of tracheo-oesophageal fistula in adults. Eur Respir Rev. 2020;29:200094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Bludau M, Fuchs HF, Herbold T, Maus MKH, Alakus H, Popp F, Leers JM, Bruns CJ, Hölscher AH, Schröder W, Chon SH. Results of endoscopic vacuum-assisted closure device for treatment of upper GI leaks. Surg Endosc. 2018;32:1906-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Laukoetter MG, Mennigen R, Neumann PA, Dhayat S, Horst G, Palmes D, Senninger N, Vowinkel T. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc. 2017;31:2687-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 7. | Famiglietti A, Lazar JF, Henderson H, Hamm M, Malouf S, Margolis M, Watson TJ, Khaitan PG. Management of anastomotic leaks after esophagectomy and gastric pull-up. J Thorac Dis. 2020;12:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Jung CFM, Müller-Dornieden A, Gaedcke J, Kunsch S, Gromski MA, Biggemann L, Seif Amir Hosseini A, Ghadimi M, Ellenrieder V, Wedi E. Impact of Endoscopic Vacuum Therapy with Low Negative Pressure for Esophageal Perforations and Postoperative Anastomotic Esophageal Leaks. Digestion. 2021;102:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Kashintsev AA, Kunda R, Proutski V. Early selective enteral feeding in combination with active decompression of duodenum in treatment of moderate and severe acute pancreatitis - A proof-of-concept clinical study. Pancreatology. 2024;24:1012-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Kashintsev AA, Rusanov DS, Antipova MV, Anisimov SV, Granstrem OK, Kokhanenko NY, Medvedev KV, Kutumov EB, Nadeeva AA, Proutski V. Hemostasis of massive bleeding from esophageal tumor: A case report. World J Gastrointest Endosc. 2022;14:636-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract. 2009;24:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29:2264-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 520] [Article Influence: 21.7] [Reference Citation Analysis (0)] |