Published online Jun 16, 2025. doi: 10.4253/wjge.v17.i6.108146
Revised: April 17, 2025
Accepted: May 20, 2025
Published online: June 16, 2025
Processing time: 66 Days and 6.6 Hours
Recently, Olympus Corporation released new scopes (XZ1200/EZ1500). However, there have been few reports on this topic, although improvement in adenoma detection rate (ADR) by texture and color enhancement imaging (TXI) or computer-aided detection system (CAD) has been reported.
To investigate the effects of the scope on the detection of adenomas and sessile serrated lesions (SSLs).
The subjects were patients who underwent pancolonic chromoendoscopy using the EVIS X1 video system center between May 2023 and October 2024. The patients were divided into the new (CF-XZ1200/CF-EZ1500) and 290 series (CF-HQ290Z/PCF-H290Z) groups. Propensity score matching was performed for age, sex, examination purpose, endoscopist, preparation, TXI use, and CAD use. The effects of the scope were analyzed in terms of the ADR, SSL detection rate (SDR), and mean number of adenomas per colonoscopy (APC).
Of the 7014 patients enrolled, 2138 pairs were extracted by propensity score matching (mean age 55.4 years, 45.5% male). The new scopes group had a significantly higher ADR than the 290 series group [51.5% vs 45.5%, odds ratio (OR) = 1.27, 95%CI: 1.13-1.43, P < 0.001]. Similarly, the new scopes group had significantly higher SDR (7.8% vs 5.7%, OR = 1.41, 95%CI: 1.11-1.80, P = 0.005) and APC (0.90 vs 0.76, OR = 1.11, 95%CI: 1.05-1.17, P < 0.001) than the 290 series group.
In conclusion, the new scope (CF-XZ1200/CF-EZ1500) enhanced the detection of adenomas and SSLs compared to the old ones (290 series).
Core Tip: Recently, Olympus Corporation released new scopes (XZ1200/EZ1500). This propensity score matching study showed that the new scopes (CF-XZ1200/CF-EZ1500) enhanced the detection of adenomas and sessile serrated lesions compared to the old ones (290 series). The improvement in image quality with this model change was remarkable.
- Citation: Nishizawa T, Toyoshima O, Yoshida S, Takahashi Y, Nakagawa H, Mizutani H, Kataoka Y, Kanazawa T, Ebinuma H, Hata K. Advantages of new generation colonoscopes on adenoma detection: A propensity-score matching study. World J Gastrointest Endosc 2025; 17(6): 108146
- URL: https://www.wjgnet.com/1948-5190/full/v17/i6/108146.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i6.108146
Colorectal cancer is the second leading cause of cancer-related deaths worldwide[1]. Adenomas and sessile serrated lesions (SSLs) are precancerous colorectal lesions, whose removal prevents colorectal cancer. The detection of adenomas and SSLs is crucial during screening endoscopy[2]. Remarkable progress has been made in endoscopy in recent years. It includes new endoscopic systems, scopes, image enhancement modes such as texture and color enhancement imaging (TXI), and computer-aided detection systems (CAD) based on artificial intelligence (AI)[3-6]. These technologies enhance the adenoma detection rate (ADR) and SSL detection rate (SDR). Improvement in ADR by TXI or CAD has been reported[7-10]. Recently, Olympus Corporation (Tokyo, Japan) released two new colonoscopy models (CF-XZ1200 and CF-EZ1500). However, there have been few reports on this topic. Therefore, we investigated the effects of the scope on the ADR and SDR.
This single-center, retrospective, propensity score-matching study, was conducted at the Toyoshima Endoscopy Clinic. Clinical data were extracted from the Toyoshima Endoscopy Clinic Database (T-file; STS Medic Inc., Tokyo, Japan). This study was approved by the Ethics Committee of the Certified Institutional Review Board of the Yoyogi Mental Clinic (No. RKK227) in accordance with the ethical guidelines for medical studies in Japan.
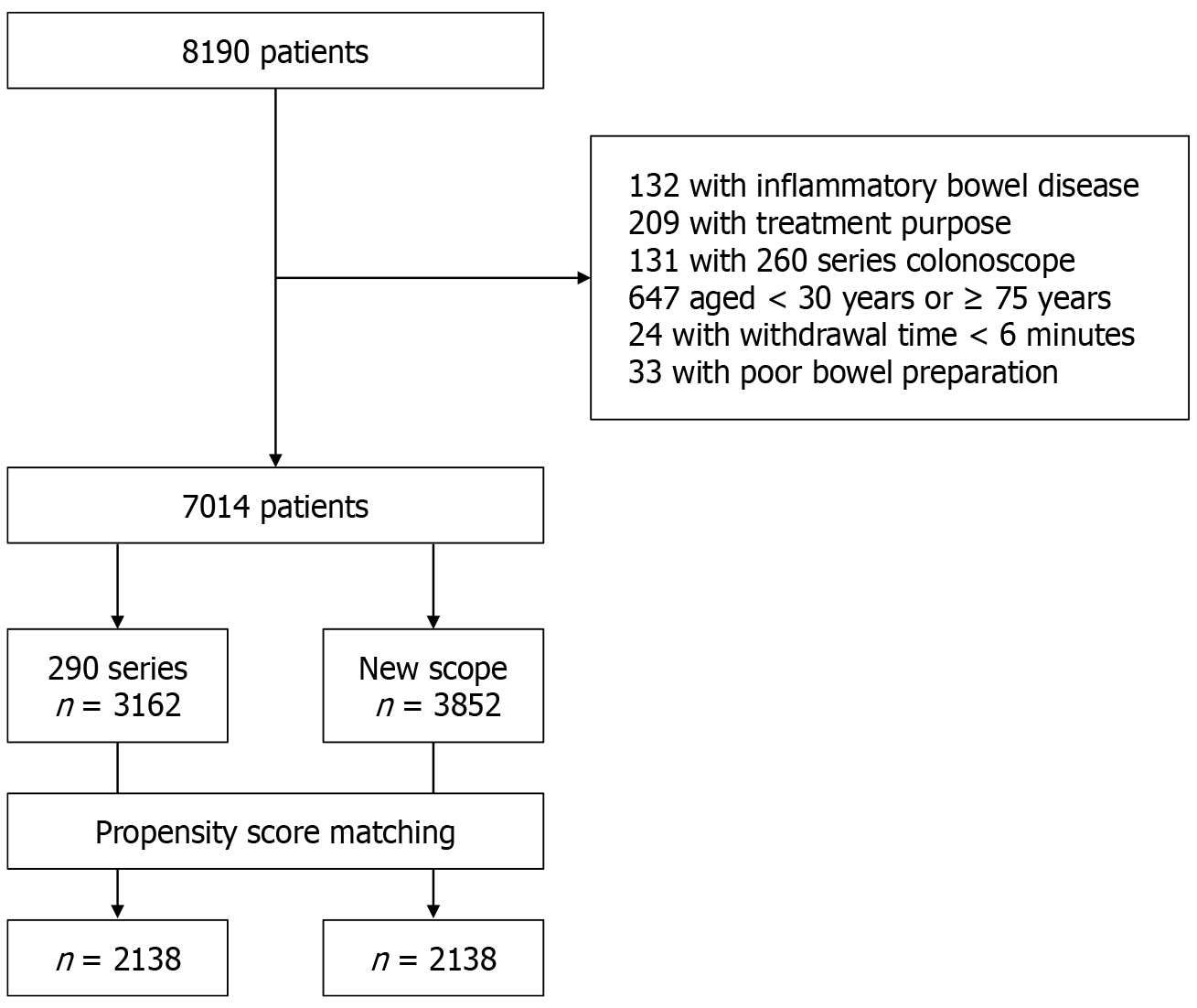
This study included patients who underwent pancolonic chromoendoscopy using the EVIS X1 video system at our clinic between May 2023 and October 2024. Patients underwent colonoscopy for screening, symptom evaluation, abnormal test results (such as positive fecal immunochemical tests), or surveillance. The exclusion criteria were inflammatory bowel diseases, treatment purposes such as polypectomy or hemostasis, use of PCF-PQ260, patients aged ≥ 75 years or < 30 years, and poor preparation classified as grade D.
We removed lesions that were diagnosed as adenomas or clinically significant serrated polyps (CSSPs)[11]. CSSPs were defined as all SSLs, all traditional serrated adenomas, hyperplastic polyps (HPs) ≥ 10 mm, and HPs ≥ 6 mm in the proximal colon[11,12]. CSSPs have dysplastic potential, and targeting CSSPs for resection is reasonable. The resection methods used were cold forceps polypectomy, cold or hot snare polypectomy, and endoscopic mucosal resection.
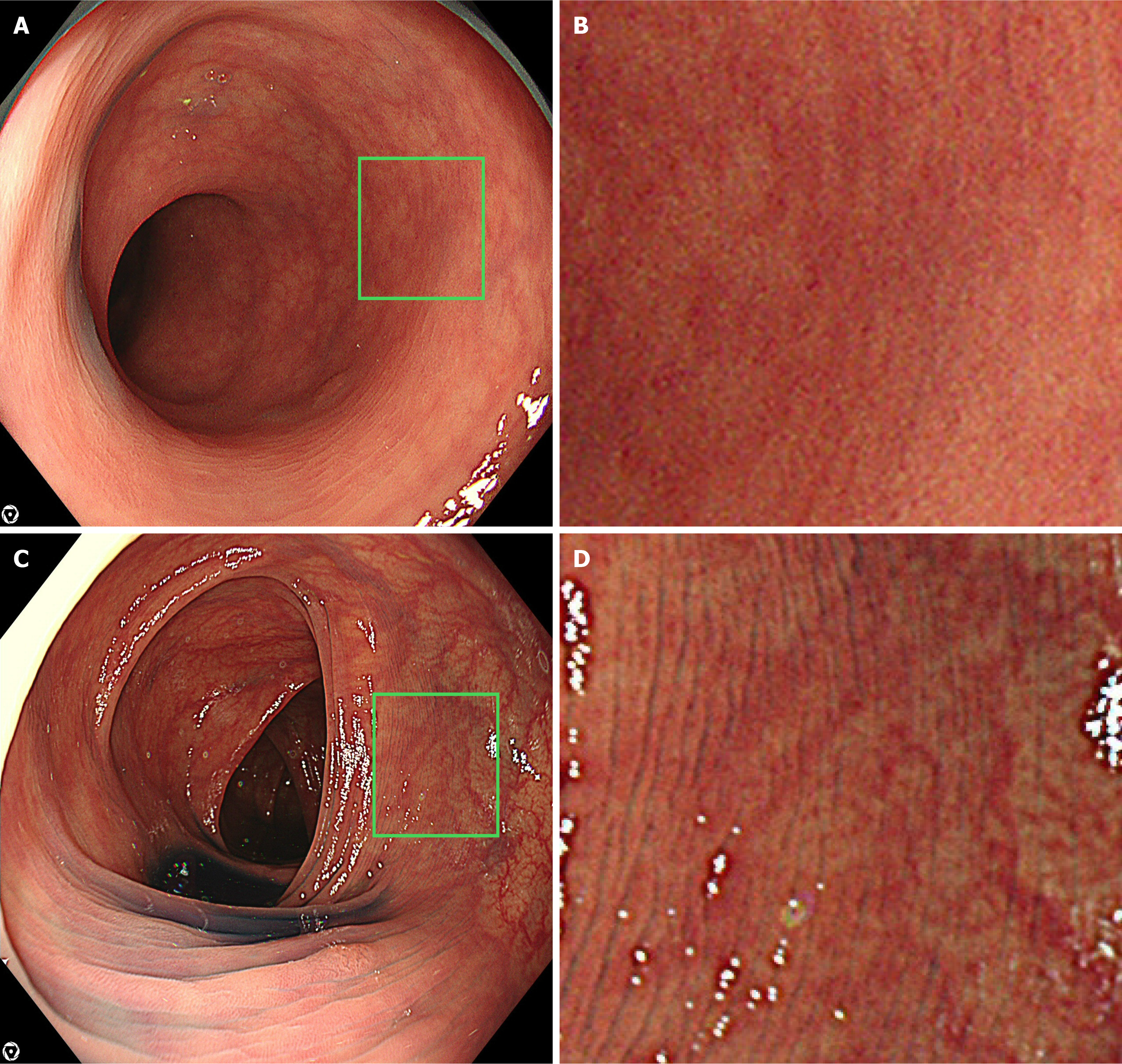
We used the EVIS X1 video system center (CV-1500) and a 4 K resolution ultra-high-definition liquid crystal display (UHD LCD) monitor (OEV321UH; Olympus, Tokyo, Japan). The colonoscopes included CF-XZ1200, CF-EZ1500, CF-HQ290Z, and PCF-H290Z. Representative images of the new and old scopes are shown in Figure 1. Pancolonic chromoendoscopy was performed by spraying 0.05% indigo carmine. ADR is a validated indicator of colonoscopy performance quality. The minimally acceptable, standard of care, and aspirational benchmarks were set at 25%, 30%, and 39%, respectively[13]. Endoscopists were divided into two groups: (1) ADR ≥ 40%; and (2) ADR < 40%[14]. Bowel preparations were classified into four groups: (1) Grade A was defined as clean or with minimal fluid in all colonic segments (good); (2) Grade B was defined as residual semi-solid stool that could be easily removed (average); (3) Grade C was defined as partially removable stool that obstructed full visualization of the mucosa (marginal); and (4) Grade D was defined as remaining solid stool that prevented examination (poor)[15]. Patients with grade D were excluded from the study. Withdrawal time included the time required to remove the polyps.
The observation modes were TXI and white light imaging. TXI mode 1 was used[16]. The endoscopists were assisted by an AI-based CAD system (EIRL Colon Polyp; ELPIXEL Inc. Tokyo, Japan). All resected specimens were pathologically diagnosed.
The data were extracted from the Toyoshima Endoscopy Clinic Database including age, sex, indications, endoscopists, bowel preparation, use of TXI or CAD, withdrawal time, and numbers of removed adenomas, SSLs, and CSSPs. We calculated the ADR, SDR, adenoma and SDR (ASDR), CSSP detection rate (CSDR), mean number of adenomas per colonoscopy (APC), and mean number of SSLs per colonoscopy (SPC).
When comparing ADRs between the two groups, the clinically significant potential confounding factors were age, sex, indications for colonoscopy (screening, symptom evaluation, abnormal test results including fecal immunochemical test, or polyp surveillance), endoscopists, bowel preparation grades, and use of TXI and/or CAD. To reduce the effects of selection bias and confounding factors, propensity score matching was adjusted for all potential confounding factors[17]. Patients who underwent colonoscopy using the new scopes (CF-XZ1200/CF-EZ1500) were identified, and the propensity score was matched with those who underwent colonoscopy using the CF290 series. Matching was performed with a 1:1 matching protocol using nearest-neighbor matching without replacement and with a caliper width of 0.25[18], which is the standard deviation of the logit of the propensity score for patients who underwent colonoscopy in the CF290 series. After propensity score matching, the effects of scope on the ADR, SDR, ASDR, CSDR, APC, SPC, and withdrawal time were analyzed.
A total of 8190 pancolonic chromoendoscopies were performed during the study period. The patient flowchart is shown in Figure 2. Finally, 7014 patients were enrolled, and 2138 pairs (4276 patients) were extracted by propensity score matching (mean age 55.4 years, 45.5% male). Twenty-two endoscopists performed colonoscopies, including 11 high-ADR and 11 low-ADR endoscopists. After propensity score matching, there were no differences in age, sex, purpose, endoscopist, bowel preparation, and TXI or CAD use rates by scope (Table 1). The new scopes group had a significantly higher ADR than the 290 series [51.5% vs 45.5%, odds ratio (OR) = 1.27, 95%CI: 1.13-1.43, P < 0.001]. Similarly, the new scopes group had significantly higher SDR (7.8% vs 5.7%, OR = 1.41, 95%CI: 1.11-1.80, P = 0.005), ASDR (55.7% vs 48.7%, OR = 1.32, 95%CI: 1.17-1.49, P < 0.001), CSDR (14.2% vs 9.8%, OR = 1.52, 95%CI: 1.26-1.84, P < 0.001), APC (0.90 vs 0.76, OR = 1.11, 95%CI: 1.05-1.17, P < 0.001) and SPC (0.10 vs 0.07, OR = 1.31, 95%CI: 1.09-1.57, P = 0.004) than the 290 series group (Table 2). There was no difference in withdrawal time between the two groups.
| Before matching | After matching | |||
| 290 series | New scope | 290 series | New scope | |
| n | 3162 | 3852 | 2138 | 2138 |
| Age (years) | 55.1 | 55.4 | 55.6 | 55.3 |
| Male sex (%) | 58.9 | 29.9 | 44.1 | 46.9 |
| Indication (screening/symptom/abnormal test results/surveillance) | 894/513/275/1480 | 945/619/550/1737 | 583/316/201/1038 | 538/347/303/950 |
| Endoscopists with high adenoma detection rate (%) | 73.0 | 87.4 | 81.0 | 81.2 |
| Preparation (excellent + good/poor) | 2740/422 | 3473/379 | 1884/254 | 1857/281 |
| Texture and color enhancement imaging (%) | 73.6 | 85.0 | 82.1 | 80.9 |
| Computer-aided detection system (%) | 38.5 | 62.5 | 48.2 | 45.9 |
| 290 series | New scope | Odds ratio | 95%CI lower | 95%CI upper | P value | |
| n | 2138 | 2138 | ||||
| Adenoma detection rate (%) | 45.5 | 51.5 | 1.2712 | 1.1273 | 1.4335 | < 0.001 |
| SDR (%) | 5.7 | 7.8 | 1.4124 | 1.1087 | 1.7992 | 0.005 |
| Adenoma and SDR (%) | 48.7 | 55.7 | 1.3228 | 1.1729 | 1.4919 | < 0.001 |
| Clinically significant serrated polyp detection rate (%) | 9.8 | 14.2 | 1.5240 | 1.2635 | 1.8383 | < 0.001 |
| Adenomas per colonoscopy | 0.76 | 0.90 | 1.1105 | 1.0542 | 1.1699 | < 0.001 |
| Sessile serrated lesions per colonoscopy | 0.07 | 0.10 | 1.3089 | 1.0891 | 1.5731 | 0.004 |
| Withdrawal time (minutes) | 13.7 | 13.4 | 0.9860 | 0.9717 | 1.0005 | 0.0575 |
This study showed that the new scopes (CF-XZ1200/CF-EZ1500) enhanced the detection of adenomas and SSLs compared to the old ones (290 series).
The video scope system centers of Olympus advanced from EVIS LUCERA CV-260 to EVIS LUCERA ELITE CV-290 to EVIS X1 CV-1500. The years of release were 2002, 2012, and 2020. The signaling methods were standard-definition television, high-definition television (HDTV), and “4K” resolution, and the resolutions are generally 640 × 480, 1920 × 1080, and 3840 × 2160, respectively. The horizontal pixels of 3840 are approximately 4000, therefore it is called “4K”. The resolution has quadrupled from HDTV to “4K”[19]. The combination of the new scopes (CF-XZ1200/CF-EZ1500), EVIS X1 CV-1500, and a 4 K UHD LCD monitor can display “4K” images. The images of “4K” are clear and detailed, making it possible to identify even minute lesions.
The CF-EZ1500 is characterized by an extended depth of field technology. This technology combines images captured in the near view with those captured in the far view to generate a single image. A wider depth of field provides greater clarity and richer detail throughout the image area, supporting superior observation.
The CF-XZ1200 was characterized using a high-speed sequential scanning method. In this method, red, green, and blue lights are illuminated sequentially, which can result in flickering and color fringing[20]. CF-XZ1200 captured images at 120 fps, surpassing 60 fps in 290 series. The high-speed sequential scanning method minimizes flickering and color fringing, and improves image quality.
We previously reported a propensity score-matched study between 260 series and 290 series for each endoscopy system and colonoscope combination[21]. In the 290 series, the amount of light increased with new lenses and mirrors, and the viewing angle widened from 140° to 170°. The 290 series feature a responsive insertion technology with a passive bending section. The scope was designed to bend naturally when it comes into contact with the colorectal wall, helping it pass smoothly through curved areas such as the sigmoid colon. In a previous study, the insertion time of the 290 series was shorter than that of the 260 series (P < 0.001). The ADR of the 290 series was higher than that of the 260 series; however, the difference was not significant (46.3% vs 44.4%)[21]. Our study demonstrated a significant improvement of 45.5%–51.5% from the 290 series to the new scopes. This model change may have a larger impact than the last one, especially in terms of image quality.
Our study has some limitations. First, this was a retrospective single-center study; however, the medical data recordings were well controlled. Second, bias might have included the learning curves of the endoscopists. However, the study period was one and a half years, and experienced endoscopists with ADRs ≥ 40% performed 80% of the examinations, and the effect of the bias might be small. Third, the economic aspects of a possible change from old to new endoscopic equipment were not discussed. This warrants discussion in the future.
The new scopes (CF-XZ1200/CF-EZ1500) enhanced the detection of adenomas and SSLs compared to the old ones (290 series). The improvement in image quality with this model change was remarkable.
| 1. | Li Q, Zhang X, Wang Y, Gao R, Zhang Y, Zheng X, Huang F, Liu W, Luo C, Liu F. Spatiotemporal trends in the burden of colorectal cancer incidence and risk factors at country level from 1990 to 2019. J Gastroenterol Hepatol. 2024;39:2616-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | Galati JS, Lin K, Gross SA. Recent advances in devices and technologies that might prove revolutionary for colonoscopy procedures. Expert Rev Med Devices. 2023;20:1087-1103. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Bond A, O'Toole P, Fisher G, Subramanian S, Haslam N, Probert C, Cox T, Sarkar S. New-Generation High-Definition Colonoscopes Increase Adenoma Detection when Screening a Moderate-Risk Population for Colorectal Cancer. Clin Colorectal Cancer. 2017;16:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Joseph J, LePage EM, Cheney CP, Pawa R. Artificial intelligence in colonoscopy. World J Gastroenterol. 2021;27:4802-4817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Suzuki T, Hara T, Kitagawa Y, Takashiro H, Nankinzan R, Sugita O, Yamaguchi T. Linked-color imaging improves endoscopic visibility of colorectal nongranular flat lesions. Gastrointest Endosc. 2017;86:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Nagai M, Suzuki S, Minato Y, Ishibashi F, Mochida K, Ohata K, Morishita T. Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials. Clin Endosc. 2023;56:553-562. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Antonelli G, Bevivino G, Pecere S, Ebigbo A, Cereatti F, Akizue N, Di Fonzo M, Coppola M, Barbaro F, Walter BM, Sharma P, Caruso A, Okimoto K, Antenucci C, Matsumura T, Zerboni G, Grossi C, Meinikheim M, Papparella LG, Correale L, Costamagna G, Repici A, Spada C, Messmann H, Hassan C, Iacopini F. Texture and color enhancement imaging versus high definition white-light endoscopy for detection of colorectal neoplasia: a randomized trial. Endoscopy. 2023;55:1072-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 8. | Biscaglia G, Cocomazzi F, Gentile M, Loconte I, Mileti A, Paolillo R, Marra A, Castellana S, Mazza T, Di Leo A, Perri F. Real-time, computer-aided, detection-assisted colonoscopy eliminates differences in adenoma detection rate between trainee and experienced endoscopists. Endosc Int Open. 2022;10:E616-E621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Tiankanon K, Aniwan S, Kerr SJ, Mekritthikrai K, Kongtab N, Wisedopas N, Piyachaturawat P, Kulpatcharapong S, Linlawan S, Phromnil P, Muangpaisarn P, Orprayoon T, Chanyaswad J, Sunthornwechapong P, Vateekul P, Kullavanijaya P, Rerknimitr R. Improvement of adenoma detection rate by two computer-aided colonic polyp detection systems in high adenoma detectors: a randomized multicenter trial. Endoscopy. 2024;56:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Sinagra E, Badalamenti M, Maida M, Spadaccini M, Maselli R, Rossi F, Conoscenti G, Raimondo D, Pallio S, Repici A, Anderloni A. Use of artificial intelligence in improving adenoma detection rate during colonoscopy: Might both endoscopists and pathologists be further helped. World J Gastroenterol. 2020;26:5911-5918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Anderson JC, Hisey W, Mackenzie TA, Robinson CM, Srivastava A, Meester RGS, Butterly LF. Clinically significant serrated polyp detection rates and risk for postcolonoscopy colorectal cancer: data from the New Hampshire Colonoscopy Registry. Gastrointest Endosc. 2022;96:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Park SK, Park SH, Yang HJ, Jung YS, Park JH, Sohn CI, Park DI. Simple proxies for detection of clinically significant serrated polyps and data for their benchmarks. J Gastroenterol Hepatol. 2020;35:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Hilsden RJ, Rose SM, Dube C, Rostom A, Bridges R, McGregor SE, Brenner DR, Heitman SJ. Defining and Applying Locally Relevant Benchmarks for the Adenoma Detection Rate. Am J Gastroenterol. 2019;114:1315-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Toyoshima O, Nishizawa T, Yoshida S, Sekiba K, Kataoka Y, Hata K, Watanabe H, Tsuji Y, Koike K. Expert endoscopists with high adenoma detection rates frequently detect diminutive adenomas in proximal colon. Endosc Int Open. 2020;8:E775-E782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 15. | Ell C, Fischbach W, Bronisch HJ, Dertinger S, Layer P, Rünzi M, Schneider T, Kachel G, Grüger J, Köllinger M, Nagell W, Goerg KJ, Wanitschke R, Gruss HJ. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. 2008;103:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Sato T. TXI: Texture and Color Enhancement Imaging for Endoscopic Image Enhancement. J Healthc Eng. 2021;2021:5518948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 17. | Zhao SQ, Wang SY, Ge N, Guo JT, Liu X, Wang GX, Su L, Sun SY, Wang S. Endoscopic full-thickness resection vs surgical resection for gastric stromal tumors: Efficacy and safety using propensity score matching. World J Gastrointest Surg. 2025;17:101002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Rosenbaum PR, Rubin DB. The bias due to incomplete matching. Biometrics. 1985;41:103-116. [PubMed] |
| 19. | Zwimpfer TA, Wismer C, Fellmann-Fischer B, Geiger J, Schötzau A, Heinzelmann-Schwarz V. Comparison of 2D 4K vs. 3D HD laparoscopic imaging systems using a pelvitrainer model: a randomized controlled study. Updates Surg. 2022;74:1137-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Vakil N, Knyrim K, Everbach EC. The appreciation of colour in endoscopy. Baillieres Clin Gastroenterol. 1991;5:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Toyoshima O, Yoshida S, Nishizawa T, Yamakawa T, Sakitani K, Hata K, Takahashi Y, Fujishiro M, Watanabe H, Koike K. CF290 for pancolonic chromoendoscopy improved sessile serrated polyp detection and procedure time: a propensity score-matching study. Endosc Int Open. 2019;7:E987-E993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |