Published online Apr 16, 2024. doi: 10.4253/wjge.v16.i4.206
Peer-review started: December 28, 2023
First decision: January 19, 2024
Revised: January 29, 2024
Accepted: March 18, 2024
Article in press: March 18, 2024
Published online: April 16, 2024
Processing time: 104 Days and 17.8 Hours
No studies have yet been conducted on changes in microcirculatory hemodynamics of colorectal adenomas in vivo under endoscopy. The microcirculation of the colorectal adenoma could be observed in vivo by a novel high-resolution magnification endoscopy with blue laser imaging (BLI), thus providing a new insight into the microcirculation of early colon tumors.
To observe the superficial microcirculation of colorectal adenomas using the novel magnifying colonoscope with BLI and quantitatively analyzed the changes in hemodynamic parameters.
From October 2019 to January 2020, 11 patients were screened for colon adenomas with the novel high-resolution magnification endoscope with BLI. Video images were recorded and processed with Adobe Premiere, Adobe Photoshop and Image-pro Plus software. Four microcirculation parameters: Microcirculation vessel density (MVD), mean vessel width (MVW) with width standard deviation (WSD), and blood flow velocity (BFV), were calculated for adenomas and the surrounding normal mucosa.
A total of 16 adenomas were identified. Compared with the normal surrounding mucosa, the superficial vessel density in the adenomas was decreased (MVD: 0.95 ± 0.18 vs 1.17 ± 0.28 μm/μm2, P < 0.05). MVW (5.11 ± 1.19 vs 4.16 ± 0.76 μm, P < 0.05) and WSD (11.94 ± 3.44 vs 9.04 ± 3.74, P < 0.05) were both increased. BFV slowed in the adenomas (709.74 ± 213.28 vs 1256.51 ± 383.31 μm/s, P < 0.05).
The novel high-resolution magnification endoscope with BLI can be used for in vivo study of adenoma superficial microcirculation. Superficial vessel density was decreased, more irregular, with slower blood flow.
Core Tip: No studies have yet been conducted on changes in microcirculatory hemodynamics of colorectal adenomas in vivo under endoscopy. Through our study, we found that the novel high-resolution magnification endoscope with BLI can be a tool for in-vivo study of adenoma superficial microcirculation. The superficial vessel density in the adenoma was decreased with more irregularity and slower blood flow. This is the first and pilot study to observe the microcirculatory hemodynamics of colorectal adenomas in vivo under endoscopy, and we believe that other doctors will be inspired by our article.
- Citation: Dong HB, Chen T, Zhang XF, Ren YT, Jiang B. In vivo pilot study into superficial microcirculatory characteristics of colorectal adenomas using novel high-resolution magnifying endoscopy with blue laser imaging. World J Gastrointest Endosc 2024; 16(4): 206-213
- URL: https://www.wjgnet.com/1948-5190/full/v16/i4/206.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i4.206
Colorectal cancer (CRC) is one of the most common malignancies in humans, ranking third in morbidity and second in mortality worldwide. The incidence of CRC is rising rapidly in the Asia–Pacific region[1]. Nearly half of the patients have a life span of < 5 years due to late diagnosis and potentially progressive disease. The majority of CRCs arise from adenomas; that is, the classical adenoma–carcinoma sequence (ACS)[2]. ACS is a series of events by which colorectal adenomas develop, initially showing low-grade dysplasia, and some progress to high-grade dysplasia and eventually invasive carcinoma[3]. Prior research has shown that identifying premalignant stage lesions (adenomas) of CRC by colonoscopy and subsequent endoscopic resection can prevent disease progression and reduce CRC-associated morbidity and mortality[4,5].
Angiogenesis, the secondary growth of blood vessels, plays an important role in tumor development[6]. Angiogenesis mediates the transition from hyperplasia to dysplasia and is a necessary condition for the growth of solid tumors[7]. The surface capillaries of colorectal tumors often show morphological changes, such as heterogeneity in vessel diameter or density and loss of hierarchical structure[8]. Although the importance of tumor angiogenesis is well known, conventional endoscopic images cannot be used to display these changes in capillaries due to inadequate resolution. No studies have yet been conducted on changes in microcirculatory hemodynamics of colorectal adenomas in vivo under endoscopy. In clinical practice, we found that a novel high-resolution magnifying colonoscope (Fujifilm EC-760ZP) with blue-laser imaging (BLI) can clearly display mucosal surface capillary networks in vivo and in real time.
In this study, we observed the superficial microcirculation of colorectal adenomas using the novel magnifying colonoscope with BLI and quantitatively analyzed the changes in hemodynamic parameters, thus providing a new insight into the early colorectal tumors.
In this prospective study, the novel magnifying colonoscopy at the endoscopic center of Beijing Tsinghua Changgung Hospital between October 2019 and January 2020 diagnosed 11 patients with colorectal adenomas. All patients gave signed informed consent and the study was approved by the Medical Ethics Committee of Beijing Tsinghua Changgung Hospital and registered in the Chinese Clinical Trial Registry (ChiCTR2000031294). All research was performed in accordance with relevant guidelines and regulations.
The patients were examined by the same endoscopist (YR) with the same high-resolution magnifying endoscope (EC-760ZP; Fujifilm, Japan). Standard bowel preparation and intravenous anesthesia were conducted in all the patients. Before each endoscopic examination, a soft black rubber cap (Olympus, Japan) was attached to the tip of the endoscope, and a microscopic ruler on a transparent glass plate (div=100 μm, Cossim, China) was used for measurement calibration at maximal magnification (145×).
Once a polyp was discovered, it was initially observed by conventional white-light imaging. A fully opened biopsy forceps (width 6 mm) was used to estimate the lesion size. Under BLI with low magnification, Japanese narrow-band imaging Expert Team (JNET) classification was used to evaluate the microsurface and microvessels of the polyps[9]. The transparent cap was attached to the surface of the polyp and the surrounding mucosa. The superficial capillary network was observed at maximal magnification (145×). The calibration and examination procedures were recorded as high-resolution videos (.mp4, 1080p, 30 frames/s) for at least 5 s and stored in a hard disk for further analysis. After observation, all polyps were resected under endoscopy and sent for pathological evaluation.
Adobe Premiere Pro 2019 software was used to export the recorded video images at 30 frames/s. Clear images were selected and the surface capillaries were identified and highlighted by Image-pro Plus 6.0 (Media Cybernetics, JNET). Mean vessel width (MVW) with width standard deviation (WSD) and microcirculation vessel density (MVD) (total vessel length per image area) were calculated with microscopic ruler calibration in Image-pro Plus software[10,11].
Time-sequential surface capillary images of each frame were imported in Adobe Photoshop CS4. The route of capillary flow was identified, marked and merged into one image without resolution loss. The distance between two marks suggested the blood flow within a certain time. The emergent image was imported in Image-pro Plus. The blood flow velocity (BFV) was calculated as the distance between two marks divided by the time between the two given sequential frames (1/30-2/30 s). BFV in three different areas were averaged.
Continuous data were expressed as mean ± SD and categorical data as percentages. The paired t test was used to compare the means. P < 0.05 was considered statistically significant. STATA 17.0 software was used for statistical analysis.
A total of 16 adenomas were discovered in the 11 patients. The mean age of the patients was 59.9 ± 7.4 years and there were six women and five men (Table 1). The average adenoma size was 8.2 ± 4.0 mm. There were four 0-Isp, 11 0-IIa adenomas and one sessile serrated lesions. Thirteen adenomas were classified as JNET type 2a and three as JNET type 2b. Pathology showed that all the adenomas were tubular.
| Variables | n (%) |
| Sex | |
| Male | 5 (45.5) |
| Female | 6 (54.5) |
| Age (mean ± SD) (yr) | 59.94 ± 7.40 |
| Lesion size (mean ± SD) (mm) | 8.19 ± 3.95 |
| Polyp morphology | |
| 0-Isp | 4 (25) |
| 0-IIa | 11 (68.7) |
| SSL | 1 (6.3) |
| JNET classification | |
| JNET 2a | 13 (81.2) |
| JNET 2b | 3 (18.8) |
| Pathology tubular adenoma | 16 (100) |
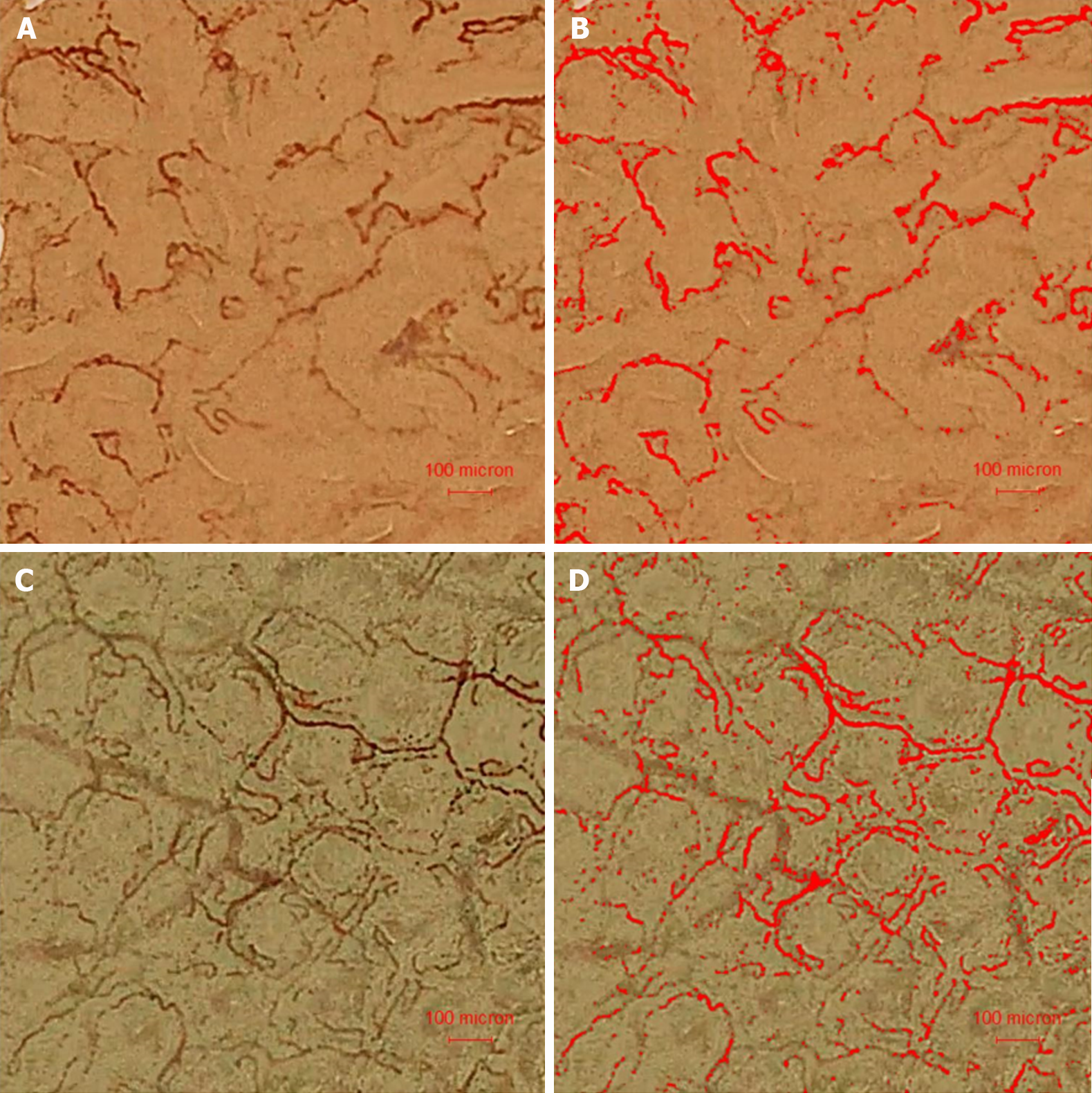
The surface blood flow in the capillaries could be clearly seen under BLI with maximal magnification, both in the adenoma and surrounding mucosa (Figure 1A and B, Videos 1 and 2). The capillaries within the adenoma appeared to be wider and more tortuous than those in the surrounding mucosa. The surface capillaries were automatically identified as red and calculated (Figure 1C and D). Mark the position of blood flow in certain time, so as to obtain the blood flow advance distance, and then calculate the BFV (Figure 2A and B).
Compared with the surrounding normal mucosa, the superficial vessel density of the adenomas was significantly decreased (MVD: 0.95 ± 0.18 vs 1.1 ± 0.28 μm/μm2, P < 0.05). MVW (5.11 ± 1.19 vs 4.16 ± 0.19 μm, P < 0.05) and WSD (11.94 ± 3.44 vs 9.04 ± 3.74, P < 0.05) were increased. The superficial blood flow slowed down remarkably in the adenomas (BFV: 709.74 ± 213.28 vs 1256.51 ± 383.31 μm/s, P < 0.05) (Table 2).
| Adenoma | Surrounding mucosa | t | P value | |
| MVD (μm/μm2) | 0.95 ± 0.18 | 1.17 ± 0.28 | 2.640 | 0.019 |
| MVW (μm) | 5.11 ± 1.19 | 4.16 ± 0.76 | 5.503 | < 0.001 |
| WSD (μm) | 11.94 ± 3.44 | 9.04 ± 3.74 | 4.494 | < 0.001 |
| BFV (μm/s) | 709.74 ± 213.28 | 1256.51 ± 383.31 | 4.986 | < 0.001 |
The pathway of CRC progression through ACS is well known, so early diagnosis and treatment of colorectal adenoma are crucial to reduce the risk of CRC development[4]. Various enhanced imaging techniques have been developed to improve the ability of doctors to recognize neoplastic lesions such as narrow-band imaging (NBI) (Olympus), i-SCAN (Pentax, Tokyo, Japan) and flexible spectral imaging color enhancement (Fujifilm)[12]. BLI (Lasereo System; Fujifilm) is another form of NBI. Instead of using filters for white light to produce narrow bandwidths, the BLI system is equipped with light sources that emit two different wavelengths of laser light. A laser with a wavelength of 450 nm can stimulate phosphors to produce white light illumination, while a BLI mode laser with a wavelength of 410 nm can be used as a high-contrast signal to obtain information on mucosal vascular patterns and surface patterns, thus achieving visual enhancement of surface vessels and structures[13,14].
Although the importance of neoplastic angiogenesis is widely recognized, there is strong evidence that induction of angiogenesis may occur early in the ACS, with angiogenic conversion occurring at the same time as tumor invasion[2,15,16]. However, there are no quantitative data on the indexes of capillary microcirculation of colorectal adenoma under endoscopy and in the surrounding normal mucosa. Blood flow in capillaries on the mucosal surface of the colon can be clearly observed under high-resolution magnification endoscopy with BLI, which provides a possibility for quantitative analysis of microcirculatory changes in colorectal adenomas.
We found that capillaries on the normal colorectal mucosal surface were arranged around the annular adenoid tube, while capillaries on the surface of the adenoma lost their normal structure. The capillary length per unit area on the surface of adenoma was shortened and vessel density was reduced. In addition, compared with the surrounding normal mucosa, the vessel width on the surface of the adenoma was increased and BFV was decreased. Nowadays, endoscopists usually use the JNET classification for microsurface structure and microvascular pattern. It is subjective in nature, and there is still some disagreement[17]. However, through our quantitative calculation with high-resolution magnifying endoscopy with BLI, we found that the width and variability of capillaries on the surface of the adenomas increased, which could confirm the rationality of the (JNET) classification. Further studies are warranted.
Previous studies have shown that image-enhanced endoscopy using NBI and BLI can be used to characterize known lesions by enhancing mucosal vessels and structures[18]. The use of BLI can significantly improve identification of adenomas, but there are no studies on microcirculation of colorectal adenoma under endoscopy[19,20]. To date, the only study on tumor vessels is a scanning electron microscopy study of cast colorectal vessels, which has provided spatial tissue information of tumor vessels, as well as quantitative results of morphological characteristics and diameter of individual vessels[21,22]. The normal mucosal capillaries of the colorectum presented a honeycomb arrangement around the mucosal glands, while the vascular layer in the adenoma was lost, which was consistent with our endoscopic observations. Further quantitative analysis showed that the vascular space in the adenoma was narrowed, the density was increased, the vascular width was increased, and the dispersion was greater. However, the vascular density of colorectal adenomas was decreased in our study. The difference in vascular density between the two studies may be related to different definitions. In the previous study, vascular density was defined as the spatial density of blood vessels in the lesion, which was related to the volume of blood vessels and the distance between them. We defined vascular density as the length of microcirculation capillary blood vessels on the adenoma surface within a unit area[11]. We only studied the changes in surface vessel length and did not involve deeper vessels. Figure 1D clearly shows that the software automatically recognized the capillaries on the surface of the adenoma, while the deeper and thicker vessels were not included in the analysis.
For the first time, we quantitatively analyzed the changes in capillary BFV of the colorectal adenomas, and the decreased velocity in the tumor may explain the failure of tumor drug therapy, because slower blood flow affects the delivery of drugs to the tumor, thus reducing their effectiveness. We found that these changes were largely related to the progress in endoscopic imaging technology. The emergence of high-resolution magnification endoscopy provides us with the possibility to study the changes in microcirculation-related indicators of colorectal adenoma.
One limitation of our study was the small sample size, which means that the results are not widely representative. In the next study, we will increase the sample size and further analyze the changes in hemodynamic indicators related to the surface vascular microcirculation of polyps, adenomas and adenocarcinomas. This will provide a new theoretical basis for the diagnosis of early colorectal tumors and the possibility of active recognition of colorectal lesions with AI technology under endoscopy.
In conclusion, high-resolution magnifying endoscopy can be used to quantitatively analyze the microcirculation on the surface of colorectal adenomas. The superficial vessel density in the adenomas was decreased, with more irregularity and slower blood flow.
The novel high-resolution magnification endoscope with BLI can be a tool for the in vivo study of adenoma superficial microcirculation. The superficial vessel density in the adenoma was decreased, with more irregularity and slower blood flow.
Colorectal cancer (CRC) is one of the most common malignancies in humans. Prior research has shown that identifying premalignant stage lesions (adenomas) of CRC by colonoscopy and subsequent endoscopic resection can prevent disease progression and reduce CRC-associated morbidity and mortality. Angiogenesis, the secondary growth of blood vessels, plays an important role in the development of tumors. The surface capillaries of colorectal tumors often show morphological changes, such as heterogeneity in vessel diameter or density and loss of hierarchical structure.
Although the importance of tumor angiogenesis is well known, conventional endoscopic images cannot be used to show these changes in capillaries due to inadequate resolution. No studies have yet been conducted on changes in microcirculatory hemodynamics of colorectal adenomas in vivo under endoscopy. In clinical practice, we found that a novel high-resolution magnifying colonoscope (Fujifilm EC-760ZP) with blue-laser imaging (BLI) clearly revealed the mucosal surface capillary network in vivo and in real time.
In this study, we observed the superficial microcirculation of colorectal adenomas using the novel magnifying colonoscope with BLI and quantitatively analyzed the changes in hemodynamic parameters, thus providing a new insight into early colorectal tumors.
From October 2019 to January 2020, 11 patients were screened for colon adenomas with the novel high-resolution magnification endoscope with BLI. Video images were recorded and processed with Adobe Premiere, Adobe Photoshop and Image-pro Plus software. Four microcirculation parameters: Microcirculation vessel density, mean vessel width with width standard deviation, and blood flow velocity, were calculated respectively for adenoma and the surrounding normal mucosa.
A total of 16 adenomas were identified. Compared with the normal surrounding mucosa, the superficial vessel density in the adenomas was decreased; the mean vessel width and vessel width deviation were both increased; and blood flow slowed down in the adenomas.
The novel high-resolution magnification endoscope with BLI can be a tool for the in vivo study of adenoma superficial microcirculation. The superficial vessel density in the adenoma was decreased, with more irregularity and slower blood flow.
High-resolution magnifying endoscopy can be used to quantitatively analyze the microcirculation on the surface of the colorectal adenomas. It provide the possibility of active recognition of colorectal lesions with AI technology under endoscopy.
Thank you to the medical staff of Endoscopy Center of Beijing Tsinghua Changgung Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe H, Japan S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Sung JJ, Lau JY, Goh KL, Leung WK; Asia Pacific Working Group on Colorectal Cancer. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 598] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 2. | Staton CA, Chetwood AS, Cameron IC, Cross SS, Brown NJ, Reed MW. The angiogenic switch occurs at the adenoma stage of the adenoma carcinoma sequence in colorectal cancer. Gut. 2007;56:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Liu H, Wu J, Liu XC, Wei N, Liu KL, Ma YH, Chang H, Zhou Q. Correlation between microvascular characteristics and the expression of MVD, IGF-1 and STAT3 in the development of colonic polyps carcinogenesis. Exp Ther Med. 2017;13:49-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2286] [Article Influence: 175.8] [Reference Citation Analysis (2)] |
| 5. | Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, Imamura Y, Willett WC, Rosner BA, Fuchs CS, Giovannucci E, Ogino S, Chan AT. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1158] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 6. | Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 1016] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 7. | Tiwari AK, Crawford SE, Radosevich A, Wali RK, Stypula Y, Kunte DP, Mutyal N, Ruderman S, Gomes A, Cornwell ML, De La Cruz M, Brasky J, Gibson TP, Backman V, Roy HK. Neo-angiogenesis and the premalignant micro-circulatory augmentation of early colon carcinogenesis. Cancer Lett. 2011;306:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Aihara H, Saito S, Tajiri H. Rationale for and clinical benefits of colonoscopy with narrow band imaging: pathological prediction and colorectal screening. Int J Colorectal Dis. 2013;28:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Sakamoto T, Takamaru H, Sekiguchi M, Yamada M, Matsuda T, Saito Y. Reliability of Japan Narrow-Band Imaging Expert Team Classification for the Diagnosis of Colorectal Neoplasms: A Pilot Study. Digestion. 2020;101:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Tian Y, Zheng Y, Teng G, Li J, Wang H. Imbalanced mucosal microcirculation in the remission stage of ulcerative colitis using probe-based confocal laser endomicroscopy. BMC Gastroenterol. 2019;19:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tascon G, Dobbe I, Ince C. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11:R101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 577] [Cited by in RCA: 626] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 12. | Subramaniam S, Hayee B, Aepli P, Schoon E, Stefanovic M, Kandiah K, Thayalasekaran S, Alkandari A, Bassett P, Coron E, Pech O, Hassan C, Neumann H, Bisschops R, Repici A, Bhandari P. Optical diagnosis of colorectal polyps with Blue Light Imaging using a new international classification. United European Gastroenterol J. 2019;7:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Yoshida N, Yagi N, Inada Y, Kugai M, Okayama T, Kamada K, Katada K, Uchiyama K, Ishikawa T, Handa O, Takagi T, Konishi H, Kokura S, Yanagisawa A, Naito Y. Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig Endosc. 2014;26:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Yoshida N, Hisabe T, Inada Y, Kugai M, Yagi N, Hirai F, Yao K, Matsui T, Iwashita A, Kato M, Yanagisawa A, Naito Y. The ability of a novel blue laser imaging system for the diagnosis of invasion depth of colorectal neoplasms. J Gastroenterol. 2014;49:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Roy HK, Gomes A, Turzhitsky V, Goldberg MJ, Rogers J, Ruderman S, Young KL, Kromine A, Brand RE, Jameel M, Vakil P, Hasabou N, Backman V. Spectroscopic microvascular blood detection from the endoscopically normal colonic mucosa: biomarker for neoplasia risk. Gastroenterology. 2008;135:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Raica M, Cimpean AM, Ribatti D. Angiogenesis in pre-malignant conditions. Eur J Cancer. 2009;45:1924-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 402] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 18. | Ang TL, Li JW, Wong YJ, Tan YJ, Fock KM, Tan MTK, Kwek ABE, Teo EK, Ang DS, Wang LM. A prospective randomized study of colonoscopy using blue laser imaging and white light imaging in detection and differentiation of colonic polyps. Endosc Int Open. 2019;7:E1207-E1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Dos Santos CEO, Malaman D, Yoshida N, Pereira-Lima JC, Onófrio FQ, Furlan RG, Tabushi FI, Malafaia O. Blue laser imaging: a new image-enhanced endoscopy for the diagnosis of colorectal lesions. Eur J Gastroenterol Hepatol. 2018;30:1514-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Yoshida N, Hisabe T, Ikematsu H, Ishihara H, Terasawa M, Inaba A, Sato D, Cho H, Ego M, Tanaka Y, Yasuda R, Inoue K, Murakami T, Inada Y, Itoh Y, Saito Y. Comparison Between Linked Color Imaging and Blue Laser Imaging for Improving the Visibility of Flat Colorectal Polyps: A Multicenter Pilot Study. Dig Dis Sci. 2020;65:2054-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer. 2001;84:1354-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Skinner SA, Frydman GM, O'Brien PE. Microvascular structure of benign and malignant tumors of the colon in humans. Dig Dis Sci. 1995;40:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |