Published online Apr 16, 2024. doi: 10.4253/wjge.v16.i4.193
Peer-review started: November 29, 2023
First decision: December 27, 2023
Revised: January 28, 2024
Accepted: March 18, 2024
Article in press: March 18, 2024
Published online: April 16, 2024
Processing time: 133 Days and 13.9 Hours
Choosing an optimal post-polypectomy management strategy of malignant colorectal polyps is challenging, and evidence regarding a surveillance-only strategy is limited.
To evaluate long-term outcomes after endoscopic removal of malignant colorectal polyps.
A single-center retrospective cohort study was conducted to evaluate outcomes after endoscopic removal of malignant colorectal polyps between 2010 and 2020. Residual disease rate and nodal metastases after secondary surgery and local and distant recurrence rate for those with at least 1 year of follow-up were investigated. Event rates for categorical variables and means for continuous variables with 95% confidence intervals were calculated, and Fisher’s exact test and Mann-Whitney test were performed. Potential risk factors of adverse outcomes were determined with univariate and multivariate logistic regression models.
In total, 135 lesions (mean size: 22.1 mm; location: 42% rectal) from 129 patients (mean age: 67.7 years; 56% male) were enrolled. The proportion of pedunculated and non-pedunculated lesions was similar, with en bloc resection in 82% and 47% of lesions, respectively. Tumor differentiation, distance from resection margins, depth of submucosal invasion, lymphovascular invasion, and budding were reported at 89.6%, 45.2%, 58.5%, 31.9%, and 25.2%, respectively. Residual tumor was found in 10 patients, and nodal metastasis was found in 4 of 41 patients who underwent secondary surgical resection. Univariate analysis identified piecemeal resection as a risk factor for residual malignancy (odds ratio: 1.74; P = 0.042). At least 1 year of follow-up was available for 117 lesions from 111 patients (mean follow-up period: 5.59 years). Overall, 54%, 30%, 30%, 11%, and 16% of patients presented at the 1-year, 3-year, 5-year, 7-year, and 9-10-year surveillance examinations. Adverse outcomes occurred in 9.0% (local recurrence and dissemination in 4 patients and 9 patients, respectively), with no difference between patients undergoing secondary surgery and surveillance only.
Reporting of histological features and adherence to surveillance colonoscopy needs improvement. Long-term adverse outcome rates might be higher than previously reported, irrespective of whether secondary surgery was performed.
Core Tip: Despite recent advancements in endoscopy and the ability to perform optical diagnoses, submucosal invasion in colorectal polyps is often diagnosed at post-polypectomy histological evaluations. The reporting of high-risk histological features cannot serve as the sole basis of optimal post-polypectomy management strategy. Long-term adverse outcomes after endoscopic resection of malignant colorectal polyps might be more common than previously reported, irrespective of whether secondary surgery was performed. Therefore, adherence to post-polypectomy surveillance colonoscopy should be improved.
- Citation: Fábián A, Bor R, Vasas B, Szűcs M, Tóth T, Bősze Z, Szántó KJ, Bacsur P, Bálint A, Farkas B, Farkas K, Milassin Á, Rutka M, Resál T, Molnár T, Szepes Z. Long-term outcomes after endoscopic removal of malignant colorectal polyps: Results from a 10-year cohort. World J Gastrointest Endosc 2024; 16(4): 193-205
- URL: https://www.wjgnet.com/1948-5190/full/v16/i4/193.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i4.193
Colorectal cancer is the third most frequently diagnosed malignancy and the second leading cause of cancer-related deaths. Hungary is among the countries with the highest global reported incidence rates[1,2]. Introduction of colorectal screening programs results in malignancies being recognized at an earlier stage, and consequently the number of malignant polyps [submucosal invasion (SMI) on histologic examination (pT1 stage according to the TNM classification), independent from lymph node involvement] is rising[3,4]. The prevalence of malignant polyps is estimated to be between 0.75%-5.60% of endoscopically removed polyps in the general population but can be as high as 15.00% in the screening population[3,5,6].
These lesions can appear macroscopically benign, and up to 40% of these lesions are not identifiable with optical macroscopic diagnostic tools[7,8]. Often, invasive adenocarcinoma is revealed by post-polypectomy histological examination. By this time, there is a risk for lymphovascular invasion and metastasis formation due to SMI. Lymph node metastases can occur in 6%-13% of T1 colorectal tumors[9], but the exact rate depends on various endoscopic and histological prognostic factors. The following histological features have been associated with a higher risk of adverse outcomes: Poorly differentiated adenocarcinoma; involvement of resection margins; deep SMI (at least 1 mm); vascular, lymphatic, and perineural invasion; and tumor budding[4,9,10].
The definition of a positive resection margin after polypectomy varies in the literature. Although most guidelines use the 1 mm cutoff value, recently some authors have proposed that resection margins should only be considered positive when tumor cells are found at the cautery line[4,11-13]. The 2023 update of National Comprehensive Cancer Network (NCCN) guidelines recommends surgical resection for both colon and rectum malignancies if one of the following features are present: Fragmented polypectomy sample; unassessable resection margins; or the presence of at least one of the histological prognostic features suggestive of an adverse outcome (lymphovascular invasion, positive resection margin, or tumor budding)[14,15].
Suboptimal reporting of histological features can make the decision-making process over further management strategy (completion surgery vs surveillance only) challenging[4,16]. The risk of surgery due to patient age and comorbidities, as well as patient preferences and tumor location, also need to be considered[17]. Evidence of long-term outcomes of a surveillance-only strategy after polypectomy is limited. Currently, there is no consensus on the timing of surveillance colonoscopies and the need for additional cross-section imaging modalities[4].
Therefore, we aimed to evaluate the long-term outcomes of endoscopic removal of malignant colorectal polyps by assessing residual malignancy and lymph node involvement rate after secondary surgery (first endpoint; Figure 1) and local and distant recurrence rate throughout the follow-up period both in cases of secondary surgery and a surveillance-only strategy (second endpoint; Figure 2).
This retrospective cohort study investigated outcomes after endoscopic resection of malignant colorectal polyps resected between January 1, 2010 and December 31, 2020 in the tertiary endoscopic center of University of Szeged. This study was carried out in accordance with the Helsinki Declaration and was approved by the Regional and Institutional Human Medical Biological Research Ethics Committee of University of Szeged (clinical trial registration number: 4137/2018).
Lesions were enrolled if the following inclusion criteria applied: (1) No invasive malignancy was suspected with pre-polypectomy examinations (histology, virtual chromoendoscopy, rectal endosonography, if performed); (2) Lesions appeared to be suitable for endoscopic resection based on their macroscopic appearance and adequate lifting sign; (3) Invasive adenocarcinoma was revealed by post-polypectomy histology; and (4) Depth of invasion was limited to the submucosa (T1). Lesions were excluded if polypectomy was not completed due to suspicion of an invasive tumor. Long-term outcomes were only assessed for lesions in cases that had least 1 year of follow-up data available. Patients with inflammatory bowel disease-associated neoplasia as well as those with a clinically suspected or verified polyposis syndrome or hereditary non-polyposis colorectal cancer based on the Amsterdam II criteria were excluded from the analysis. During the study period, tumor testing for microsatellite instability was not routinely available for early-stage colorectal cancer.
Demographic data of patients, polyp characteristics [size, location and morphology (pedunculated vs non-pedunculated, Paris classification)], method of endoscopic resection, completeness of resection based on endoscopic assessment, and rate of adverse events were collected from the electronic medical record system. Post-polypectomy histological reports were reviewed for the following features considered to be related to high risk of adverse outcomes: Determinability and involvement of resection margins (tumor cells in the cautery line, distance from resection margin reaching 1 mm), absolute depth of SMI (superficial SMI < 1mm, deep SMI ≥ 1 mm), tumor differentiation [low grade (well or moderately differentiated) vs high grade (poorly differentiated)], tumor budding (Bd1: 1-4 buds, Bd2: 5-9 buds, Bd3: ≥ 10 buds at the invasion front), and lymphovascular invasion (possibly assessing lymphatic and vascular invasion separately). Reporting of Haggitt and Kikuchi classification was also assessed, but because of their limited determinability due to the common lack of muscular propria in polypectomy specimens, these were not included in quantitative analyses. Tumor markers [carcinoembryonic antigen (CEA) and cancer antigen (CA) 19-9] at the time of endoscopic polyp removal were also assessed as potential predictors of adverse outcomes.
Patients were divided into two groups according to the post-polypectomy management strategy applied (secondary surgery for completion vs surveillance only). The decision between the two strategies was made on tumor board discussions considering post-polypectomy histological results, age, comorbidities, and preferences of patients. The rate of residual malignancy and lymph node involvement was investigated in patients undergoing secondary surgery. Local and distant recurrence during the follow-up period were investigated as adverse outcome measures in cases of both secondary surgery and surveillance-only strategies. Adverse outcome rates were compared between the two strategies to assess the potential risk derived from not having completion surgery after endoscopic resection of malignant polyps.
The follow-up period was defined as the time interval between the polypectomy date and the last registered date of a patient visit recorded in the electronic medical record system. Cause of death (if available) was registered for patients who died during the follow-up period. Length of colonoscopic surveillance (i.e. last registered colonoscopy date) was also assessed. Clinical data of patients with distant metastases were reviewed searching for other, more advanced malignancies as a potential primary focus of dissemination.
Categorical variables were reported as event rates and relative frequencies, and continuous variables as the means with 95% confidence intervals (CI). Fisher’s exact test was used to analyze categorical data, whereas the Mann-Whitney test was used in cases of continuous data. Potential risk factors of adverse outcomes were determined with univariate and multivariate logistic regression models. Statistical tests were performed using R statistical software version 3.1.2 (R Foundation, Vienna, Austria) and jamovi software version 2.3.24[18,19]. P values < 0.05 were considered significant.
In total, 135 endoscopically resected malignant colorectal polyps from 129 patients [age: 67.7 years (95%CI: 66.0–69.4 years); 56% male] were enrolled during the 10-year study period. The proportion of pedunculated and non-pedunculated lesions was similar (48% vs 45%). En bloc resection could be achieved in 82% of pedunculated polyps, whereas it was feasible in only 47% of non-pedunculated lesions. Polyp characteristics are summarized in Table 1. Endoscopic polypectomy was performed with snare polypectomy and endoscopic mucosal resection in most of the cases. Endoscopic submucosal dissection (ESD) and endoscopic full-thickness resection were not routinely available in our institution during the study period.
| Characteristic | n | Value |
| Location, n (%) | ||
| Colon | 80 (59) | |
| Right colon | 12 (9) | |
| Left colon | 68 (50) | |
| Rectum | 55 (41) | |
| Morphology (Paris classification), n (%) | ||
| Pedunculated | ||
| 0-Ip | 65 (48) | |
| Non-pedunculated | 60 (45) | |
| 0-Is | 34 (25) | |
| 0-Isp | 3 (2) | |
| 0-IIa | 13 (10) | |
| 0-IIb | 3 (2) | |
| 0-IIc | 7 (5) | |
| Not available | 10 (7) | |
| Polyp size in mm, mean (95%CI) | 22.1 (20.0–24.2) | |
| Pedunculated | 20.7 (18.0–23.4) | P = 0.0041 |
| Non-pedunculated | 24.6 (21.0–28.2) | |
| En bloc resection, n (%) | 89 (66) | |
| Pedunculated | 53 (82) | |
| Non-pedunculated | 28 (47) | |
| Polyp morphology not available | 8 |
Adverse events occurred in 21 cases (15.6%) and included post-polypectomy bleeding in 14 cases (transfusion was required in 2 cases), perforation in 6 cases (surgical intervention was necessary in 3 cases, the others could be managed by endoscopic closure), and post-polypectomy syndrome requiring antibiotics in 1 case.
Tumor marker values (CEA or CA 19-9) were available for 37 out of the 129 patients at the time of endoscopic polyp removal. CEA and CA 19-9 were elevated in 5 and 3 patients, respectively, and both were elevated in 1 patient. It should be noted that the latter patient also had a synchronous advanced-stage colorectal tumor in addition to the T1 stage malignant colorectal polyp. Elevated tumor marker values did not exceed 2× the upper limit of normal values for CEA and CA 19-9.
Although endoscopic removal was considered complete based on endoscopic assessment in 87% of the cases, histology revealed complete resection in only 56%. Completeness of resection could not be determined in 26 cases (19%) due to thermal injury of resection margins, tissue fragmentation, or lack of adequate specimen orientation after piecemeal resection.
Throughout the entire study period, high-risk histologic features were adequately reported as follows: Tumor differentiation in 89.6%; tumor distance from resection margins in 45.2%; absolute depth of SMI in 58.5%; Haggitt/Kikuchi classification in 31.9%; lymphovascular invasion in 31.9%; and tumor budding in 25.2%. Reporting of all features (except Haggitt/Kikuchi classification) was adequate in only 26 cases (19%). Only one feature was reported in 36 cases (27%) and no features in 3 cases (2%).
Based on the available data, at least one high-risk histological feature was present in 60 cases (44%). If considering only R1 resection margin cases (tumor cells can be detected at the cautery line) as high risk (as proposed by recent studies[11]), this rate changed to 39% (53 cases). If unassessable resection margins and piecemeal resection were considered high-risk features as well, 77 cases (57%), and 88 cases (65%), respectively, were in the high-risk category.
Secondary surgery was performed for 45 lesions (33.3%) in 41 patients (31.8%) 90 d (95%CI: 22.4–158.9 d) after the polypectomy on average. Overall, 53% of these lesions were located in the rectum and 47% in the colon. At least one high risk feature was present in 82.2% (including unassessable resection margins as high-risk features as well). This percentage increased to 91.1% if piecemeal resection was also considered a high-risk feature according to the most recent NCCN guideline[14,15]. On the other hand, only 48% of lesions (37/77 cases) with at least one high-risk feature (considering unassessable margins as high-risk as well) underwent secondary surgery for completion.
Surgery-related adverse events occurred in 5 cases (12.2%) and included postoperative confusion in 1 case, necessary reoperation in 3 cases because of mechanical occlusion due to adhesions, wound dehiscence, and enterocutaneous fistula, and 1 patient death due to aspiration-induced bronchopneumonia as a consequence of paralytic bowel obstruction. Therefore, surgical mortality was 2.4% in our cohort.
Histological examination of surgically resected specimens revealed residual malignancy in 15 lesions in 10 patients (24.4%) and lymph node involvement in 4 patients (9.8%) [3 of them (6.7%) had residual malignancy as well]. All patients with residual malignancy (in whom endoscopic resection margins were assessable) had tumor cells in the cautery line (R1) after endoscopic resection. In univariate logistic regression analysis, piecemeal resection was found to be a risk factor for residual malignancy [odds ratio (OR): 1.74, P = 0.042], but the multivariate model did not confirm this (Tables 2 and 3).
| Investigated parameter | Residual malignancy | Lymph node involvement | ||
| OR | P value | OR | P value | |
| Size | 0.05 | 0.055 | 0.003 | 0.936 |
| Location: Rectum | 1.10 | 0.148 | 1.34 | 0.268 |
| Morphology: Non-pedunculated | 1.74 | 0.116 | 17.90 | 0.995 |
| En bloc resection: No | 1.74 | 0.042 | 1.10 | 0.362 |
| Tumor differentiation | NA | NA | ||
| Positive resection margins: Negative-R1 | 17.60 | 0.998 | 17.20 | 0.998 |
| Positive resection margins: Negative-critical | 3.58E-08 | 1.000 | -6.93E-09 | 1.00 |
| Depth of submucosal invasion: Deep | -1.18 | 0.227 | -0.32 | 0.773 |
| Lymphatic invasion | 2.40 | 0.173 | -17.80 | 0.997 |
| Vascular invasion | -16.96 | 0.997 | -16.06 | 0.997 |
| Tumor budding | 5.17E-15 | 1.000 | -16.62 | 0.998 |
| Investigated parameter | Residual malignancy | Lymph node involvement | ||
| OR | P value | OR | P value | |
| Size | 0.04 | 0.179 | -0.02 | 0.654 |
| Location: Rectum | 0.48 | 0.602 | 0.82 | 0.518 |
| Morphology: Non-pedunculated | 1.32 | 0.332 | 17.45 | 0.996 |
| En bloc resection: No | 0.81 | 0.409 | 0.96 | 0.477 |
| At least one high-risk feature | 17.7 | 0.994 | -0.48 | 1 |
As described above, 45 lesions from 41 patients underwent secondary surgery, and surveillance-only strategy was chosen for the other 90 lesions from 88 patients. However, only 117 lesions from 111 patients had at least 1 year of follow-up data available and were taken into consideration when assessing long-term outcomes. The mean follow-up period for this subgroup was 5.59 years [95%CI: 5.02–6.16 years]. In total, 40 lesions from 36 patients underwent secondary surgery for completion, and surveillance-only strategy was chosen for 77 lesions from 75 patients.
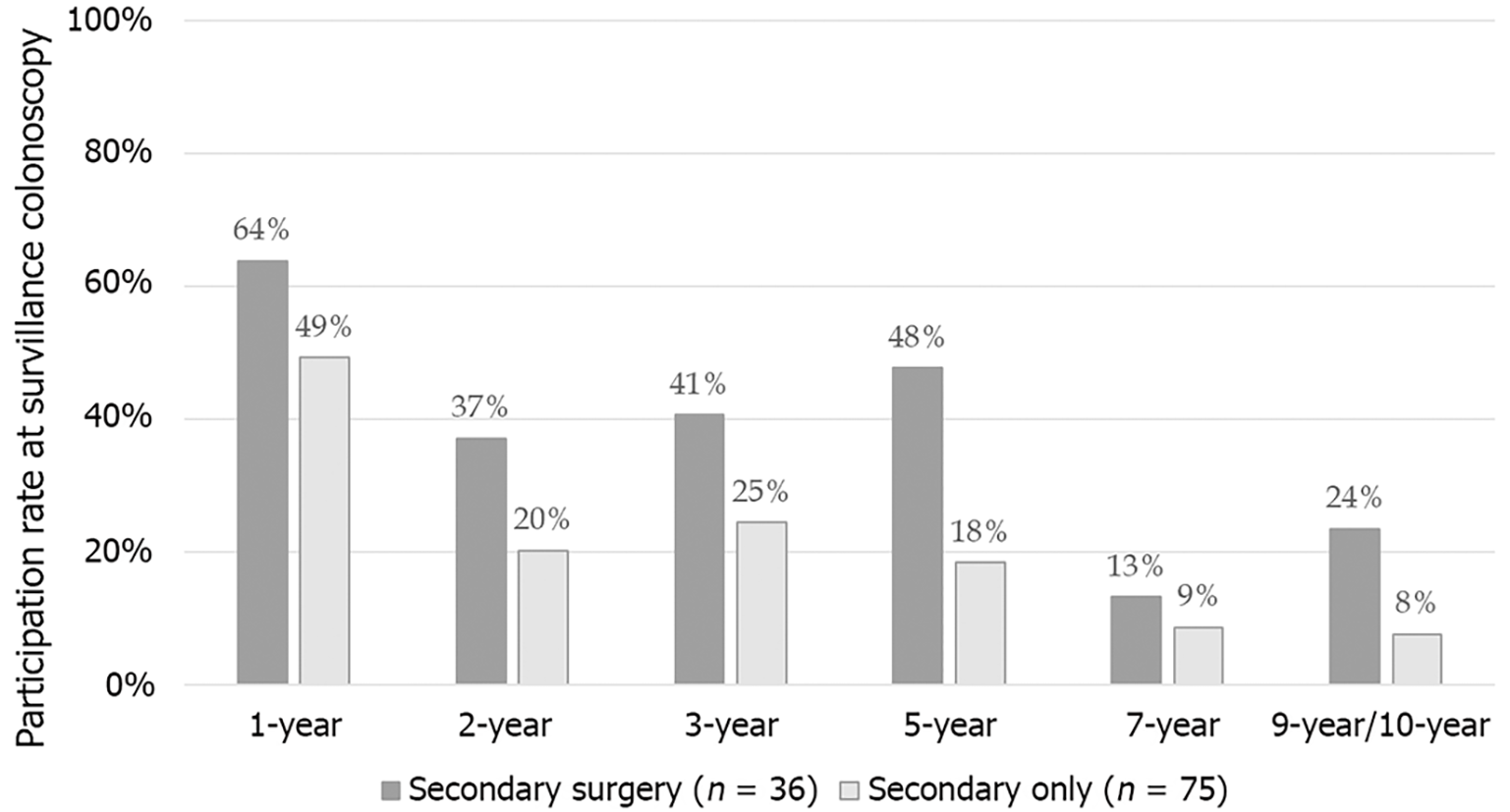
During the follow-up period, participation rates at surveillance colonoscopy showed a gradually decreasing tendency. While 54% of patients presented at the 1-year surveillance colonoscopy, participation rates for 3-year, 5-year, 7-year, and 9-10-year examinations were 30%, 30%, 11%, and 16%, respectively. For each time point, participation rate was determined as the number of patients who underwent surveillance colonoscopy compared to the number of patients for whom follow-up information was available and who were alive. Remarkably, patients undergoing secondary surgery were more likely to participate in surveillance colonoscopies than those with a surveillance-only strategy after polypectomy (Figure 2).
During the follow-up period, distant metastasis without any other, more advanced malignancy as a potential primary focus was detected in 9 patients (8.1%). Local recurrence was also detected in 3 of these patients and was reported in 1 additional patient without distant metastasis (local recurrence rate: 3.6%). The mean occurrence of local recurrence was 3.98 years (range: 1.84–7.53 years). The total rate of adverse outcomes (dissemination or local recurrence) in the entire study population was 9.0%. Cancer-related deaths were reported in 2 patients; therefore, tumor progression-related mortality rate was 1.8%. There was no significant difference in adverse outcome rates between the two patient groups (i.e. secondary surgery vs surveillance only) (Table 4).
| Feature | Secondary surgery for completion, n = 36 | Surveillance-only strategy, n = 75 | P value |
| Adverse outcomes: Dissemination and/or local recurrence | 5 (13.9) | 5 (6.7) | 0.289 |
| Dissemination | 5 (13.9) | 4 (5.3) | 0.147 |
| Local recurrence | 2 (5.6) | 2 (2.7) | 0.594 |
| Both | 2 (5.6) | 1 (1.3) | |
| Tumor progression | 1 (2.8) | 1 (1.3) | 0.546 |
Non-pedunculated polyp morphology was determined as a risk factor of distant metastases with logistic regression (OR: 2.51, P = 0.020), although it was not confirmed by multivariate analysis (Tables 5 and 6). None of the patients with elevated initial tumor marker values presented with adverse outcomes.
| Investigated parameter | Distant metastasis | Local recurrence | ||
| OR | P value | OR | P value | |
| Size | 0.03 | 0.278 | 0.001 | 0.985 |
| Location: Rectum | 0.70 | 0.276 | 1.35 | 0.251 |
| Morphology: Non-pedunculated | 2.51 | 0.020 | 18.30 | 0.995 |
| En bloc resection: No | 0.19 | 0.776 | -0.55 | 0.641 |
| Tumor differentiation | -13.16 | 0.993 | NA | |
| Positive resection margins: Negative-R1 | 0.43 | 0.715 | 1.75E-14 | 1.000 |
| Positive resection margins: Negative-critical | -17.26 | 0.995 | -8.97E-30 | 1.000 |
| Depth of submucosal invasion: Deep | -0.486 | 0.578 | -17.41 | 0.995 |
| Lymphatic invasion | -16.62 | 0.995 | -7.37E-14 | 1.000 |
| Vascular invasion | 1.70 | 0.212 | 5.19E-15 | 1.000 |
| Tumor budding | -17.17 | 0.997 | NA | |
| Surgery for completion: No | -0.61 | 0.343 | -0.429 | 0.677 |
| Investigated parameter | Distant metastasis | Local recurrence | ||
| OR | P value | OR | P value | |
| Size | -0.02 | 0.572 | 1.26E-15 | 1.000 |
| Location: Rectum | 0.32 | 0.648 | 19.5 | 0.998 |
| Morphology: Non-pedunculated | 1.19 | 0.136 | 17.7 | 0.998 |
| At least one high-risk feature | 0.59 | 0.514 | 18.0 | 0.999 |
| Surgery for completion | -0.39 | 0.613 | -19.4 | 0.998 |
It was also investigated how outcomes would have been affected if the need for resection surgery following endoscopic polypectomy during the study period had been assessed according to the current NCCN recommendation[14,15]. Overall, 64% of patients were managed according to the NCCN recommendation (resection surgery or surveillance only). However, of the patients for whom surgical resection was recommended, only 53% underwent resection surgery. No significant difference was observed in adverse event rates between the groups (Table 7).
| Recommendation according to NCCN guideline | Surgery for completion, n = 70 | Surveillance was sufficient, n = 41 | ||
| Resection was performed? | Yes | No | Yes | No |
| 33 | 37 | 3 | 38 | |
| Adverse outcome: Dissemination or local recurrence | 5 (15) | 2 (5.4) | 0 | 3 (7.9) |
| Dissemination | 5 | 2 | 0 | 2 |
| Local recurrence | 2 | 0 | 0 | 2 |
To the best of our knowledge, the results from this single-center, retrospective cohort study are the first data from the Central-European region regarding long-term outcomes of endoscopic removal of malignant colorectal polyps. The relatively longer follow-up period in our study compared to that reported in the majority of previous studies[20-25] and inclusion of only those with at least 1 year of follow-up allowed for adequate assessment of adverse outcomes. Patient selection limited to those with a submucosally invasive malignant polyp was another strength of our study, as inclusion of intramucosal cancer (pTis) might falsely result in more favorable long-term outcomes.
Prepolypectomy identification of SMI in colorectal polyps is often challenging, even with the application of advanced optical diagnostic tools, e.g., virtual chromoendoscopy[7,8]. In the community setting, the availability, feasibility, and minimum standard of advanced imaging use are unknown according to the current European Society of Gastrointestinal Endoscopy guidelines on performance measures for lower gastrointestinal endoscopy[26]. In our tertiary center, advanced imaging techniques were not routinely available and applied during the study period, which might have resulted in underassessment of SMI, resulting in suboptimal resection choice. Macroscopic assessment of completeness of endoscopic resection of malignant colorectal polyps is often unreliable, especially in cases of non-pedunculated lesions (the majority of which were resected with the piecemeal technique).
Selecting the optimal post-polypectomy management strategy is mainly based on the presence of histological risk factors, but their reporting shows great variations[16,27]. In our study, only tumor differentiation was reported in most of the cases, and reporting of histological features was inadequate in 30% of cases (maximum one feature was reported). A recent large volume study assessing the quality of histological reports after endoscopic resection of malignant polyps also highlighted the incomplete reporting of high-risk features. Tumor differentiation, distance from resection margins, and lymphovascular invasion was reported in 82.4%, 86.8%, and 75.6% of cases, respectively. Tumor budding was only reported in 14.4% of cases.
As quantification of the depth of SMI is not required routinely by histologic guidelines, sufficient information for making an optimal post-polypectomy management decision may be lacking, even in cases of reports containing otherwise complete and adequate information on the other high-risk features[12]. Recently, there has been a shift regarding the type of information on the depth of SMI where absolute depth of invasion is preferred over Haggitt/Kikuchi classification. This is also reflected in the availability of information in our study. The Haggitt/Kikuchi classification was reported in only 33.8% of cases. Absolute depth of SMI was proposed by Ueno et al[28] and was reported in 56.6% of cases.
The definition of a positive resection margin varies greatly in the literature. In our study, residual malignancy and lymph node involvement could only be detected in cases when post-polypectomy histology revealed tumor cells in the cautery line. Although the difference was not statistically significant, this seems to support the proposition that tumor involvement of the cautery line alone carries a high risk. Brown et al[11] also detected no residual carcinoma in surgical specimens of malignant polyps previously endoscopically resected with a 0.1-1.0 mm distance from resection margins. Berg et al[12] found significantly higher lymph node involvement in cases of tumor involvement of endoscopic resection margins than in cases of tumors approaching but not reaching the cautery line. The residual malignancy and lymph node involvement rate during secondary surgery for completion was found to be in accordance with literature data[17,27-31].
In our study, only piecemeal resection was found to be a potential risk factor for residual malignancy, although it was not confirmed by multivariate analysis. Richards et al[30] identified incomplete polypectomy as a high-risk factor for residual tumor detection, and only lymphovascular invasion was found to be a risk factor for lymph node involvement. Systematic review and meta-analysis by Dykstra et al[9] identified lymphovascular invasion, tumor differentiation, and tumor budding as independent risk factors of lymph node involvement. In terms of depth of SMI, 1500 μm depth was found to have the strongest association (OR = 4.37). A multicentric study investigating the role of lymphatic and vascular invasion stated that lymphatic invasion is a stronger predictor of lymph node involvement than vascular invasion or histological differentiation[32].
Neither initial CEA nor CA 19-9 (at the time of the endoscopic polypectomy) can serve as a basis for outcome prediction of malignant colorectal polyps based on our data, as none of the patients with adverse outcomes had elevated markers. On the other hand, none of the patients with elevated markers presented with adverse outcomes.
The adverse outcome rate was somewhat higher than the one reported in the literature. Local recurrence rate after endoscopic resection of malignant polyps was found to be 2.2% over a 100-mo follow-up in the study by Asayama et al[20]. It should be underlined that intramucosal adenocarcinoma cases without SMI were also included in this study; this may explain the lower adverse outcome rate. The adverse outcome rate was 4.6% over a median 36.5-mo follow-up by Backes et al[31]. The 5-year cumulative rate of recurrence was determined to be 5.1% (2.0-13.1%) by Lopez et al[33] among patients treated only with endoscopic polypectomy. According to Dang et al[34], the pooled cumulative incidence rate of recurrence after endoscopic removal of T1 colorectal cancer was 3.3% (95%CI: 2.6%-4.3%, I2 = 54.9%) based on meta-analytic calculations, with similar rates for local and distant recurrence (1.9% and 1.6%, respectively). However, the recurrence rate can be higher in cases of high-risk T1 tumors [7.0% (95%CI: 4.9-9.9%, I2 = 48.1%)]. Recurrence was detected within 72 mo in 95.6% of the cases.
Differences in polypectomy techniques might also serve as an explanation to variations in adverse outcome rates. Most of the previously mentioned studies involved cases of endoscopic mucosal resection and ESD, whereas ESD was not routinely performed in our institute during the study period. Tumor testing for microsatellite instability was also not routinely available for early-stage colorectal cancer during the study period. Therefore, in order to homogenize the patient population, those with suspected hereditary colorectal tumor or polyposis syndrome, as well as those with inflammatory bowel disease-associated neoplasia, were excluded. Therefore, the relatively higher adverse outcome rate in our study cannot be contributed to these.
Based on these results, the follow-up time of our study can be considered appropriate to assess adverse outcomes. However, it should be highlighted that local recurrence was detected more than 7 years after the polypectomy in one of our cases, even with adequate participation in surveillance colonoscopies. No uniform recommendation is available for the timing of surveillance examinations during the follow-up of malignant colorectal polyps undergoing endoscopic resection only. The European Society of Gastrointestinal Endoscopy guidelines published in 2019 recommend a surveillance strategy similar to that of other colorectal cancers after R0 endoscopic resection of low-risk T1 stage colorectal cancers[10]. For malignant polyps with high-risk features that were endoscopically resected and no consequent completion surgery, most authors recommend the initial surveillance colonoscopy to be performed within 3-6 months, and further follow-up should be based on these results. However, no additional advice on surveillance examinations is given[3].
Recently, based on their meta-analysis, Dang et al[34] recommended initial surveillance colonoscopy for low-risk lesions with complete endoscopic resection 1 year after the polypectomy and advised against the use of cross-sectional imaging modalities in this group. Individualized follow-up strategy was advocated for high-risk lesions, both in terms of surveillance colonoscopies and cross-sectional imaging modalities. The authors call for intensive surveillance strategies (surveillance colonoscopy at 3 months, 6 months, 12 months, semiannually in the second year, then annually from year 2 to year 5).
A recent questionnaire-based study investigating follow-up strategies applied in the Scandinavian countries reported the use of 3-year (38%-59%) and 5-year (26%-38%) surveillance strategies in most of the institutes with a different strategy applied based on tumor location (mainly in terms of the use of cross-sectional imaging modalities). They found that 34% of respondents would consider a surveillance strategy for malignant polyps removed endoscopically with ≤ 1 mm resection margin[6]. Although a surveillance-only strategy was applied in the majority of our patients, only half of these patients presented at the 1-year follow-up, and less than 20% showed up at the 5-year follow-up. Given the recurrence patterns detailed above, this should be considered insufficient. However, participation rates on follow-up are still more favorable than those in the United Kingdom cohort reported by Sharma et al[27], where only 61% of patients had a 3-months surveillance colonoscopy, and information at the 1-year follow-up was available only in 6.6%.
The greatest limitation of our study was its retrospective nature, in terms of data on endoscopic polypectomies, surveillance colonoscopies, and histological data. Many high-risk histological features were identified during the study period, and histological guidelines for their reporting were also published in this period. This may account for incomplete histological data in the initial study period. Virtual chromoendoscopy that may assist the recognition of deep SMI was not available in our institute at the earlier study period. Tumor testing for microsatellite instability was not routinely available for early-stage colorectal cancer during the study period. Therefore, the potential differences in adverse outcomes of sporadic and hereditary malignant colorectal polyps could not be assessed. The single center nature of the study reflects only local practice and might be contributed to the relatively smaller sample size compared to multicentric studies; on the other hand, it guarantees uniform management strategies.
Adequate knowledge of high-risk histological features is essential for the selection of the optimal post-polypectomy management strategy after endoscopic resection of malignant colorectal polyps. Appropriate reporting of high-risk endoscopic and histological features is necessary to improve the quality of endoscopic and histological reports and is expected to optimize the selection of post-polypectomy management strategy. Secondary surgery for completion was only performed for half of the cases with high-risk histological features. The residual malignancy and lymph node involvement rates were 25% and 10% of these cases, respectively. Considering that residual malignancy and lymph node involvement could exclusively be detected in surgical specimens after R1 endoscopic resection, revision of the definition of a positive resection margin needs to be considered.
The adverse outcome rate during the follow-up period was found to be somewhat elevated compared to literature data, irrespective of whether secondary surgery for completion after endoscopic polypectomy was performed. This might be attributed to suboptimal prepolypectomy assessment and therefore suboptimal polypectomy choice. Routine use of advanced optical diagnostic tools and implementation of advanced polypectomy techniques (e.g., ESD and endoscopic full-thickness resection) for en bloc resection is expected to reduce adverse outcome rates and needs to be encouraged. Tumor markers cannot serve as a basis of adverse outcome prediction after endoscopic removal of malignant colorectal polyps.
Improving reduced patient adherence to surveillance colonoscopy is essential to detect adverse outcomes as soon as possible. In selected cases, extension of the follow-up period and incorporating cross-sectional imaging studies into the follow-up strategy to detect the disseminated process may be considered. There is a pressing need for further, long-term, multicentric studies considering optimal timing and participation rate of surveillance examinations.
The incidence of malignant colorectal polyps is increasing with the introduction of colorectal screening programs. Even with the application of optical diagnostic tools, many of these lesions are diagnosed only after endoscopic polyp removal. Submucosal invasion that is already present by this time can result in lymphovascular invasion and metastasis formation. Choosing the management strategy (completion surgery vs surveillance only) is mainly based on histological prognostic factors.
Suboptimal reporting of prognostic histological features might lead to inadequate post-polypectomy management choice (including both over-treatment resulting in unnecessary bowel resection and under-treatment leading to an increased risk of disease recurrence and dissemination). The decision over post-polypectomy management is further complicated by the fact that evidence about long-term outcomes of a surveillance-only strategy is limited.
This study aimed to assess the long-term outcomes of endoscopic removal of malignant colorectal polyps by comparing local and distant recurrence rates between the two post-polypectomy management strategies (completion surgery and surveillance-only strategy). We also assessed the residual malignancy and lymph node involvement rate after secondary surgery as well as the adequacy of reporting of post-polypectomy prognostic histological features and investigated the adherence to post-polypectomy surveillance colonoscopies.
A retrospective cohort study over a 10-year study period was conducted. Residual disease rate and nodal metastases after secondary surgery and local and distant recurrence rates for those with at least 1 year of follow-up were investigated. The relatively longer follow-up period in our study compared to previous reports allowed for adequate assessment of adverse outcomes.
Reporting of high-risk histological features varies greatly. While tumor differentiation was reported in almost 90% of cases, budding was only reported in 25% of cases. The residual malignancy and lymph node involvement rates were 25% and 10%, respectively, but could only be detected in surgical specimens after R1 endoscopic resection. The long-term post-polypectomy adverse outcome rate was 9.0%, which was somewhat elevated compared to previously reported rates. Secondary surgery for completion after endoscopic polypectomy did not affect the occurrence of adverse outcomes. Adherence to surveillance colonoscopy was low with only half of the patients presenting at the 1-year follow-up.
Reporting of high-risk features is often inadequate to serve as a basis for the decision of the optimal management strategy and needs to be improved. The definition of a positive resection margin after endoscopic resection needs to be reconsidered, as residual malignancy and lymph node involvement were found only in surgical specimens after R1 endoscopic resection. The relatively higher long-term adverse outcome rate draws attention to the importance of adequate prepolypectomy assessment and implementation of advanced polypectomy techniques. Tumor markers cannot serve as a basis of adverse outcome prediction. Improving adherence to surveillance colonoscopy is essential.
There is a pressing need for further, long-term, multicentric studies considering optimal timing and participation rate of surveillance examination. Our study mainly focused on sporadic malignant colorectal polyps, but any potential differences between adverse outcomes of hereditary and sporadic lesions might further be investigated.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shim CN, South Korea; Yu J, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Cai YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64628] [Article Influence: 16157.0] [Reference Citation Analysis (176)] |
| 2. | Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335-349.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1229] [Article Influence: 245.8] [Reference Citation Analysis (0)] |
| 3. | Saraiva S, Rosa I, Fonseca R, Pereira AD. Colorectal malignant polyps: a modern approach. Ann Gastroenterol. 2022;35:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Shaukat A, Kaltenbach T, Dominitz JA, Robertson DJ, Anderson JC, Cruise M, Burke CA, Gupta S, Lieberman D, Syngal S, Rex DK. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;159:1916-1934.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 5. | Williams JG, Pullan RD, Hill J, Horgan PG, Salmo E, Buchanan GN, Rasheed S, McGee SG, Haboubi N; Association of Coloproctology of Great Britain and Ireland. Management of the malignant colorectal polyp: ACPGBI position statement. Colorectal Dis. 2013;15 Suppl 2:1-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Asheer ZE, Bisgaard T, Mjåland O, Angenete E, Bulut O, Souzani KL. Scandinavian surveillance follow-up programmes in patients with malignant colorectal polyps. Dan Med J. 2021;68. [PubMed] |
| 7. | Puig I, López-Cerón M, Arnau A, Rosiñol Ò, Cuatrecasas M, Herreros-De-Tejada A, Ferrández Á, Serra-Burriel M, Nogales Ó, Vida F, de Castro L, López-Vicente J, Vega P, Álvarez-González MA, González-Santiago J, Hernández-Conde M, Díez-Redondo P, Rivero-Sánchez L, Gimeno-García AZ, Burgos A, García-Alonso FJ, Bustamente-Balén M, Martínez-Bauer E, Peñas B, Pellise M; EndoCAR group; Spanish Gastroenterological Association and the Spanish Digestive Endoscopy Society. Accuracy of the narrow-band imaging international colorectal endoscopic classification system in identification of deep invasion in colorectal polyps. Gastroenterology. 2019;156:75-87. [DOI] [Full Text] |
| 8. | Brunori A, Daca-Alvarez M, Pellisé M. pT1 colorectal cancer: A treatment dilemma. Best Pract Res Clin Gastroenterol. 2023;66:101854. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Dykstra MA, Gimon TI, Ronksley PE, Buie WD, MacLean AR. Classic and Novel Histopathologic Risk Factors for Lymph Node Metastasis in T1 Colorectal Cancer: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2021;64:1139-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Hassan C, Wysocki PT, Fuccio L, Seufferlein T, Dinis-Ribeiro M, Brandão C, Regula J, Frazzoni L, Pellise M, Alfieri S, Dekker E, Jover R, Rosati G, Senore C, Spada C, Gralnek I, Dumonceau JM, van Hooft JE, van Cutsem E, Ponchon T. Endoscopic surveillance after surgical or endoscopic resection for colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Digestive Oncology (ESDO) Guideline. Endoscopy. 2019;51:266-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 11. | Brown IS, Bettington ML, Bettington A, Miller G, Rosty C. Adverse histological features in malignant colorectal polyps: a contemporary series of 239 cases. J Clin Pathol. 2016;69:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Berg KB, Telford JJ, Gentile L, Schaeffer DF. Re-examining the 1-mm margin and submucosal depth of invasion: a review of 216 malignant colorectal polyps. Virchows Arch. 2020;476:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Zammit AP, Brown I, Hooper JD, Clark DA, Riddell AD. Missing parameters in malignant polyp histology reports: can appropriate decisions be made? Pathology. 2023;55:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 409] [Article Influence: 136.3] [Reference Citation Analysis (0)] |
| 15. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 956] [Article Influence: 239.0] [Reference Citation Analysis (16)] |
| 16. | Gimon TI, Dykstra MA, Chezar K, Buie WD, MacLean A. Malignant Colorectal Polyp Pathology: Are We Getting Sufficient Information to Make Decisions? Dis Colon Rectum. 2020;63:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Solon JG, Oliva K, Farmer KC, Wang W, Wilkins S, McMurrick PJ. Rectum versus colon: should malignant polyps be treated differently? ANZ J Surg. 2021;91:927-931. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | jamovi. The jamovi project (2022). Jamovi. (Version 2.3) [Computer Software]. Available from: https://www.jamovi.org. |
| 19. | R Core Team. 2R: A Language and environment for statistical computing. (Version 4.1). 2021. Available from: https://www.R-project.org. |
| 20. | Asayama N, Oka S, Tanaka S, Ninomiya Y, Tamaru Y, Shigita K, Hayashi N, Egi H, Hinoi T, Ohdan H, Arihiro K, Chayama K. Long-term outcomes after treatment for T1 colorectal carcinoma. Int J Colorectal Dis. 2016;31:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Oka S, Tanaka S, Kanao H, Ishikawa H, Watanabe T, Igarashi M, Saito Y, Ikematsu H, Kobayashi K, Inoue Y, Yahagi N, Tsuda S, Simizu S, Iishi H, Yamano H, Kudo SE, Tsuruta O, Tamura S, Cho E, Fujii T, Sano Y, Nakamura H, Sugihara K, Muto T. Mid-term prognosis after endoscopic resection for submucosal colorectal carcinoma: summary of a multicenter questionnaire survey conducted by the colorectal endoscopic resection standardization implementation working group in Japanese Society for Cancer of the Colon and Rectum. Dig Endosc. 2011;23:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Choi DH, Sohn DK, Chang HJ, Lim SB, Choi HS, Jeong SY. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum. 2009;52:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Kobayashi H, Higuchi T, Uetake H, Iida S, Ishikawa T, Ishiguro M, Sugihara K. Resection with en bloc removal of regional lymph node after endoscopic resection for T1 colorectal cancer. Ann Surg Oncol. 2012;19:4161-4167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Nishikawa Y, Horimatsu T, Nishizaki D, Kohno A, Yokoyama A, Yoshioka M, Hida K, Sakanaka K, Minamiguchi S, Seno H, Sakai Y, Nakayama T. Qualitative and Quantitative Analysis of Posttreatment Strategy After Endoscopic Resection for Patients with T1 Colorectal Cancer at High Risk of Lymph Node Metastasis. J Gastrointest Cancer. 2020;51:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | van de Ven SEM, Backes Y, Hilbink M, Seerden TCJ, Kessels K, de Vos Tot Nederveen Cappel WH, Groen JN, Wolfhagen FHJ, Geesing JMJ, Borg FT, van Bergeijk J, Spanier BWM, Mundt MW, Pullens HJM, Boonstra JJ, Opsteeg B, van Lent AUG, Schrauwen RWM, Laclé MM, Moons LMG, Terhaar Sive Droste JS; Dutch T1 CRC Working Group. Periprocedural adverse events after endoscopic resection of T1 colorectal carcinomas. Gastrointest Endosc. 2020;91:142-152.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, Hoff G, Jover R, Suchanek S, Ferlitsch M, Anderson J, Roesch T, Hultcranz R, Racz I, Kuipers EJ, Garborg K, East JE, Rupinski M, Seip B, Bennett C, Senore C, Minozzi S, Bisschops R, Domagk D, Valori R, Spada C, Hassan C, Dinis-Ribeiro M, Rutter MD. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49:378-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 485] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 27. | Sharma V, Junejo MA, Mitchell PJ. Current Management of Malignant Colorectal Polyps Across a Regional United Kingdom Cancer Network. Dis Colon Rectum. 2020;63:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H, Ozawa K, Yoshimura K, Bekku S. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 527] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 29. | Levic K, Kjær M, Bulut O, Jess P, Bisgaard T. Watchful waiting versus colorectal resection after polypectomy for malignant colorectal polyps. Dan Med J. 2015;62:A4996. [PubMed] |
| 30. | Richards CH, Ventham NT, Mansouri D, Wilson M, Ramsay G, Mackay CD, Parnaby CN, Smith D, On J, Speake D, McFarlane G, Neo YN, Aitken E, Forrest C, Knight K, McKay A, Nair H, Mulholland C, Robertson JH, Carey FA, Steele R; Scottish Surgical Research Group. An evidence-based treatment algorithm for colorectal polyp cancers: results from the Scottish Screen-detected Polyp Cancer Study (SSPoCS). Gut. 2018;67:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Backes Y, de Vos Tot Nederveen Cappel WH, van Bergeijk J, Ter Borg F, Schwartz MP, Spanier BWM, Geesing JMJ, Kessels K, Kerkhof M, Groen JN, Wolfhagen FHJ, Seerden TCJ, van Lelyveld N, Offerhaus GJA, Siersema PD, Lacle MM, Moons LMG. Risk for Incomplete Resection after Macroscopic Radical Endoscopic Resection of T1 Colorectal Cancer: A Multicenter Cohort Study. Am J Gastroenterol. 2017;112:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Kudo SE, Ichimasa K, Villard B, Mori Y, Misawa M, Saito S, Hotta K, Saito Y, Matsuda T, Yamada K, Mitani T, Ohtsuka K, Chino A, Ide D, Imai K, Kishida Y, Nakamura K, Saiki Y, Tanaka M, Hoteya S, Yamashita S, Kinugasa Y, Fukuda M, Kudo T, Miyachi H, Ishida F, Itoh H, Oda M, Mori K. Artificial Intelligence System to Determine Risk of T1 Colorectal Cancer Metastasis to Lymph Node. Gastroenterology. 2021;160:1075-1084.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 33. | Lopez A, Bouvier AM, Jooste V, Cottet V, Romain G, Faivre J, Manfredi S, Lepage C. Outcomes following polypectomy for malignant colorectal polyps are similar to those following surgery in the general population. Gut. 2019;68:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Dang H, Dekkers N, le Cessie S, van Hooft JE, van Leerdam ME, Oldenburg PP, Flothuis L, Schoones JW, Langers AMJ, Hardwick JCH, van der Kraan J, Boonstra JJ. Risk and Time Pattern of Recurrences After Local Endoscopic Resection of T1 Colorectal Cancer: A Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:e298-e314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (1)] |