Published online Feb 16, 2024. doi: 10.4253/wjge.v16.i2.83
Peer-review started: October 28, 2023
First decision: December 29, 2023
Revised: January 4, 2024
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: February 16, 2024
Processing time: 95 Days and 3 Hours
Gastric phytobezoars (GPBs) are very common in northern China. Combined therapy involving carbonated beverage consumption and endoscopic lithotripsy has been shown to be effective and safe. Existing studies on this subject are often case reports highlighting the successful dissolution of phytobezoars through Coca-Cola consumption. Consequently, large-scale prospective investigations in this domain remain scarce. Therefore, we conducted a randomized controlled trial to examine the effects of Coca-Cola consumption on GPBs.
To evaluate the impact of Coca-Cola on GPBs, including the dissolution rate, medical expenses, ulcer rate, and operation time.
A total of 160 consecutive patients diagnosed with GPBs were allocated into two groups (a control group and an intervention group) through computer-generated randomization. Patients in the intervention group received a Coca-Cola-based regimen (Coca-Cola 2000-4000 mL per day for 7 d), while those in the control group underwent emergency fragmentation.
Complete dissolution of GPBs was achieved in 100% of the patients in the intervention group. The disparity in expenses between the control group and intervention group (t = 25.791, P = 0.000) was statistically significant, and the difference in gastric ulcer occurrence between the control group and intervention group (χ2 = 6.181, P = 0.013) was also statistically significant.
Timely ingestion of Coca-Cola yields significant benefits, including a complete dissolution rate of 100%, a low incidence of gastric ulcers, no need for fragmentation and reduced expenses.
Core Tip: The timely and sufficient ingestion of Coca-Cola by patients with phytobezoars yields significant benefits, including a high rate of complete dissolution, a low incidence of gastric ulcers, no need for surgery, and reduced medical expenses.
- Citation: Liu FG, Meng DF, Shen X, Meng D, Liu Y, Zhang LY. Coca-Cola consumption vs fragmentation in the management of patients with phytobezoars: A prospective randomized controlled trial. World J Gastrointest Endosc 2024; 16(2): 83-90
- URL: https://www.wjgnet.com/1948-5190/full/v16/i2/83.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i2.83
Gastric bezoars are defined as foreign objects that develop within the gastrointestinal tract due to the accumulation of ingested material[1]. Specifically, gastric phytobezoars (GPBs) were consisted of indigestible cellulose, tannin, and lignin derived from the consumption of persimmons, hawthorn fruits, or date plum persimmons. Tannin undergoes polymerization, resulting in a coagulum that includes protein, pepsins, cellulose, and hemicellulose, forming bezoars[2]. In recent years, a combined therapy approach involving litholysis with carbonated beverages and endoscopic lithotripsy has emer
This meticulously designed study followed a prospective, single-blinded approach with balanced randomization at a 1:1 ratio. Ethical approval for the study was obtained from the Institutional Review Board of the Affiliated Hospital of Qingdao University (QYFYWZLL 26293), and the study was registered in the ClinicalTrial.gov Protocol Registration System with the registration number NCT05645263. The study was conducted at the Affiliated Hospital of Qingdao University from January 1st, 2018, to December 1st, 2022. All participants who expressed willingness to take part in the study provided digital informed consent. The study strictly adhered to the principles outlined in the World Medical Association Declaration of Helsinki.
The sample size was calculated using the following website: http://riskcalc.org:3838/samplesize/. Ultimately, a total of 160 patients (2 arms) were enrolled in the study and randomly allocated to the control or intervention group at a 1:1 ratio.
The study employed specific inclusion and exclusion criteria to ensure the selection of appropriate participants. The inclusion criteria encompassed the absence of contraindications (such as severe heart diseases, suspected shock, or digestive tract perforation), suspected mental diseases, or infectious diseases of the digestive tract that could hinder gastroscopy. Additionally, participants were required to have a bezoar history of no more than 14 d, no history of peptic ulcer diseases and an age range between 14 and 80 years. The exclusion criteria comprised individuals who had history of upper gastrointestinal surgery, underwent previous therapies before enrollment or individuals who did not consent to random allocation.
The following instruments and materials were used in this study: CV-290 (Olympus Co. Ltd), GIF-Q260J (Olympus Co. Ltd), the WF-DTH fragmentation kit (Wilson Shanghai Co. Ltd), disposable snares (Micro-Tech Nanjing Co. Ltd), and Coca-Cola.
A total of 160 consecutive patients were enrolled in the study by doctors in the outpatient department and randomly assigned at a 1:1 ratio to either the control group, which underwent emergency fragmentation with gastroscopy, or the intervention group, which consumed Coca-Cola in the endoscopy center. The enrollment period spanned from January 1, 2018, to December 1, 2022. Randomization was conducted by the investigator using a web-based computer-generated random number system (www.randomization.com). In the intervention group, patients consumed Coca-Cola to treat GPBs. The amount consumed was 250 mL-500 mL every 2 h until bedtime, tailored to individual health conditions and lifestyle habits. Close attention was given to the patients' bowel movements to assess the excretion of bezoars (which are usually harder than normal stool and not scattered by flushing water). The duration of Coca-Cola ingestion was 7 d for the intervention group. Upon completion of the Coca-Cola therapy, patients underwent another endoscopy because endoscopy can observe the mucosa of the stomach directly and further intervention if fragmentation was necessary. The endoscopist recorded all images for every patient. The volume of GPBs was estimated under endoscopy and the evaluation for gastric ulcer was performed in the first endoscopy. Prior to endoscopy, all patients provided digital informed consent.
Statistical analysis was performed using SPSS version 22 (IBM, Inc. Armonk, NY, United States). Continuous variables are presented as the mean and standard deviation (SD). Independent samples t test was utilized for continuous variable analysis. Categorical variables were compared using the chi-square test or Fisher's exact test, as appropriate. A significance level of P < 0.05 was considered statistically meaningful.
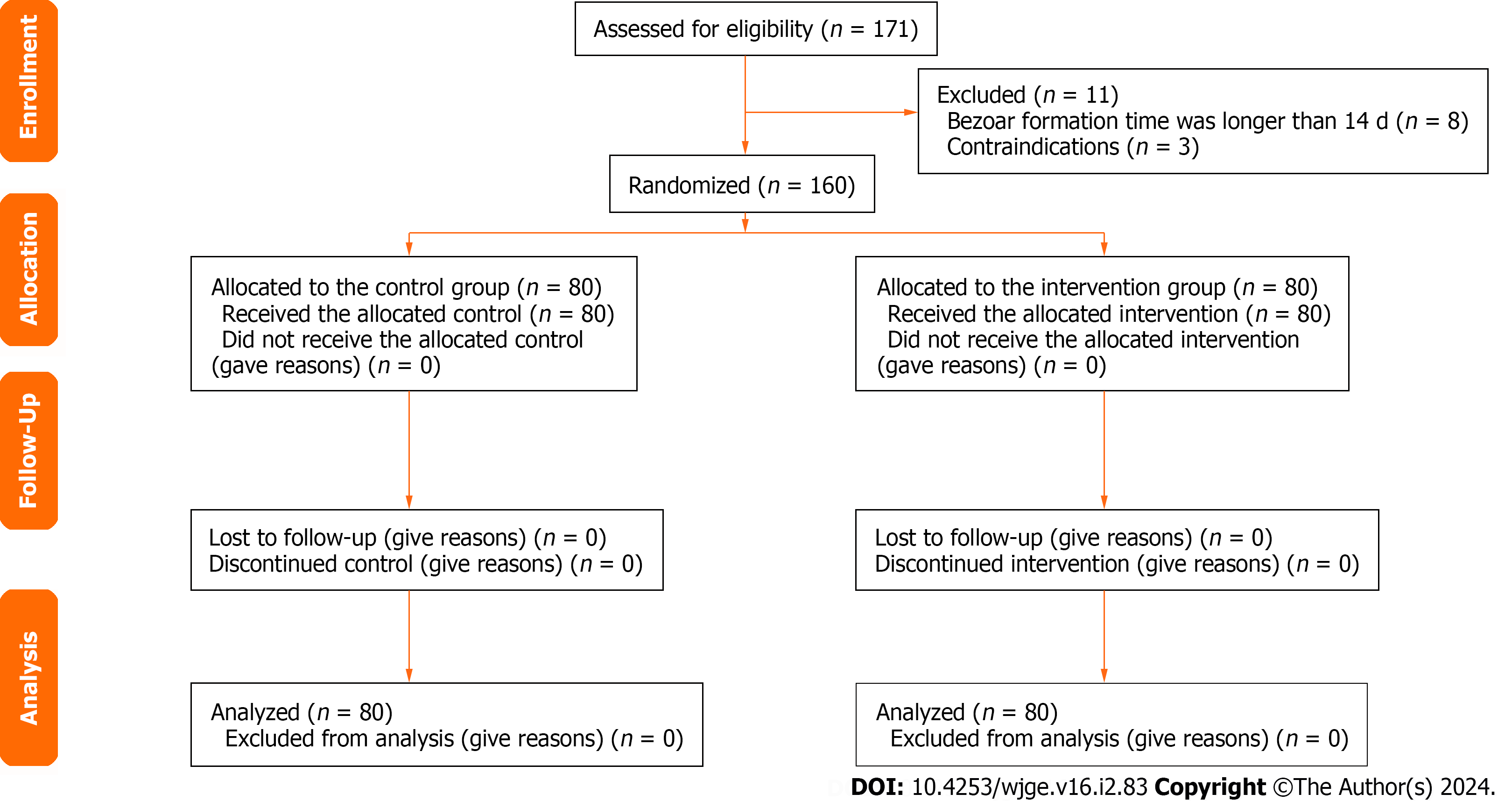
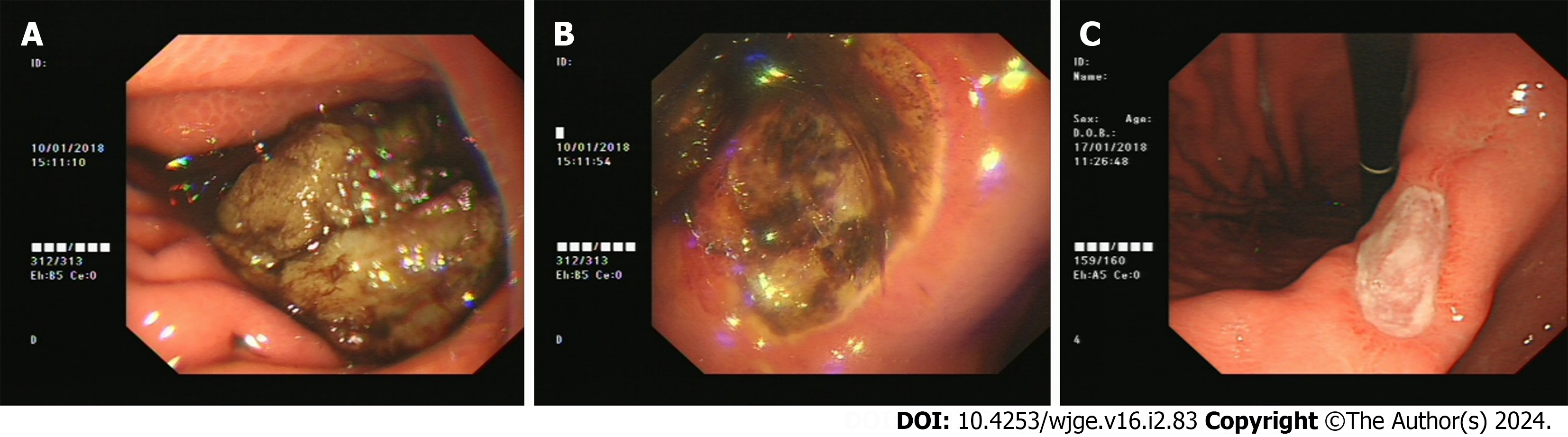
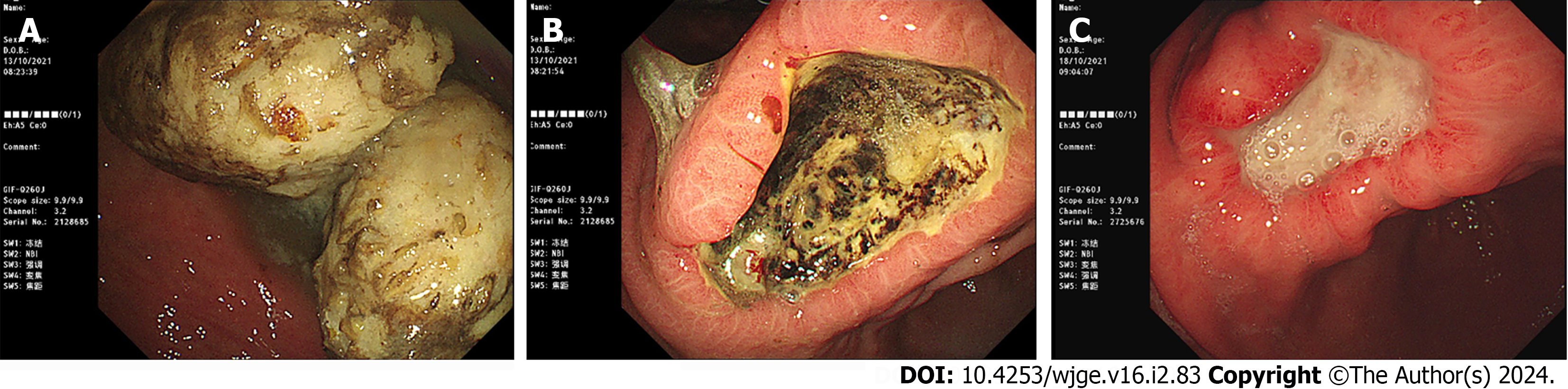
The CONSORT 2010 Flow Diagram is illustrated in Figure 1. Atypical images pertaining to the study findings are shown in Figures 2 and 3. The images of Figures 2 and 3 are all from the intervention group. The patient in Figure 2 has a GPB history of 7 d and the patient in Figure 3 has a GBPs history of 14 d. The former 2 images of Figure 2 shows the GPBs in the stomach and an ulcer which is shallow and bleeding located in the angulus in the first endoscopy and the last image shows nothing anomaly except the ulcer in the second endoscopy after Coca-Cola therapy. The former 2 images of Figure 3 shows the GPBs in the stomach and a deep ulcer located in the angulus in the first endoscopy and the last image shows nothing anomaly except the shallower ulcer in the second endoscopy after Coca-Cola therapy.
Data on age, bezoar volume and medical expenses are presented in Table 1. There were no statistically significant differences in age or bezoar volume between the 2 groups, but there was a significant difference in medical expenses.
| Group | n | Age (yr) | Volume of bezoar (cm × cm) | Expenses (RMB) |
| Control group | 80 | 53.80 ± 11.76 | 18.07 ± 8.16 | 1540.01 ± 520.381 |
| Intervention group | 80 | 50.79 ± 12.95 | 18.38 ± 8.31 | 27.59 ± 7.96 |
| t value | 1.54 | -0.233 | 25.791 | |
| P value | 0.327 | 0.487 | 0.000 |
Remarkably, a complete dissolution rate of 100% was achieved in the intervention group, highlighting the effectiveness of the intervention in treating GPBs.
Table 1 provides information regarding the medical expenses incurred during the study. The statistical analysis revealed significant differences in the medical expenses between the control and intervention groups (1540.01 ± 250.81 RMB vs 27.59 ± 7.96 RMB, t = 25.971, P = 0.000).
The gastric fragmentation time of the control group was 31.23 ± 9.62 min; however, no patients had gastric fragmentation in the intervention group.
Information on the occurrence of gastric ulcers between the control and intervention groups is presented in Table 2. The rate of gastric ulcer occurrence was 86.25% in the control group and 70.0% in the intervention group. Notably, a statistically significant difference was observed between the control group and intervention group in terms of gastric ulcer occurrence (χ2 = 6.181, P = 0.021). The results shed light on the varying rates of gastric ulcer occurrence associated with different treatment approaches.
| Group | n | Ulcer | No ulcer | χ2 | P value |
| Control group | 80 | 69 | 11 | 6.181 | 0.013 |
| Intervention group | 80 | 56 | 24 |
GPBs often arise from the ingestion of persimmons, hawthorn fruits, or date plums on an empty stomach. They can also be associated with conditions such as diabetic gastroparesis[7] and endoscopic sclerotherapy, which can lead to delayed gastric emptying[8,9]. In the present study[10], patients were successfully treated using a combination of endoscopic fragmentation and pharmacotherapy. While this combined therapy has been indicated to be effective, it involves high costs, discomfort, and long operation time.
A significant milestone in the use of Coca-Cola for phytobezoar treatment was the publication of the article "Gastric phytobezoars may be treated by nasogastric cola lavage" in 2002 by Ladas et al[11]. This study documented complete success in the treatment of five phytobezoar patients using Coca-Cola lavage. In a systematic review conducted by Ladas et al[5] in 2013, it was concluded that Coca-Cola can effectively dissolve GPBs and could be considered a first-line treatment option, with a complete dissolution rate of 50% and a favorable outcome observed in 91.3% of patients. It has been reported that the consumption of 3 Liters of Coca-Cola every 12 h has a satisfactory effect on bezoar lysis[5,12,13]. Our study demonstrated a complete phytobezoar dissolution rate of 100%, which aligns with previous findings.
The article "In Vitro Analysis of Gastric Phytobezoar Dissolubility by Cola, Cola Zero, Cellulose and Papain" reported that Coca-Cola exhibited the highest phytolytic activity, with an 18.5% ± 5.8% decrease in weight, while Coke Zero also demonstrated substantial phytolytic action (16.1% ± 0.4%). In patients who consumed carbonated beverages, bezoars were easily broken when grasped with forceps, which was not observed in patients who consumed water, cellulose or papain. These findings provide scientific evidence supporting the efficacy of Coca-Cola in bezoar lysis[14]. Although the exact mechanism of the dissolution of bezoars by Coca-Cola has not been fully explained, it may be attributed to the low acidity. The pH of Coca-Cola is 1.9, which is more acidic than that of 0.010 mol/L hydrochloric acid (pH 2.0). Further
In terms of adverse effects, short-term Coca-Cola therapy may cause temporary bloating, reflux, and gastritis. However, the long-term use of Coca-Cola can lead to tooth decay and osteoporosis[17,18]. These issues should be taken into account when utilizing Coca-Cola as a therapeutic intervention for GPBs. The participants in our study had no obvious side effects because of the short regimen.
Intestinal obstructions caused by phytobezoars are a rare occurrence in adults with normally functioning intestinal tracts[19]. However, in cases where ileus is present or when patients have refractory bezoars, surgical removal becomes necessary[20]. Avoiding severe complications associated with phytobezoars poses a challenge for medical professionals. Partially dissolved bezoars have the potential to pass through the pylorus and obstruct the small bowel, particularly in the presence of ankylosis, enterostasis, or poor gastric motility[13,21-27]. Therefore, it is crucial for patients receiving Coca-Cola therapy to be vigilant about their stool and monitor symptoms of ileus. Once a patient is confident that the bezoar has been discharged in the stool, they can discontinue the Coca-Cola diet to prevent small bowel obstruction. The duration of Coca-Cola consumption can be prolonged as necessary without any set limitations. Additionally, a low-fiber diet during Coca-Cola therapy is recommended to support the treatment process.
GPBs as foreign bodies located in the stomach, can cause mechanical friction and/or compression, leading to erosion, ulceration, or bleeding of the gastric mucosa[2]. The incidence of gastric ulcers associated with phytobezoars is approximately 80%[28]. The presence of a gastric bezoar can result in gastric mucosal injury, but as the bezoar dissolves, the gastric mucosa becomes less affected. In our study, the gastric ulcer occurrence rate in the intervention group (70.00%) was lower than that in the control group (86.25%). This lower rate of gastric ulcers in the intervention group may be attributed to the consumption of Coca-Cola. Coca-Cola softens bezoars[29], and the gastric mucosa becomes less irritated once bezoars are softened. Gastric ulcers can manifest as single or multiple lesions located in various regions of the stomach, including the angulus, body, or antrum. The overall occurrence of gastric ulcers in our study was 78.13%, which aligns with previous findings. The timely administration of Coca-Cola appears to yield better outcomes, as the occurrence rate of gastric ulcers decreases with the dissolution of bezoars.
Treating GPBs with Coca-Cola consumption offers a practical, cost-effective, and effective approach. Patients with GPBs should consume Coca-Cola at the earliest opportunity to minimize the occurrence of gastric ulcers, making it a routine practice in clinical settings. However, the optimal dosage and duration of Coca-Cola ingestion for treating GPBs have not been definitively established through large-scale experimental studies. Therefore, further extensive research is warranted to investigate and establish guidelines regarding the appropriate dose and duration of Coca-Cola consumption in the treatment of GPBs.
With the publication of Ladas SD’s article (Systematic review: Coca-Cola can effectively dissolve gastric phetobezoars as a first-line treatment), Coca-Cola dissolution therapy in patients with gastric phytobezoars (GPBs) is gradually being accepted in clinical practice. However, existing studies on this subject are often case reports highlighting the successful dissolution of phytobezoars using Coca-Cola. Consequently, large-scale prospective investigations in this domain remain scarce. Therefore, we conducted a randomized controlled trial to examine the effects of Coca-Cola administration on GPBs.
This study evaluated the intervention treatment of patients with Coca-Cola dissolution therapy, including the complete resolution rate, gastric ulcer rate, medical expenses and endoscopic operation time. Additionally, this study aimed to find a treatment plan that can attain the expected results and minimize the side effects.
The aim was to evaluate the impact of Coca-Cola on GPBs, including the dissolution rate, medical expenses, ulcer rate, and operation time.
In this study, a total of 160 consecutive patients diagnosed with GPBs were allocated into two groups (a control group and an intervention group) through computer-generated randomization. Patients in the intervention group were receive a Coca-Cola-based regimen (Coca-Cola 2000-4000 mL per day for 7 d), while those in the control group underwent emergency fragmentation.
Complete dissolution of GPBs was achieved in 100% of the patients in the intervention group. The disparity in expenses between the control group and intervention group (t = 25.791, P = 0.000) was statistically significant, and the difference in the gastric ulcer occurrence rate between the control group and intervention group (χ2 = 6.181, P = 0.013) was also statistically significant.
Timely ingestion of Coca-Cola yields significant benefits, including a complete dissolution rate of 100%, a low incidence of gastric ulcers, no need for fragmentation and reduced expenses.
This treatment is beneficial for relieving patients’ pain, reducing the need for emergency gastroscopy, decreasing medical expenses and lowering the gastric ulcer rate. Therefore, Coca-Cola dissolution therapy for GPBs is a safe, feasible, simple and effective method that is worthy of clinical application and promotion.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Integrative and complementary medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anestiadou E, Greece S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Awawda A, Al Ashhab H, Shammas I, Al Mohtasib M, Abu Asbeh Y. Endoscopic Management of Unusual Bezoar in a Prader-Willi Syndrome Patient. Cureus. 2022;14:e29900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Zhang RL, Yang ZL, Fan BG. Huge gastric disopyrobezoar: a case report and review of literatures. World J Gastroenterol. 2008;14:152-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Hu X, Guo Q, Xu QW, Zhang RY, Yang YC, Han SX, Liu WH. Novel endoscopic tangential sawing technique in treatment of giant gastric bezoars: a retrospective single-center study (with video). Gastrointest Endosc. 2022;96:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 4. | Tabesh E, Dehghan A, Tahmasebi M, Javadi N. Gastric phytobezoars as a very unusual cause of gastric outlet obstruction. J Res Med Sci. 2021;26:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Ladas SD, Kamberoglou D, Karamanolis G, Vlachogiannakos J, Zouboulis-Vafiadis I. Systematic review: Coca-Cola can effectively dissolve gastric phytobezoars as a first-line treatment. Aliment Pharmacol Ther. 2013;37:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Nelson A, Romo N, Levanon D, Blumfield E, Gershel J. Gastric Bezoar Treatment Using Oral Coca-Cola. Clin Pediatr (Phila). 2017;56:485-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Whitson BA, Asolati M, Kandaswamy R, Sutherland DE. Diabetic gastroparesis-associated bezoar resolution via "cola-lysis". Clin Transplant. 2008;22:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Bi D, Choi C, League J, Camilleri M, Prichard DO. Food Residue During Esophagogastroduodenoscopy Is Commonly Encountered and Is Not Pathognomonic of Delayed Gastric Emptying. Dig Dis Sci. 2021;66:3951-3959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Davion T, Delamarre J, Reix N, Lambert A, Capron JP. Gastric bezoar: another side effect of endoscopic variceal sclerotherapy. Scand J Gastroenterol. 1989;24:818-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Gayà J, Barranco L, Llompart A, Reyes J, Obrador A. Persimmon bezoars: a successful combined therapy. Gastrointest Endosc. 2002;55:581-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Ladas SD, Triantafyllou K, Tzathas C, Tassios P, Rokkas T, Raptis SA. Gastric phytobezoars may be treated by nasogastric Coca-Cola lavage. Eur J Gastroenterol Hepatol. 2002;14:801-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Komaki Y, Kanmura S, Tanaka A, Nakashima M, Komaki F, Iwaya H, Arima S, Sasaki F, Nasu Y, Tanoue S, Hashimoto S, Ido A. Cola Dissolution Therapy via Ileus Tube Was Effective for Ileus Secondary to Small Bowel Obstruction Induced by an Enterolith. Intern Med. 2019;58:2473-2478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 13. | Lee BJ, Park JJ, Chun HJ, Kim JH, Yeon JE, Jeen YT, Kim JS, Byun KS, Lee SW, Choi JH, Kim CD, Ryu HS, Bak YT. How good is cola for dissolution of gastric phytobezoars? World J Gastroenterol. 2009;15:2265-2269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Iwamuro M, Kawai Y, Shiraha H, Takaki A, Okada H, Yamamoto K. In vitro analysis of gastric phytobezoar dissolubility by coca-cola, coca-cola zero, cellulase, and papain. J Clin Gastroenterol. 2014;48:190-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Okamoto Y, Yamauchi M, Sugihara K, Kato H, Nagao M. Is coca-cola effective for dissolving phytobezoars? Eur J Gastroenterol Hepatol. 2007;19:611-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | McCloy RF, Greenberg GR, Baron JH. Duodenal pH in health and duodenal ulcer disease: effect of a meal, Coca-Cola, smoking, and cimetidine. Gut. 1984;25:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Maladkar SR, Yadav P, Muniraja ANA, Uchil GS, George LV, Augustine D, Rao RS, Patil S, Sowmya SV, Haragannavar VC. Erosive Effect of Acidic Beverages and Dietary Preservatives on Extracted Human Teeth-An In Vitro Analysis. Eur J Dent. 2022;16:919-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Mondelli J, Sene F, Ramos RP, Benetti AR. Tooth structure and fracture strength of cavities. Braz Dent J. 2007;18:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Pitiakoudis M, Tsaroucha A, Mimidis K, Constantinidis T, Anagnostoulis S, Stathopoulos G, Simopoulos C. Esophageal and small bowel obstruction by occupational bezoar: report of a case. BMC Gastroenterol. 2003;3:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Iwamuro M, Okada H, Matsueda K, Inaba T, Kusumoto C, Imagawa A, Yamamoto K. Review of the diagnosis and management of gastrointestinal bezoars. World J Gastrointest Endosc. 2015;7:336-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 214] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (5)] |
| 21. | Aydin I, Sengul I, Sengul D. Phytobezoar: An Unusual Condition Leading to Small Bowel Obstruction. Cureus. 2022;14:e23885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Bouali M, Ballati A, El Bakouri A, Elhattabi K, Bensardi F, Fadil A. Phytobezoar: An unusual cause of small bowel obstruction. Ann Med Surg (Lond). 2021;62:323-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Kannan NL, Singaraju H, Sim SW. Laparoscopic-assisted removal of gastric trichobezoar: a novel technique to reduce operative complications and time. J Pediatr Surg. 2013;48:1826-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Serpa E, Luciano E, Pacheco F, Solh W. Phytobezoar causing small bowel obstruction in a patient with Crohn's disease: A case report. Int J Surg Case Rep. 2022;99:107615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Somuncu E, Solak IHA. Colonic Obstruction Secondary to Phytobezoar Caused by Vitex Agnus-Castus Seeds: A Case Report. J Coll Physicians Surg Pak. 2022;32:S115-S117. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Toka B, Eminler AT, Karacaer C, Uslan MI, Koksal AS, Parlak E. A Simple Method for Endoscopic Treatment of Large Gastric Phytobezoars: "Hand-Made Bezoaratome". Turk J Gastroenterol. 2021;32:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Wei KY, Sung CC, Lin SH. Phytobezoar-induced small bowel obstruction in an elderly patient undergoing dialysis: a case report. J Int Med Res. 2020;48:300060520962942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Liu LN, Wang L, Jia SJ, Wang P. Clinical Features, Risk Factors, and Endoscopic Treatment of Bezoars: A Retrospective Analysis from a Single Center in Northern China. Med Sci Monit. 2020;26:e926539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Park SE, Ahn JY, Jung HY, Na S, Park SJ, Lim H, Choi KS, Lee JH, Kim DH, Choi KD, Song HJ, Lee GH, Kim JH. Clinical outcomes associated with treatment modalities for gastrointestinal bezoars. Gut Liver. 2014;8:400-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |