Published online Jun 16, 2023. doi: 10.4253/wjge.v15.i6.469
Peer-review started: March 6, 2023
First decision: April 28, 2023
Revised: May 8, 2023
Accepted: May 22, 2023
Article in press: May 22, 2023
Published online: June 16, 2023
Processing time: 99 Days and 18.4 Hours
Endoscopic sleeve gastroplasty (ESG) is an effective therapy for class I-II obesity, but there are knowledge gaps in the published literature about its implementation in patients with class III obesity [body mass index (BMI) ≥ 40 kg/m2].
To evaluate the safety, clinical efficacy, and durability of ESG in adults with class III obesity.
This was a retrospective cohort study that used prospectively collected data on adults with BMI ≥ 40 kg/m2 who underwent ESG and longitudinal lifestyle counseling at two centers with expertise in endobariatric therapies from May 2018-March 2022. The primary outcome was total body weight loss (TBWL) at 12 mo. Secondary outcomes included changes in TBWL, excess weight loss (EWL) and BMI at various time points up to 36 mo, clinical responder rates at 12 and 24 mo, and comorbidity improvement. Safety outcomes were reported through the study duration. One-way ANOVA test was performed with multiple Tukey pairwise comparisons for TBWL, EWL, and BMI over the study duration.
404 consecutive patients (78.5% female, mean age 42.9 years, mean BMI 44.8 ± 4.7 kg/m2) were enrolled. ESGs were performed using an average of 7 sutures, over 42 ± 9 min, and with 100% technical success. TBWL was 20.9 ± 6.2% at 12 mo, 20.5 ± 6.9% at 24 mo, and 20.3 ± 9.5% at 36 mo. EWL was 49.6 ± 15.1% at 12 mo, 49.4 ± 16.7% at 24 mo, and 47.1 ± 23.5% at 36 mo. There was no difference in TBWL at 12, 15, 24, and 36 mo from ESG. TBWL exceeding 10%, 15%, and 20% was achieved by 96.7%, 87.4%, and 55.6% of the cohort at 12 mo, respectively. Of the cohort with the relevant comorbidity at time of ESG, 66.1% had improvement in hypertension, 61.7% had improvement in type II diabetes, and 45.1% had improvement in hyperlipidemia over study duration. There was one instance of dehydration requiring hospitalization (0.2% serious adverse event rate).
When combined with longitudinal nutritional support, ESG induces effective and durable weight loss in adults with class III obesity, with improvement in comorbidities and an acceptable safety profile.
Core Tip: Patients with obesity wishing to avoid bariatric surgery can benefit from endoscopic sleeve gastroplasty (ESG), but little has been published about the safety and efficacy of ESG in those with class III obesity (body mass index ≥ 40 kg/m2). Based on this appraisal of a large, international cohort, ESG can be safely performed in adults with class III obesity, with clinically meaningful weight loss at one year that can be maintained over the subsequent two years, as well as improvement in weight-related comorbidities. Patients and medical providers should be made aware that ESG combined with longitudinal nutritional support is a promising weight loss tool for those with class III obesity.
- Citation: Maselli DB, Hoff AC, Kucera A, Weaver E, Sebring L, Gooch L, Walton K, Lee D, Cratty T, Beal S, Nanduri S, Rease K, Gainey CS, Eaton L, Coan B, McGowan CE. Endoscopic sleeve gastroplasty in class III obesity: Efficacy, safety, and durability outcomes in 404 consecutive patients. World J Gastrointest Endosc 2023; 15(6): 469-479
- URL: https://www.wjgnet.com/1948-5190/full/v15/i6/469.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i6.469
Obesity is a chronic, progressive, multifactorial disease spectrum of excess adiposity with detrimental effects on patients’ health and well-being[1]. Those with class III obesity [body mass index (BMI) ≥ 40 kg/m2] have 1.5 times greater risk of all-cause mortality than those with class I (BMI 30.0-34.9 kg/m2) or class II (BMI 35.0-39.9 kg/m2) obesity, and compared to individuals of normal weight, they have over double the risk of all-cause mortality, with a loss of 7-14 years of life expectancy[2,3]. While adults with class III obesity account for nearly 6% of the United States adult population, they constitute one-fifth of per-capita healthcare expenditures and thus represent a population in need of effective and safe weight loss strategies[4,5].
Bariatric and metabolic surgeries are the most effective weight loss interventions for patients with obesity[6]. However, the reach of these surgeries is constrained by a variety of barriers, most notably patient perception of risks and desire to avoid invasive procedures; accordingly, only 1% or less of eligible patients pursue bariatric surgery[7,8]. For patients with class III obesity, this rate is estimated to be 1 in 400[9]. Failure to provide such patients with effective surgical weight loss has been linked to development of additional obesity-associated medical problems[10]. These challenges widen the treatment gap in the global burden of obesity, especially among those at the high ranges of BMI.
Over the past decade, endoscopic bariatric therapies have entered the therapeutic landscape, hypothesized to have greater patient acceptance due to their minimally invasive, anatomy-preserving nature[11]. The endoscopic sleeve gastroplasty (ESG) involves incisionless, per-oral gastric remodeling via full thickness sutures placed along the stomach’s greater curvature to create a sleeve-like configuration that reduces stomach volume by 80%[12]. It has shown considerable promise in those with class I and II obesity, inducing a total body weight loss of approximately 16% at one year[13,14].
In July 2022, the United States Food and Drug Administration (FDA) granted De Novo Market Authorization for the creation of the ESG using the Apollo ESG™ (formerly OverStitch device, Apollo Endosurgery, Austin, TX, United States) for treatment of obesity in those with BMI from 30 kg/m2 to 50 kg/m2. However, due to the relatively recent emergence of endoscopic bariatric therapies, as well as preceding expert level recommendations that they be employed in lower classes of obesity, little has been published on the use of ESG in class III obesity[11,15]. A retrospective review that included 146 adults with class III obesity who underwent ESG at a single center in Spain observed similar weight loss and adverse event outcomes as subjects with class I and II obesity, suggesting ESG is an appropriate therapy in patients with BMIs exceeding 40 kg/m2, but further study is required to validate the findings to bolster confidence in widespread clinical adoption[16].
To address this, we examined weight loss and safety outcomes up to three years in 404 consecutive patients with class III obesity who underwent ESG, without concomitant weight loss medications, at two centers with expertise in endoscopic bariatric therapies. We hypothesized that ESG in subjects with class III obesity would achieve clinically significant weight loss with an acceptable safety profile.
This was an international, multicenter, retrospective analysis of prospectively followed consecutive patients with class III obesity who underwent ESG. This study was approved by an Institutional Review Board (WCG IRB, Puyallup, WA). The study was conducted following ethical principles outlined in the Declaration of Helsinki and was consistent with the Good Clinical Practices recommendation. All authors had access to the study data and reviewed and approved the final manuscript.
Study participants were enrolled if they were ≥ 20 years of age, had BMI ≥ 40 kg/m2, had failed to lose weight through diet/exercise alone, were interested in an endobariatric procedure for weight loss, could provide informed consent, and were willing to comply with a structured lifestyle program and dietary modification. Subjects were excluded for concomitant use of weight loss medications, prior bariatric surgery (except for history of laparoscopic adjusted gastric band status post removal), bleeding disorder or coagulopathy, non-steroidal anti-inflammatory drug dependence, poorly controlled diabetes, and severe cardiopulmonary disease, as well as if hiatal hernia > 4 cm, and/or active peptic ulcer disease was noted at time of ESG.
All subjects underwent self-financed ESGs between May 2018 and March 2022 at True You Weight Loss (Cary, NC, United States) and Clinica Angioskope (Sao Paulo, Brazil). All ESGs were performed by two providers with expertise in endoscopic bariatric therapies, each having performed over five hundred ESG procedures by the start of the study (CM and AH). Procedures were performed using the OverStitch Endoscopic Suturing System (Apollo Endosurgery, Austin, TX, United States) under general anesthesia with endotracheal intubation. Procedural technique was performed as previously published[17]. Subjects were discharged the same day. After the procedure, all patients were enrolled in a comprehensive lifestyle program with long-term nutritional support and monitoring at monthly virtual or in-person visits with registered dieticians who provided counseling on dietary and exercise behaviors to reinforce weight loss. Follow up with a physician or nurse practitioner was also offered as needed during the first year after ESG to provide further support and address symptoms. Patient weights were collected at each visit, either in person or virtually by standardized Bluetooth-enabled digital scale, while safety outcomes were monitored longitudinally.
The primary outcome of the study was total body weight loss (TBWL) at 12 mo, expressed as a percentage of weight lost in comparison to baseline weight on the day of the ESG procedure. The expectation was that the mean TBWL was at least 10%, which is the expected TBWL following an endobariatric procedure[11]. Secondary endpoints included TBWL at 3, 6, 15, 24, and 36 mo; clinical responder rates (defined as ≥ 10% TBWL, ≥ 15% TBWL, ≥ 20% TBWL, ≥ 25% TBWL, and ≥ 30% TBWL) at 12 and 24 mo; excess weight loss (EWL) and BMI at 3, 6, 12, 15, 24, and 36 mo; number of sutures used to create the ESG; and technical success (defined as completed procedure without early termination due to technical challenges or complications), as reported in similar studies of ESG[18]. The presence of hypertension, type II diabetes, and hyperlipidemia at time of ESG was defined as any of the following: Established diagnosis by a primary or referring provider; use of medication/devices to treat the condition. Additionally, type II diabetes was diagnosed if hemoglobin A1c ≥ 6.5% within 3 mo prior to ESG, and hyperlipidemia was diagnosed if low-density lipoprotein ≥ 160 mg/dL or total cholesterol ≥ 200 mg/dL within 3 mo of ESG. Improvement in comorbidity was defined as reduction in or complete discontinuation of medications used to treat the condition by a referring provider at any point in time during study duration. It was not standard practice to repeat laboratory values after ESG in our centers, but improvement in comorbidity was also reported if a patient obtained labs elsewhere that showed hemoglobin A1c < 6.5% (type II diabetes), total cholesterol < 200 mg/dL (hyperlipidemia), and/or low-density lipoprotein < 160 mg/dL (hyperlipidemia) at any point in time during study duration. Safety data were collected throughout the three-year study duration and were graded according to the American Society for Gastrointestinal Endoscopy lexicon[19].
The statistical components of this study were performed and reviewed by a biomedical statistician. Descriptive statistics were used for analyses. All data were tested for normality using the Kolmogorov-Smirnov test, Q-Q plot, and Levene’s test. Continuous variables were expressed as means with standard deviations or medians with ranges and 95% confidence intervals. One-way ANOVA test was performed with multiple Tukey pairwise comparisons with months from the procedure as the grouping variable for TBWL, EWL, and BMI. Categorical variables were expressed as frequencies. The Kruskal-Wallis test was performed to evaluate differences in clinical responder rates with months from procedure as the grouping variable. This test was only performed for 10% and 20% clinical responders. If a difference was detected, then Wilcoxan Rank Sum test with Bonferroni Correction was performed to determine comparisons with significant differences in clinical responders. Follow up was reported as a percentage, calculated as number of patients with available data at a time point, divided by number of patients expected to have available data at that time point. Adverse event rate frequency was based on the number of patients treated. P values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS (version 29.0).
Four hundred and four patients (mean age 42.9 years, 78.5% female, mean pre-procedural weight 127.3 ± 20.1 kg, mean pre-procedural BMI 44.8 ± 4.7 kg/m2) underwent ESG between May 2018 and March 2022. Technical success rate was 100%. Mean procedure duration was 42 ± 9 min and used a median of 7 sutures, with a range of 4 to 12 sutures. Prior to ESG, the cohort had the following obesity-associated comorbidities: Hypertension (35.4%), type II diabetes (17.8%), and hyperlipidemia (16.8%). Table 1 shows the baseline demographic and anthropometric characteristics of the study cohort.
| Characteristic | Value |
| Age (yr) | 42.9 ± 9.4 |
| % Female | 78.5 |
| Weight (kg) | 127.3 ± 20.1 |
| BMI (kg/m2) | 44.8 ± 4.7 |
| Comorbidities, n (%) | |
| Hypertension | 143 (35.4%) |
| Type II diabetes | 72 (17.8%) |
| Hyperlipidemia | 68 (16.8%) |
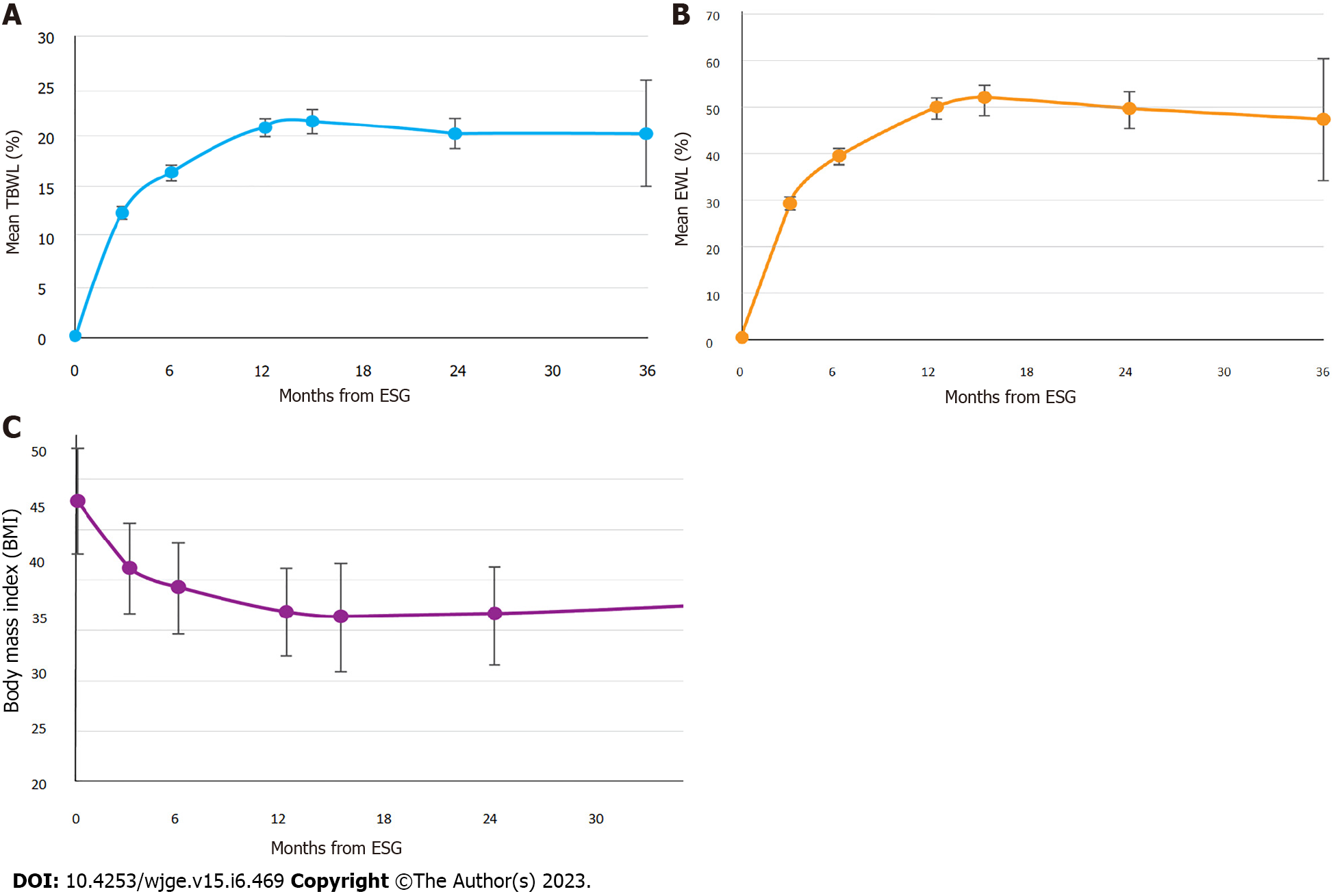
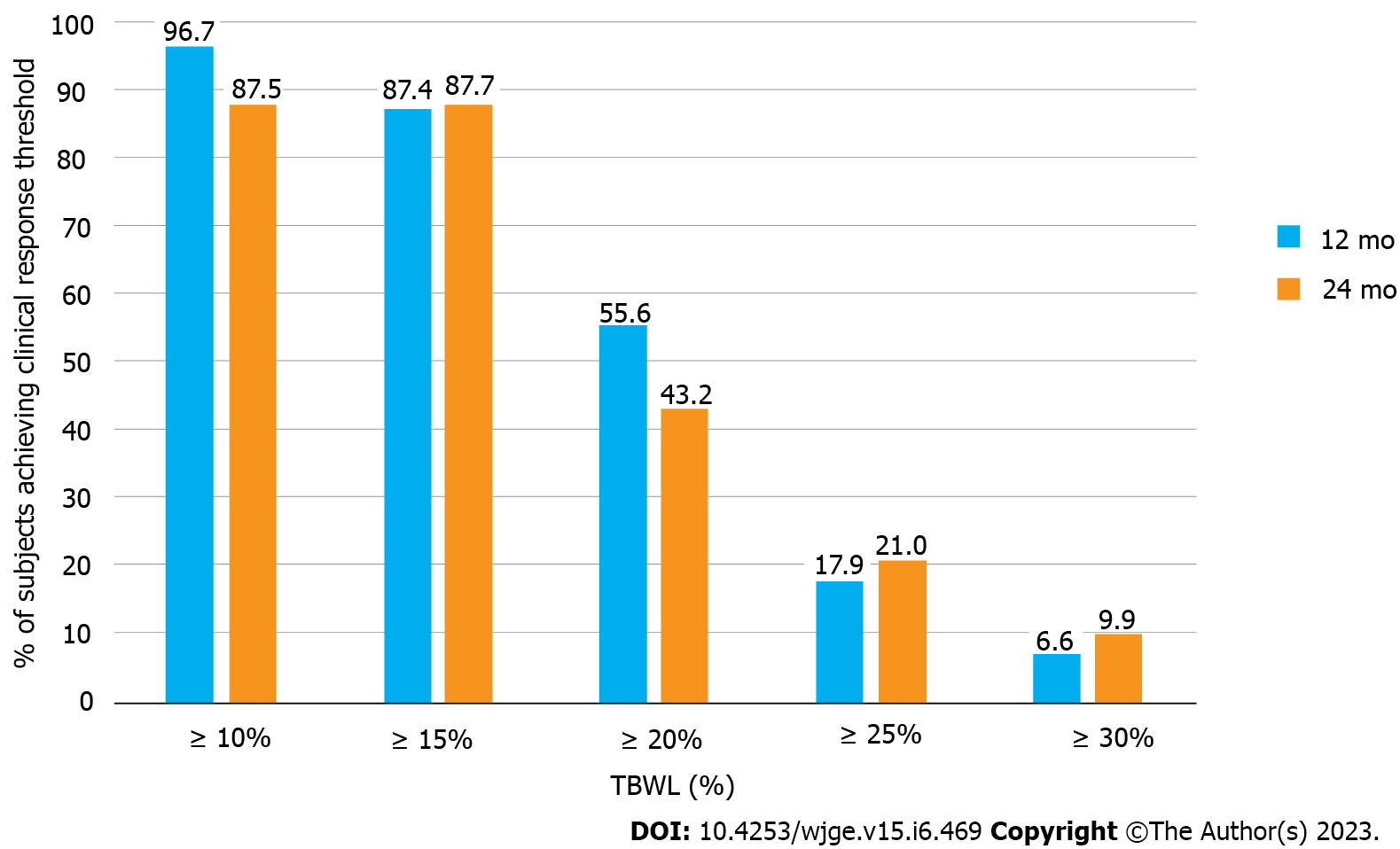
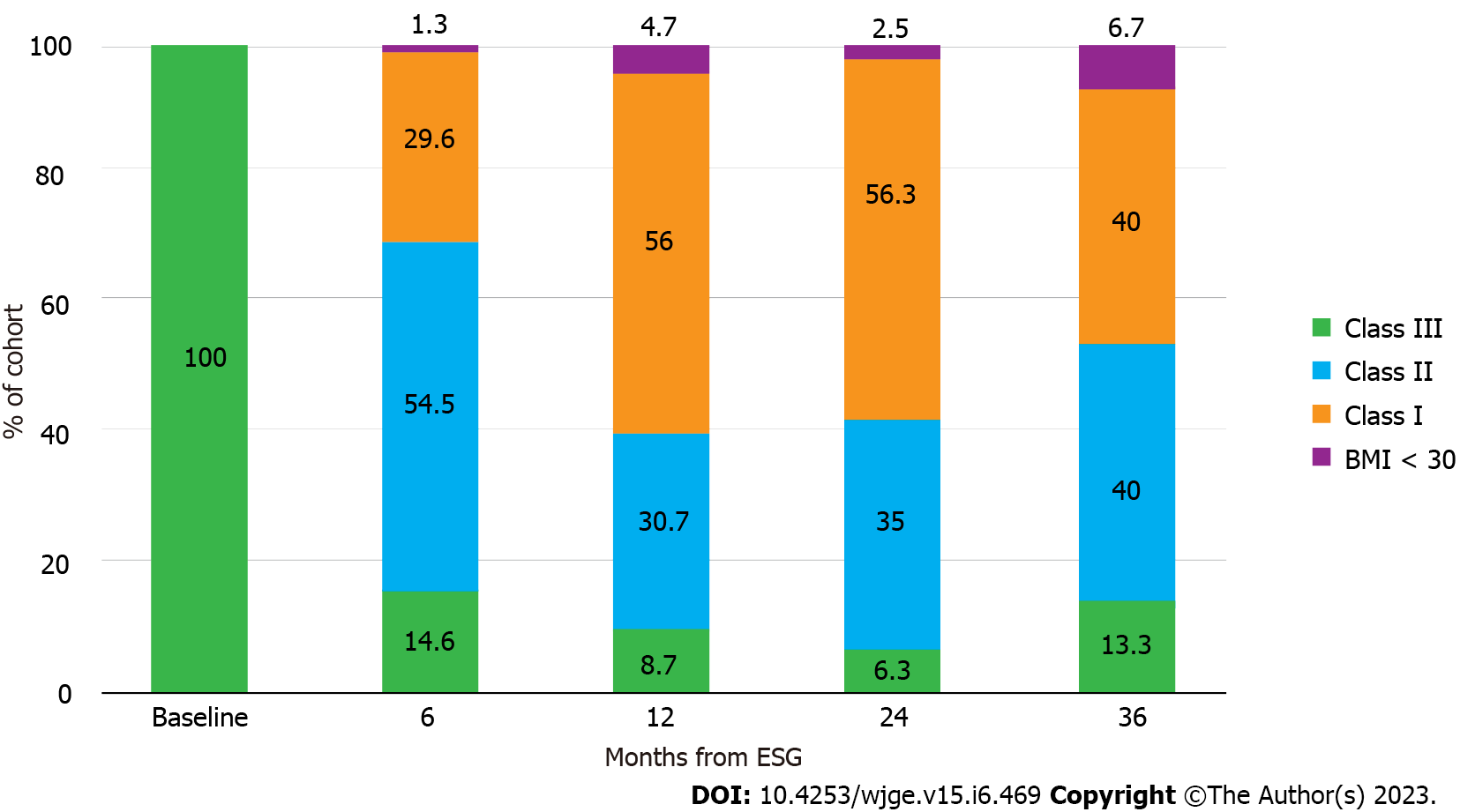
Table 2 shows subject accountability by visit, with greater than 80% follow-up achieved at all time points. TBWL was 12.5 ± 3.7% at 3 mo, 16.5 ± 4.8% at 6 mo, 20.9 ± 6.2% at 12 mo, 21.6 ± 7.2% at 15 mo, 20.5 ± 6.9% at 24 mo, and 20.3 ± 9.5% at 36 mo (Figure 1A). EWL was 29.5 ± 9.6% at 3 mo, 39.2 ± 12.7% at 6 mo, 49.6 ± 15.1% at 12 mo, 51.6 ± 16.8% at 15 mo, 49.4 ± 16.7% at 24 mo, and 47.1 ± 23.5% at 36 mo (Figure 1B). BMI decreased from 44.8 ± 4.7 kg/m2 at baseline to 38.8 ± 3.1 kg/m2 at 3 mo, 37.0 ± 4.0 kg/m2 at 6 mo, 35.0 ± 4.0 kg/m2 at 12 mo, 34.5 ± 4.7 kg/m2 at 15 mo, 34.0 ± 4.7 kg/m2 at 24 mo, and 35.6 ± 5.5 kg/m2 at 36 mo (Figure 1C). One-way ANOVA results for TBWL and EWL revealed statistically significant differences in values between preceding and subsequent timepoints through month 12, with no differences noted between 12, 15, 24, and 36 mo. For BMI, there were statistically significant differences in values between preceding and subsequent time points through month 6, with no statistical differences noted from time points spanning 6 to 36 mo. Figure 2 illustrates the distribution of obesity classes during the study. While less than 10% of subjects were cured of obesity during study duration, most subjects (85.4%) exited class III obesity by 6 mo, without notable increase in the proportion of class III obesity in the study duration. A plurality of the cohort had class I obesity by 12 and 24 mo. 12-mo clinical response rates showed 96.7% achieved at least 10% TBWL, 87.4% achieved at least 15% TBWL, and 55.6% achieved at least 20% TBWL, with similar proportions of clinical responders observed at 24 mo (Figure 3). Kruskal-Wallis confirmed no difference in ≥ 10% TBWL rates at 12 vs 24 mo or ≥ 20% TBWL rates at 12 vs 24 mo (P < 0.001 for both). Of the cohort with the respective comorbidity at the time of ESG, 66.1% had improvement in hypertension, 61.7% had improvement in type II diabetes, and 45.1% had improvement in hyperlipidemia over study duration. No patient underwent an additional endoscopic procedure for repeat suturing. One subject (starting BMI 50.5 kg/m2) converted to a Roux-en-Y gastric bypass at 22 mo after achieving 22% TBWL.
There were no instances of death, gastrointestinal perforation, abscess/sepsis, gastrointestinal bleeding, intensive care unit admission, or need for endoscopic or surgical intervention for management of procedural complications. There were two instances of dehydration requiring emergency room presentation 2 d and 8 d after the ESG, one of which required 3-day hospitalization for acute kidney injury, which resolved with intravenous fluids. This yielded an overall adverse event rate of 0.5% and a 0.2% serious adverse event (SAE) rate.
This is one of the first studies—and the largest to date—that examines the novel application of ESG in patients with class III obesity, a demographic traditionally relegated to surgery for weight loss. The data presented here help address misperceptions about ESG in patients with class III obesity that ostensibly are founded on concerns about insufficient efficacy, increased risk of adverse outcomes, and technical challenges in a high BMI population. Crucially, these findings support the United States FDA’s recent authorization for use of the ESG in patients with obesity with BMI spanning 30 kg/m2 to 50 kg/m2.
This study demonstrates that ESG, without concomitant weight loss medications, and in conjunction with prescribed diet/exercise counseling, can induce clinically significant weight loss in patients with BMI ≥ 40 kg/m2. Our cohort achieved a TBWL of nearly 21% at 12 mo, exceeding the 16% TBWL reported in multiple meta-analyses of ESG, and which was sustained at years 2 and 3[13,14,20]. Our results were concordant with a recently published study by Lopez-Nava et al[16] in which 146 subjects with class III obesity achieved 20.5% TBWL at one year. Weight loss appears to be most pronounced in year one after ESG, with efforts later focused on weight loss maintenance in years two and three, in line with the weight loss trajectory from ESG previously observed by Sharaiha and colleagues[21].
An important finding in our study is that most patients with class III obesity who undergo ESG will exit class III obesity by 6 mo and further improve their weight at 12 mo. Compellingly, very few subjects return to class III obesity at years 2 and 3. However, while weight loss was clinically significant, very few in the cohort were cured of obesity during the duration of the study. This underscores the challenges of managing a chronic, progressive, relapsing disorder and may provide the rationale for concomitant or sequential treatment with weight loss medications in this patient population to achieve even greater weight loss; in fact, early success with incretin-based pharmacotherapy and ESG has been reported[22]. This observation additionally supports the concept of ESG as a bridging procedure in patients with markedly elevated BMI but with surgical contraindications or elevated operative risk, as has been published in a small case series by Zorron and colleagues, with a subsequent larger cohort showing safe revision of ESG to laparoscopic sleeve gastrectomy (LSG) by Alqahtani and colleagues[23,24]. Ultimately, the clinical response to ESG in our cohort remains substantive, particularly given that traditional bariatric surgeries have a limited penetrance for eligible patients, and ESG provides a minimally invasive alternative for those who are not interested in pursuing surgical weight loss[7,8].
The Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds recommend that an endoscopic bariatric and metabolic therapy facilitate at least 25% EWL at 12 mo[25]. In this cohort, EWL was nearly double this threshold, at almost 50% at 12 and 24 mo. This is less than the approximately 60% 12-mo EWL reported in meta-analyses of ESG, but most subjects in the ESG literature were closer to ideal body weight, which augments EWL for a given magnitude of weight loss[13,14,26]. The EWL of our cohort fell short of EWL observed following LSG, which is approximately 86% at one year; however, this does diminish to around 63% at 3 years[27]. A recently published study comparing ESG and LSG, in which ESG subjects had a baseline BMI of 32.5 ± 3.1 kg/m2, showed a mean difference in TBWL of 9.7% at 1 year and 4.8% at 3 years in favor of LSG[28]. While both interventions create a narrowed, restricted gastric reservoir, this discrepancy may result from differences in hormonal influences (LSG involves resection of the fundus and thus diminishes ghrelin, whereas ESG does not) and distinct foregut sensorimotor effects (LSG accelerates gastric emptying to impact proximal small intestine-mediated satiation pathways, where ESG delays gastric emptying to impact gastric-mediated peripheral appetite signals)[29-32]. Further exploration of weight loss and safety outcomes in ESG vs LSG warrant direct head-to-head trials, primarily to better inform patients of their available options and the differences between current surgical and endoscopic bariatric therapies.
As approximately 75% of adults with class III obesity have at least one obesity-associated comorbidity, improvement in comorbidities is a valuable measure[25]. Throughout the study duration, comorbidity improvement was observed in over half of those with hypertension and type II diabetes, and nearly half of those with hyperlipidemia. We attribute this phenomenon to clinical responder rates, as almost all subjects achieved at least 10% TBWL at one year. This appears to be a meaningful inflection point for improvement in obesity-related comorbidities[26]. This phenomenon may help reduce the side effects, interactions, and cost associated with comorbidity-related polypharmacy often observed in patients with class III obesity.
Performance of the ESG in patients with BMI ≥ 40 kg/m2 demonstrates an acceptable safety profile for clinical adoption, with an observed 0.2% SAE rate that is in line with the expert consensus that an endoscopic bariatric and metabolic therapy not have a SAE rate exceeding 5%[25]. The majority of severe SAEs from ESG—particularly the accounts of gastric perforation, fluid collection/abscess, venous thromboembolism, and gastrointestinal bleeding reported in the literature—are expected to occur within the first month after the ESG, and thus our inclusion of all 404 patients permitted a suitable and robust ability to capture these outcomes[26]. Both adverse events in this study were graded as mild in severity according to the lexicon[19]. We suspect that these favorable safety outcomes stem from a variety of factors: performance of ESG by highly experienced endobariatric physicians; procedural technique that maintains full-thickness tissue acquisition while avoiding extra-gastric structures; avoidance of the thin-walled gastric fundus; regular follow-up visits and physician contact for symptom assessment; and exclusion of patients with severe systemic disease.
While the safety profile of ESG in patients with class III obesity is appealing compared to that of bariatric surgery, ambitions of narrowing the management gap in class III obesity with the ESG are tempered by barriers more unique to endoscopic bariatric therapies[33]. Despite the recent United States FDA authorization, the ESG is not covered by most insurances. This puts a financial burden on patients as they navigate a cash pay model. Moreover, the technical implementation of ESG remains heterogenous, and while dedicated training programs exist for bariatric surgeries, there are ongoing discussions about how best to develop and standardize endobariatric training programs and establish credentialing requirements for interested endoscopists[34]. Thus, while demonstrating favorable efficacy, safety, and acceptance, ESG still faces practical challenges that must be addressed for successful clinical adoption.
From a technical standpoint, there was little difference between creation of ESGs in our subjects with class III obesity and our patients with class I and II obesity. Patients required a median of 7 sutures, which is typical for our ESGs in lower classes of obesity, and our suture pattern was not modified for this patient population. While same-day discharge was feasible for all subjects in this cohort, there are still precautions regarding anesthesia risk, airway management, and equipment and facility factors that have to be considered by institutions aiming to offer ESG in this patient population.
Our study has several strengths. The cohort is the largest studied to date and was derived from two high-volume, experienced endobariatric centers that utilize the same procedural technique and aftercare protocols. Both study endoscopists are highly trained, having performed more than 3500 combined ESG procedures, reducing the impact of technical variability and inexperience. Finally, nutritional support with dieticians at both centers was comprehensive, and follow-up was near-complete, despite the impact of the coronavirus disease 2019 (COVID-19) pandemic.
This study also had certain limitations. Regarding trial design, this was a retrospective review of subjects that lacked a comparator arm, so the true difference in weight loss outcomes relative to a similar population using diet and exercise for weight loss is not known; however, all patients treated at both centers had failed to lose weight or maintain prior weight loss by the time they sought ESG. Second, the prevalence of medical comorbidities in this cohort was lower than would be expected for class III obesity. This may have been because diagnosis of comorbidities relied largely on indirect report from primary care physicians and patients or medication lists rather than direct lab measurement in all instances, and comorbidity improvement was limited insofar as post-ESG lab values were not widely available given that this is not standard practice in our centers. Nevertheless, this cohort was, in essence, a “healthy” population of patients with class III obesity, which is not unusual for those seeking non-surgical treatment but may have led to an under-assessment of metabolic impacts. Third, the external validity of this study may be limited considering the high level of experience of the involved centers, both in terms of procedural volume and longitudinal aftercare capabilities. Additionally, 281 (69%) of the 404 patients had their ESG procedure within 6-months of the onset of the COVID-19 pandemic, which may have impacted their overall weight loss. Finally, though patient adherence throughout the study duration was greater than 80%, the absolute number of subjects who reached the 24- and 36-month timepoints was small, meaning we must be cautious when interpreting these later outcomes.
Based on the promising results presented in this study, ESG in combination with a prescribed nutritional program should be offered to patients with class III obesity. Given the global burden of obesity, compounded by limited therapeutic options that are both accessible and appealing to patients, ESG can be a useful tool for reducing the substantial management gap in this disease when performed by experienced endobariatric physicians with reliable, long-term aftercare. Further study of ESG in class III obesity should assess improvement in associated medical problems, the effects of combination ESG-pharmacotherapy, and directly compare ESG to traditional bariatric surgeries.
When combined with longitudinal nutritional support, ESG is a safe and effective tool for adults with class III obesity, with clinically-meaningfully weight loss at one year that was sustained in the subsequent two years, as well as improvement in weight-related comorbidities. Patients may need additional therapy to reduce body mass index out of obesity range.
Endoscopic sleeve gastroplasty (ESG) is a minimally invasive weight loss tool that narrows and shortens the stomach into a tubular construct through full-thickness suturing. The majority of published data on the ESG focus on patients with class I [(Body mass index (BMI) 30.0-34.9 kg/m2] or class II (BMI 35.0-39.9 kg/m2) obesity.
Patients with class III obesity (BMI ≥ 40 kg/m2) face greater mortality risk and increased emergence of weight-related comorbidities compared those of lower obesity classes; however, the vast majority of patients with class III obesity do not pursue bariatric and metabolic surgery, leading to a substantial therapeutic gap in this patient population, which ESG may help address.
To address knowledge gaps in the clinical adoption of ESG as a weight loss tool in adults at higher ranges of body mass index, we sought to evaluate the clinical efficacy of ESG in patients with class III obesity based on weight loss and resolution of comorbidities, as well as safety outcomes, over the course of three years.
This was a retrospective evaluation of prospective collected data of adult patients undergoing ESG from May 2018-March 2022 at two centers with expertise in endobariatric therapies.
404 adult patients with class III obesity underwent ESG and achieved 20.9 ± 6.2% total body weight loss and 49.6 ± 15.1% excess weight loss at one year, which was maintained at two and three years. 87.4% of patients achieved > 15% total body weight loss by one year. Of the cohort, 66.1% had improvement in hypertension, 61.7% had improvement in type II diabetes, and 45.1% had improvement in hyperlipidemia over the study duration. There was a 0.2% serious adverse event rate.
When combined with longitudinal nutritional support, ESG facilitates safe and effective weight loss at one year in adults with class III obesity, which is maintained at years two and three. ESG should be considered for patients with class III obesity wishing to avoid metabolic and bariatric surgery.
While safe and effective in the treatment of class III obesity, ESG did not cure patients of obesity within the confines of this study, and future research should evaluate practices that enhance weight loss from ESG in this population, including procedural modifications or combination therapy with pharmacologic agents.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu D, China; Zhang J, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 891] [Article Influence: 111.4] [Reference Citation Analysis (1)] |
| 2. | Kitahara CM, Flint AJ, Berrington de Gonzalez A, Bernstein L, Brotzman M, MacInnis RJ, Moore SC, Robien K, Rosenberg PS, Singh PN, Weiderpass E, Adami HO, Anton-Culver H, Ballard-Barbash R, Buring JE, Freedman DM, Fraser GE, Beane Freeman LE, Gapstur SM, Gaziano JM, Giles GG, Håkansson N, Hoppin JA, Hu FB, Koenig K, Linet MS, Park Y, Patel AV, Purdue MP, Schairer C, Sesso HD, Visvanathan K, White E, Wolk A, Zeleniuch-Jacquotte A, Hartge P. Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 2014;11:e1001673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 3. | Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju ShN, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson ChL, Joshy G, Lewington S, Manson JE, Murphy N, Patel AV, Samet JM, Woodward M, Zheng W, Zhou M, Bansal N, Barricarte A, Carter B, Cerhan JR, Smith GD, Fang X, Franco OH, Green J, Halsey J, Hildebrand JS, Jung KJ, Korda RJ, McLerran DF, Moore SC, O'Keeffe LM, Paige E, Ramond A, Reeves GK, Rolland B, Sacerdote C, Sattar N, Sofianopoulou E, Stevens J, Thun M, Ueshima H, Yang L, Yun YD, Willeit P, Banks E, Beral V, Chen Zh, Gapstur SM, Gunter MJ, Hartge P, Jee SH, Lam TH, Peto R, Potter JD, Willett WC, Thompson SG, Danesh J, Hu FB. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1610] [Cited by in RCA: 1750] [Article Influence: 194.4] [Reference Citation Analysis (0)] |
| 4. | Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4734] [Cited by in RCA: 4473] [Article Influence: 298.2] [Reference Citation Analysis (0)] |
| 5. | Arterburn DE, Maciejewski ML, Tsevat J. Impact of morbid obesity on medical expenditures in adults. Int J Obes (Lond). 2005;29:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S-209S. |
| 7. | Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using Andersen's Model of Health Services Use. Surg Obes Relat Dis. 2018;14:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Campos GM, Khoraki J, Browning MG, Pessoa BM, Mazzini GS, Wolfe L. Changes in Utilization of Bariatric Surgery in the United States From 1993 to 2016. Ann Surg. 2020;271:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 9. | Martin M, Beekley A, Kjorstad R, Sebesta J. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surg Obes Relat Dis. 2010;6:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | Al Harakeh AB, Burkhamer KJ, Kallies KJ, Mathiason MA, Kothari SN. Natural history and metabolic consequences of morbid obesity for patients denied coverage for bariatric surgery. Surg Obes Relat Dis. 2010;6:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | ASGE/ASMBS Task Force on Endoscopic Bariatric Therapy. A pathway to endoscopic bariatric therapies. Surg Obes Relat Dis. 2011;7:672-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Kumar N, Abu Dayyeh BK, Lopez-Nava Breviere G, Galvao Neto MP, Sahdala NP, Shaikh SN, Hawes RH, Gostout CJ, Goenka MK, Orillac JR, Alvarado A, Jirapinyo P, Zundel N, Thompson CC. Endoscopic sutured gastroplasty: procedure evolution from first-in-man cases through current technique. Surg Endosc. 2018;32:2159-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Hedjoudje A, Abu Dayyeh BK, Cheskin LJ, Adam A, Neto MG, Badurdeen D, Morales JG, Sartoretto A, Nava GL, Vargas E, Sui Z, Fayad L, Farha J, Khashab MA, Kalloo AN, Alqahtani AR, Thompson CC, Kumbhari V. Efficacy and Safety of Endoscopic Sleeve Gastroplasty: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18:1043-1053.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 14. | de Miranda Neto AA, de Moura DTH, Ribeiro IB, Khan A, Singh S, da Ponte Neto AM, Madruga Neto AC, do Monte Junior ES, Tustumi F, Bernardo WM, de Moura EGH. Efficacy and Safety of Endoscopic Sleeve Gastroplasty at Mid Term in the Management of Overweight and Obese Patients: a Systematic Review and Meta-Analysis. Obes Surg. 2020;30:1971-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Neto MG, Silva LB, de Quadros LG, Grecco E, Filho AC, de Amorim AMB, de Santana MF, Dos Santos NT, de Lima JHF, de Souza TF, de Morais HWP, Vieira FM, Moon R, Teixeira AF; Brazilian Endoscopic Sleeve Gastroplasty Collaborative. Brazilian Consensus on Endoscopic Sleeve Gastroplasty. Obes Surg. 2021;31:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Lopez-Nava G, Laster J, Negi A, Fook-Chong S, Bautista-Castaño I, Asokkumar R. Endoscopic sleeve gastroplasty (ESG) for morbid obesity: how effective is it? Surg Endosc. 2022;36:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | James TW, Reddy S, Vulpis T, McGowan CE. Endoscopic Sleeve Gastroplasty Is Feasible, Safe, and Effective in a Non-academic Setting: Short-Term Outcomes from a Community Gastroenterology Practice. Obes Surg. 2020;30:1404-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Sarkar A, Tawadros A, Andalib I, Shahid HM, Tyberg A, Alkhiari R, Gaidhane M, Kedia P, John ES, Bushe B, Martinez GM, Zamarripa F, Carames MC, Carames JC, Casarodriguez F, Bove V, Costamagna G, Boskoski I, Kahaleh M. Safety and efficacy of endoscopic sleeve gastroplasty for obesity management in new bariatric endoscopy programs: a multicenter international study. Ther Adv Gastrointest Endosc. 2022;15:26317745221093883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1849] [Article Influence: 123.3] [Reference Citation Analysis (1)] |
| 20. | Storm AC, Abu Dayyeh BK. Endoscopic sleeve gastroplasty for obesity: defining the risk and reward after more than 1600 procedures. Gastrointest Endosc. 2019;89:1139-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Sharaiha RZ, Hajifathalian K, Kumar R, Saunders K, Mehta A, Ang B, Skaf D, Shah S, Herr A, Igel L, Dawod Q, Dawod E, Sampath K, Carr-Locke D, Brown R, Cohen D, Dannenberg AJ, Mahadev S, Shukla A, Aronne LJ. Five-Year Outcomes of Endoscopic Sleeve Gastroplasty for the Treatment of Obesity. Clin Gastroenterol Hepatol. 2021;19:1051-1057.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 22. | Badurdeen D, Hoff AC, Hedjoudje A, Adam A, Itani MI, Farha J, Abbarh S, Kalloo AN, Khashab MA, Singh VK, Oberbach A, Neto MG, Barrichello S, Kumbhari V. Endoscopic sleeve gastroplasty plus liraglutide vs endoscopic sleeve gastroplasty alone for weight loss. Gastrointest Endosc. 2021;93:1316-1324.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Zorron R, Veltzke-Schlieker W, Adler A, Denecke C, Dziodzio T, Pratschke J, Benzing C. Endoscopic sleeve gastroplasty using Apollo Overstitch as a bridging procedure for superobese and high risk patients. Endoscopy. 2018;50:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Alqahtani AR, Elahmedi M, Alqahtani YA, Al-Darwish A. Laparoscopic Sleeve Gastrectomy After Endoscopic Sleeve Gastroplasty: Technical Aspects and Short-Term Outcomes. Obes Surg. 2019;29:3547-3552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee; Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, Sullivan S, Thompson CC, Banerjee S. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82:425-38.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 288] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 26. | Singh S, Hourneaux de Moura DT, Khan A, Bilal M, Ryan MB, Thompson CC. Safety and efficacy of endoscopic sleeve gastroplasty worldwide for treatment of obesity: a systematic review and meta-analysis. Surg Obes Relat Dis. 2020;16:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Alvarenga ES, Lo Menzo E, Szomstein S, Rosenthal RJ. Safety and efficacy of 1020 consecutive laparoscopic sleeve gastrectomies performed as a primary treatment modality for morbid obesity. A single-center experience from the metabolic and bariatric surgical accreditation quality and improvement program. Surg Endosc. 2016;30:2673-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Alqahtani AR, Elahmedi M, Aldarwish A, Abdurabu HY, Alqahtani S. Endoscopic gastroplasty vs laparoscopic sleeve gastrectomy: a noninferiority propensity score-matched comparative study. Gastrointest Endosc. 2022;96:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 29. | McCarty TR, Jirapinyo P, Thompson CC. Effect of Sleeve Gastrectomy on Ghrelin, GLP-1, PYY, and GIP Gut Hormones: A Systematic Review and Meta-analysis. Ann Surg. 2020;272:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 30. | Lopez-Nava G, Negi A, Bautista-Castaño I, Rubio MA, Asokkumar R. Gut and Metabolic Hormones Changes After Endoscopic Sleeve Gastroplasty (ESG) Vs. Laparoscopic Sleeve Gastrectomy (LSG). Obes Surg. 2020;30:2642-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Rapaka B, Maselli DB, Lopez-Nava G, Bautista-Castaño I, Matar R, Jaruvongvanich V, Vargas EJ, Storm AC, Acosta A, Abu Dayyeh BK. Effects on physiologic measures of appetite from intragastric balloon and endoscopic sleeve gastroplasty: results of a prospective study. Chin Med J (Engl). 2022;135:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 32. | Vargas EJ, Bazerbachi F, Calderon G, Prokop LJ, Gomez V, Murad MH, Acosta A, Camilleri M, Abu Dayyeh BK. Changes in Time of Gastric Emptying After Surgical and Endoscopic Bariatrics and Weight Loss: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18:57-68.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 33. | Fayad L, Adam A, Schweitzer M, Cheskin LJ, Ajayi T, Dunlap M, Badurdeen DS, Hill C, Paranji N, Lalezari S, Kalloo AN, Khashab MA, Kumbhari V. Endoscopic sleeve gastroplasty vs laparoscopic sleeve gastrectomy: a case-matched study. Gastrointest Endosc. 2019;89:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 34. | Jirapinyo P, Thompson CC. Training in Bariatric and Metabolic Endoscopic Therapies. Clin Endosc. 2018;51:430-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |