Published online Jun 16, 2023. doi: 10.4253/wjge.v15.i6.458
Peer-review started: February 20, 2023
First decision: April 13, 2023
Revised: May 12, 2023
Accepted: May 31, 2023
Article in press: May 31, 2023
Published online: June 16, 2023
Processing time: 114 Days and 7.9 Hours
While colon endoscopic mucosal resection (EMR) is an effective technique, removal of larger polyps often requires piecemeal resection, which can increase recurrence rates. Endoscopic submucosal dissection (ESD) in the colon offers the ability for en bloc resection and is well-described in Asia, but there are limited studies comparing ESD vs EMR in the West.
To evaluate different techniques in endoscopic resection of large polyps in the colon and to identify factors for recurrence.
The study is a retrospective comparison of ESD, EMR and knife-assisted endo
A total of 376 patients and 428 polyps were included. Mean polyp size was greatest in the ESD group (35.8 mm), followed by knife-assisted endoscopic resection (33.3 mm) and EMR (30.5 mm) (P < 0.001). ESD achieved highest en bloc resection (90.4%) followed by knife-assisted endoscopic resection (31.1%) and EMR (20.2%) (P < 0.001). A total of 287 polyps had follow-up (67.1%). On follow-up analysis, recurrence rate was lowest in knife-assisted endoscopic resection (0.0%) and ESD (1.3%) and highest in EMR (12.9%) (P = 0.0017). En bloc polyp resection had significantly lower rate of recurrence (1.9%) compared to non-en bloc (12.0%, P = 0.003). On multivariate analysis, ESD (in comparison to EMR) adjusted for polyp size was found to significantly reduce risk of recurrence [adjusted hazard ratio 0.06 (95%CI: 0.01-0.57, P = 0.014)].
In our study, EMR had significantly higher recurrence compared to ESD and knife-assisted endoscopic resection. We found factors including resection by ESD, en bloc removal, and use of circumferential incision were associated with significantly decreased recurrence. While further studies are needed, we have demonstrated the efficacy of ESD in a Western population.
Core Tip: Endoscopic submucosal dissection is an effective and safe technique. Compared to endoscopic mucosal resection, we find that endoscopic submucosal dissection as well as knife-assisted endoscopic resection to achieve higher en bloc resection, circumferential incision, R0 resection as well as lower recurrence rate. While further studies are needed, we have demonstrated the efficacy of endoscopic submucosal dissection in a Western population.
- Citation: Wei MT, Zhou MJ, Li AA, Ofosu A, Hwang JH, Friedland S. Multicenter evaluation of recurrence in endoscopic submucosal dissection and endoscopic mucosal resection in the colon: A Western perspective. World J Gastrointest Endosc 2023; 15(6): 458-468
- URL: https://www.wjgnet.com/1948-5190/full/v15/i6/458.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i6.458
Large non-pedunculated colorectal polyps are currently removed primarily through endoscopic muc
We performed a retrospective study evaluating endoscopic resection performed of polyps ≥ 20 mm at two centers (Stanford University Medical Center and Veterans Affairs Palo Alto Health Care System) by two practitioners (JHH and SF), between January 1, 2016, and December 31, 2020. Inclusion criteria included adults age ≥ 18 who presented for colonoscopy with endoscopic removal of polyp ≥ 20 mm in size. Exclusion criteria included age < 18 and pregnancy.
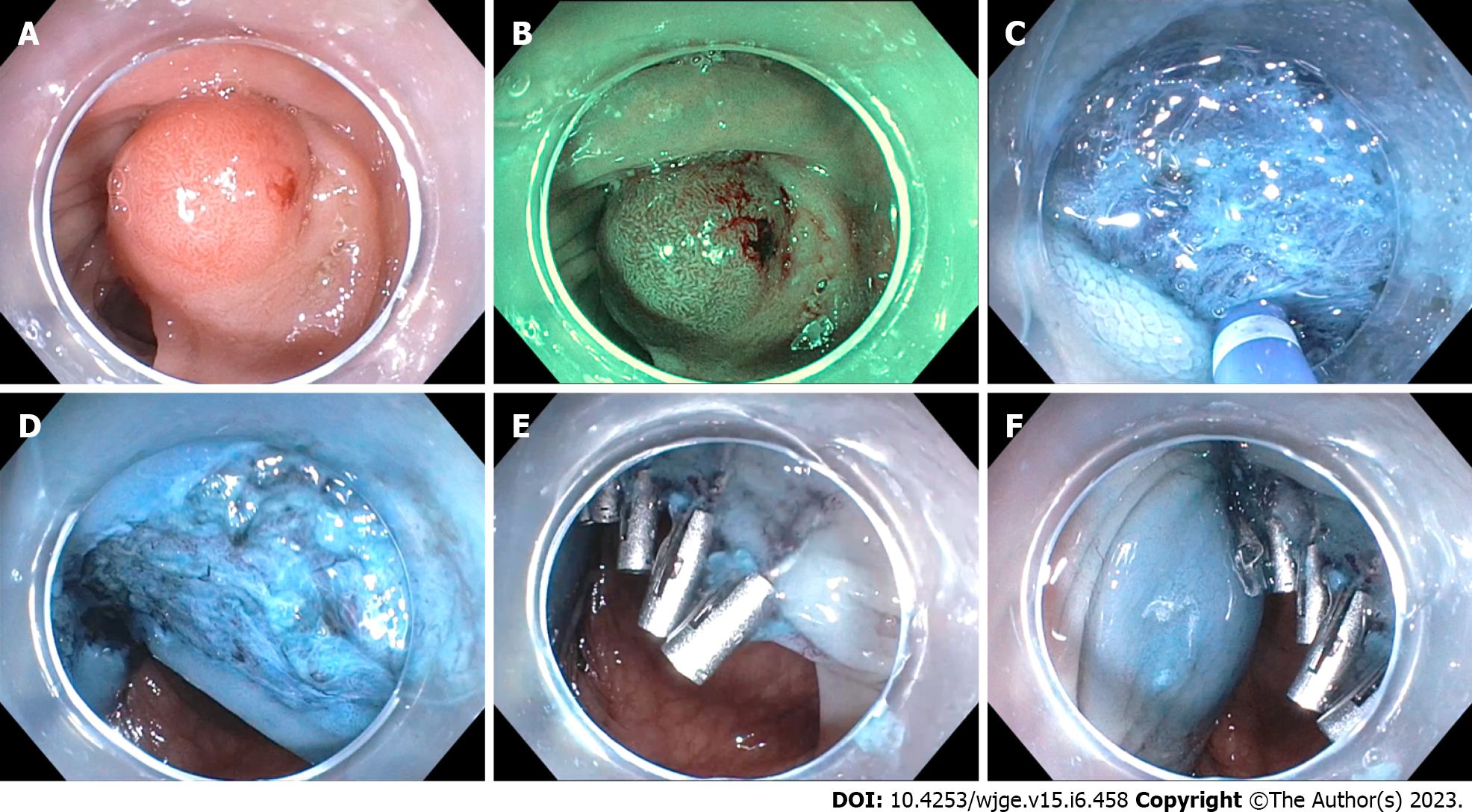
Endoscopic resection was categorized as EMR, knife-assisted endoscopic resection and ESD. EMR was defined by hot or cold snare resection of the polyp with or without submucosal injection. Knife-assisted endoscopic resection was defined as use of electrosurgical knife to facilitate snare resection, such as for circumferential incision and minimal submucosal dissection with an ESD knife. ESD was defined as use of electrosurgical knife for circumferential incision and submucosal dissection with the intention of performing a complete en bloc resection using the knife (Figure 1)[4]. En bloc resection was defined as removal of the polyp in its entirety in one singular piece. Determination of each technique is up to the discretion of the endoscopist. Knife-assisted endoscopic resection was performed when the endoscopist determined at the initial submucosal injection step that full ESD would be too dangerous, typically due to fibrosis or poor scope stability, but that there was a clinical benefit to utilizing an ESD knife to perform selected parts of the procedure.
Endoscopy was performed using high-definition video endoscopes (e.g. PCF-H190DL; GIF-1TH190). A transparent cap was attached to the tip of the endoscope for each procedure. Each polyp was carefully examined under both white light and narrow band imaging (NBI)[5] and evaluated to predict histopathological diagnosis and invasion depth. Polyps were characterized by Paris classification[6] as well as by Japan NBI Expert Team (JNET)[7,8]. Submucosal injection was performed using hydroxyethyl starch with dye, saline with dye, ORISE™ gel (Boston Scientific), Eleview™ liquid composition (Aries Pharmaceuticals), or EverLiftTM (GI Supply). Lesion marking, mucosal incision, and submucosal dissection were performed using an DualKnife (Olympus), FlushKnife (Fujinon), Hybrid Knife (ERBE) or ProKnife (Boston Scientific) with an electrosurgical generator (ERBE Elektromedizin, Tübingen, Germany). In select cases, the resection site was closed with hemostatic clips, X-Tac (Apollo Endosurgery), or OverStitch (Apollo Endosurgery). Resected specimens were pinned on cork or foam board for better pathologic analysis. The specimens were fixed with formalin[1].
The size of the polyp was determined by using the snare as reference, or if the polyp was removed en bloc, was measured against a ruler when it was retrieved from the colon.
All procedures performed by SF (Stanford and Veterans Affairs Palo Alto Health Care System) and JHH (Stanford) between January 1, 2016 and December 31, 2020 were reviewed. Data collected included patient demographics (age, sex, race/ethnicity), sedation, bowel preparation, polyp size, location, Paris and JNET classification, history of prior resection, method of resection, en bloc removal of polyp, and pathology of the polyp. Bowel preparation was characterized as adequate or inadequate. 30-d complications recorded included bleeding with or without intervention, perforation, small bowel obstruction, abdominal pain, as well as complications unrelated to procedure. Follow-up endoscopic evaluation was measured for presence or absence of recurrence. Follow-up was reviewed up to December 31, 2022. Recurrence was defined as evidence of polyp in the area of the prior resection. During follow-up endoscopy, careful examination was performed in the area of the resection, with both white light and NBI, to evaluate for recurrence. When there was suspicion for recurrence, resection or biopsies were performed of the area. The primary outcome was recurrence on follow-up. Secondary outcomes included en bloc resection and complication rates
Specimen from knife-assisted endoscopic resection and ESD were spread and pinned onto cork or Styrofoam boards immediately following endoscopic resection. The specimens were fixed in 10% buffered formalin, paraffin embedded, and cut into 2-mm-thick slices, prior to evaluation by a pathologist.
All analyses were performed with P-value < 0.05 considered significant. All tests were 2-tailed. χ2 test was performed to compare the frequencies of categorical outcomes and student’s t-test was performed to evaluate averages of normally distributed continuous variables. Cox regression analysis was performed to estimate unadjusted and adjusted hazard ratios (HR and aHR) relating potential confounders such as resection technique, age, sex, race, polyp location, prior resection attempt, polyp size, with polyp recurrence.
This study was performed under the approval of the Institutional Review Board at Stanford University, Stanford, California, United States.
There were 376 patients included in the study, 122 of whom received ESD, 44 received knife-assisted endoscopic resection and 216 received EMR. A total of 38 patients had more than one ≥ 20 mm polyp removed. There were 6 patients who underwent two resection techniques (e.g. EMR and ESD). There was similar distribution in age, sex, race/ethnicity across the three categories of procedures (Table 1). Patients undergoing ESD had a higher likelihood of receiving the procedure under general anesthesia or monitored anesthesia care (85.2%) compared to knife-assisted endoscopic resection (70.5%) and EMR (57.4%).
| N = 376 | ESD (n = 122) | Knife-assisted endoscopic resection (n = 44) | EMR (n = 216) | P value |
| Mean age (mean ± SD) | 66.9 (11.8) | 64.5 (11.8) | 66 (9.7) | 0.452 |
| Male (%) | 78 (63.9) | 23 (52.3) | 130 (60.2) | 0.395 |
| Race/Ethnicity | 0.113 | |||
| White | 78 (63.9) | 28 (63.6) | 144 (66.7) | |
| Asian | 19 (15.6) | 6 (13.6) | 12 (5.6) | |
| African American | 4 (3.3) | 1 (2.3) | 11 (5.1) | |
| Latino | 14 (11.5) | 6 (13.6) | 25 (11.6) | |
| Other | 7 (5.7) | 3 (6.8) | 24 (11.1) | |
| Sedation | < 0.001 | |||
| General anesthesia | 11 (9.0) | 2 (4.5) | 7 (3.2) | |
| Monitored anesthesia care | 93 (76.2) | 29 (65.9) | 117 (54.2) | |
| Moderate sedation | 17 (13.9) | 13 (29.5) | 82 (38.0) | |
| None | 1 (0.8) | 0 (0.0) | 10 (4.6) | |
| Adequate bowel preparation | 121 (99.2) | 41 (93.2) | 210 (97.2) | 0.100 |
A total of 428 polyps underwent endoscopic resection, with 258 by EMR and 125 by ESD (Supple
EMR (69.0%) and knife-assisted endoscopic resection (71.1%) had greater proportion of patients that underwent follow-up compared to in the ESD group (61.6%), though this was not statistically significant (P = 0.266). On evaluation of polyps that received follow-up evaluation (Table 2), ESD had highest rate of en bloc resection (89.7%) followed by knife-assisted endoscopic resection (25.0%), followed by EMR (15.2%) (P < 0.001). A higher proportion (44.2%) of polyps undergoing ESD were identified in the rectum compared to knife-assisted endoscopic resection and EMR, while a higher percentage of polyps were removed in the right colon by knife-assisted endoscopic resection or EMR. A higher proportion (74.0%) removed by ESD were identified as Paris classification Is, compared to 56.3% for knife-assisted endoscopic resection and 36.0% for EMR. EMR had the longest mean follow-up (516.2 d) compared to ESD (456.8) and knife-assisted endoscopic resection (365.0), though this was not statistically significant (P = 0.061). ESD (74.0%) and knife-assisted endoscopic resection (18.8%) had higher R0 resection compared to EMR (4.5%) (P < 0.001). There was no recurrence in the knife-assisted endoscopic removal group (0/30). Recurrence rate was lowest in knife-assisted endoscopic resection (0.0%), followed by ESD (1.3%), and highest in EMR (12.9%) (P = 0.002).
| N = 287 | ESD (n = 77) | Knife-assisted endoscopic resection (n = 32) | EMR (n = 178) | P value |
| Size of polyp, mm (mean ± SD) | 37.2 (19.7) | 32.7 (8.7) | 31.4 (11.5) | 0.010 |
| En bloc | 69 (89.7) | 8 (25.0) | 27 (15.2) | < 0.001 |
| Location of polyp | < 0.001 | |||
| Cecum | 10 (13.0) | 7 (21.9) | 47 (26.4) | |
| Ascending | 13 (16.9) | 12 (37.5) | 63 (35.4) | |
| Transverse | 8 (10.4) | 6 (18.8) | 45 (25.3) | |
| Descending | 2 (2.6) | 4 (12.5) | 12 (6.7) | |
| Sigmoid | 10 (13.0) | 1 (3.1) | 5 (2.8) | |
| Rectum | 34 (44.2) | 2 (6.3) | 6 (3.4) | |
| Paris classification | < 0.001 | |||
| Is | 57 (74.0) | 18 (56.3) | 64 (36.0) | |
| IIa | 16 (20.8) | 9 (28.1) | 102 (57.3) | |
| IIb | 0 (0.0) | 1 (3.1) | 2 (1.1) | |
| IIa+c | 2 (2.6) | 1 (3.1) | 2 (1.1) | |
| IIc | 0 (0.0) | 1 (3.1) | 0 (0.0) | |
| Isp | 2 (2.6) | 2 (6.3) | 8 (4.5) | |
| Pathology | < 0.001 | |||
| Non-neoplastic | 0 (0.0) | 1 (3.1) | 10 (5.6) | |
| Neoplastic, no high-grade dysplasia | 50 (64.9) | 25 (78.1) | 152 (85.4) | |
| High-grade dysplasia | 17 (22.1) | 6 (18.8) | 12 (6.7) | |
| Cancer | 10 (13.0) | 0 (0.0) | 4 (2.2) | |
| First follow-up, days (mean ± SD) | 456.8 (326.1) | 365.0 (230.2) | 516.2 (377.7) | 0.061 |
| Recurrence | 1 (1.3) | 0 (0.0) | 23 (12.9) | 0.0017 |
| Complete resection | < 0.001 | |||
| R0 | 57 (74.0) | 6 (18.8) | 8 (4.5) | |
| R1 | 18 (23.4) | 26 (81.3) | 156 (87.6) | |
| Rx | 2 (2.6) | 0 (0.0) | 14 (7.9) |
In categorizing polyps by presence of recurrence (Table 3), there was overall a low proportion of polyps with recurrence (8.4%). Polyps with recurrence had greater mean average size (37.4 vs 32.7 mm, P = 0.202), though this was not statistically significant. Polyps with recurrence more often had non en bloc resection (91.7% vs 61.2%, P = 0.003). Polyps with recurrence more often did not undergo circumferential incision (95.8% vs 62.7%, P = 0.001). Of note, polyps removed with circumferential incision had higher proportion of en bloc removal (76.8% vs 14.9%, P < 0.0001). Recurrence polyps had a higher proportion of polyps that had prior attempt at removal (17.6% vs 5.3%), though this was not statistically significant (P = 0.154). There was no significant difference in pathology or mean follow-up between the two groups. Compared to no recurrence, polyps with recurrence had higher proportion of R1 (91.7% vs 67.7%) and lower proportion of R0 (4.2% vs 26.6%) (P = 0.041).
| Recurrence, n (%) | No recurrence, n (%) | P value | |
| Size of polyp, mm (mean ± SD) | 37.4 (17.1) | 32.7 (13.8) | 0.202 |
| Procedure | 0.002 | ||
| ESD | 1/24 (4.2) | 76/263 (28.9) | |
| Knife-assisted endoscopic removal | 0/24 (0.0) | 32/263 (12.2) | |
| EMR | 23/24 (95.8) | 155/263 (58.9) | |
| En bloc resection | 0.003 | ||
| En bloc | 2/24 (8.3) | 102/263 (38.8) | |
| Non en bloc | 22/24 (91.7) | 161/263 (61.2) | |
| Circumferential incision | 0.001 | ||
| Yes | 1/99 (1.0) | 98/99 (99.0) | |
| No | 23/188 (12.2) | 165/188 (87.8) | |
| Prior resection | 0.154 | ||
| Prior attempt | 3/24 (12.5) (17.6) | 14/263 (5.3) | |
| No prior attempt | 21/24 (87.5) | 249/263 (94.7) | |
| Pathology | 0.691 | ||
| Non-neoplastic | 0/24 (0.0) | 11/263 (4.2) | |
| Neoplastic, no high-grade dysplasia | 19/24 (79.2) | 208/263 (79.1) | |
| High-grade dysplasia | 4/24 (16.7) | 31/263 (11.8) | |
| Cancer | 1/24 (4.2) | 13/263 (4.9) | |
| First follow-up, days (mean ± SD) | 498.0 (406.9) | 482.0 (348.7) | 0.854 |
| Complete resection | 0.041 | ||
| R0 | 1/24 (4.2) | 70/263 (26.6) | |
| R1 | 22/24 (91.7) | 178/263 (67.7) | |
| Rx | 1/24 (4.2) | 15/263 (5.7) |
Overall, there was a low patient complication rate [25 patients (6.6%)], with similar proportion of complication (6.5%-6.8%) among the three procedures (Table 4). There were 3 cases of perforation (two ESD and one knife-assisted endoscopic resection). One patient received knife-assisted endoscopic resection of a > 50 mm polyp in cecum involving the ileocecal valve. There was only partial lifting of the lesion with submucosal injection. Dense fibrosis was encountered, and as such the remainder of the resection was performed by piecemeal EMR. Following the procedure, the patient had abdominal pain, and was found to have pneumoperitoneum. The patient underwent exploratory laparotomy with resection of the terminal ileum and proximal colon. Pathology returned as tubular adenoma with focal high-grade dysplasia. In the second case, the patient had a fungating partially obstructing 50 mm mass in ascending colon. Following ESD, five hemostatic clips placed to close the wound. The patient had worsening abdominal pain following the procedure, and perforation was seen on computed tomography, leading to hemicolectomy. Pathology was consistent with tubulovillous adenoma. In the third case, a 30 mm fungating non-obstructing mass was found in the cecum, encasing the appendiceal orifice. A 40 mm specimen was resected en bloc. A single small perforation (< 2 mm) occurred, which was closed with a single clip followed by full mucosal closure with an Endoloop and clips. The patient recovered uneventfully.
| N = 376 | ESD (n = 122) | Knife-assisted endoscopic resection (n = 44) | EMR (n = 216) |
| Complication | 8 (6.6) | 3 (6.8) | 14 (6.5) |
| Bleeding without intervention | 3 (2.5)1 | 1 (2.3)1 | 5 (2.3) |
| Bleeding with intervention | 3 (2.5) | 1 (2.3) | 1 (0.5) |
| SBO/partial SBO | 0 (0.0) | 0 (0.0) | 2 (0.9) |
| Bowel perforation | 2 (1.6) | 1 (2.3) | 0 (0.0) |
| Abdominal pain | 1 (0.8)1 | 1 (2.3)1 | 3 (1.4) |
| Unrelated complication | 0 (0.0) | 0 (0.0) | 3 (1.4) |
On univariate Cox regression, age, sex, race and polyp location were not significant risk factors. Relative to EMR, ESD was found to decrease risk of recurrence [hazard ratio (HR): 0.12 (95%CI: 0.02-0.92), P = 0.041]. Completion of circumferential incision, en bloc resection as well as R0 resection were found to significantly reduce risk of recurrence (Table 5). On multivariable Cox regression adjusted for polyp size and type of resection (ESD vs EMR), ESD significantly reduced risk of recurrence [adjusted HR (aHR): 0.06 (95%CI: 0.01-0.57, P = 0.014)] (Table 6). In this analysis, we did not include en bloc resection, R0 resection, and presence of circumferential incision as these are factors closely tied with performance of ESD. When evaluating EMR compared to knife-assisted endoscopic resection combined with ESD, on multivariate analysis ESD and knife-assisted endoscopic resection also demonstrated significant decrease in risk of recurrence [aHR: 0.05 (95%CI: 0.01-0.45), P = 0.008] (Supplementary Tables 2 and 3). Knife-assisted endoscopic resection was unable to evaluated independently of ESD as there were no cases of recurrence.
| Covariates | Unadjusted hazard ratio (95%CI) | P value |
| Treatment type | 0.041 | |
| EMR, pure | Reference | |
| ESD, pure | 0.12 (0.02-0.92) | |
| Age, per year | 1.04 (0.99-1.08) | 0.109 |
| Sex | 0.898 | |
| Female | Reference | |
| Male | 1.06 (0.46-2.40) | |
| Race | 0.139 | |
| White | Reference | |
| Non-White | 1.85 (0.82-4.19) | |
| Polyp location | 0.376 | |
| Non-rectum | Reference | |
| Rectum | 0.52 (0.12-2.22) | |
| Prior resection attempt | 2.65 (0.76-9.29) | 0.127 |
| Polyp size, by mm | 1.03 (1.01-1.05) | 0.001 |
| Presence of circumferential incision | 0.12 (0.02-0.92) | 0.041 |
| En bloc resection | 0.15 (0.03-0.63) | 0.010 |
| JNET classification | ||
| Type 1 | Reference | |
| Type 2A | 3.07 (0.41-22.92) | 0.273 |
| Type 2B or 3 | 4.57 (0.41-50.74) | 0.216 |
| R0 resection | 0.13 (0.02-0.93) | 0.042 |
| Covariates | Adjusted hazard ratio (95%CI) | P value |
| Treatment type | 0.014 | |
| EMR, pure | Reference | |
| ESD, pure | 0.06 (0.01-0.57) | |
| Polyp size, by mm | 1.05 (1.02-1.07) | < 0.001 |
The development of advanced polypectomy techniques has allowed patients to avoid colorectal surgeries. While ESD is frequently performed in Asia, it is not commonly performed elsewhere including in the West. However, there are several compelling arguments for performance of ESD over EMR in large (≥ 20 mm) polyps. In a recent meta-analysis, Lim et al[9] found that ESD of polyps ≥ 20 mm was associated to higher en bloc resection [relative risk (RR): 1.9, 95%CI: 1.4-2.7; P < 0.001] and lower recurrence (RR 0.19, 95%CI: 0.09-0.43; P < 0.001) compared to EMR[9]. Given the benefits of ESD, this has culminated in a multicenter randomized controlled trial based in France led by Jacques et al[10] which found ESD to be superior to EMR in en bloc resection as well as decreased recurrence[10]. Given advantages seen with ESD, we performed the first North American study comparing ESD to EMR.
In our retrospective comparison of ESD, EMR and knife-assisted endoscopic resection, ESD was able to achieve the highest en bloc resection, followed by knife-assisted endoscopic resection; EMR had the lowest en bloc resection rate. Recurrence rate was lowest in the ESD (1.3%) and knife-assisted endoscopic resection group (0.0%), and highest in the EMR group (12.9%). On multivariate regression, we found that performance of ESD (in comparison to EMR) significantly decreased recurrence. Increased polyp size significantly increased risk of recurrence. We were able to achieve en bloc resection rate of 90.4% with ESD. This is comparable to work by Gupta et al[1], in which overall en bloc resection rate was 73.1%, and the rate for the second half of their study was 84.6%. Similarly, our study had ESD recurrence rate of 1.3%, slightly lower than the 4.3% (n = 2) by Gupta et al[1] Overall, there was low risk of complication across the three procedures. Under appropriate training, we feel the three procedures to be safe techniques.
While operational proficiency is related to study outcome, in this study we try to evaluate the specific factors that lead to success in reducing polyp recurrence. Specifically, we look at factors such as en bloc resection and performance of circumferential incision. Circumferential incision was found to be associated with decreased recurrence. In one evaluation of ESD compared to hybrid ESD (circumferential mucosal incision followed by snare resection), hybrid ESD trended towards lower en bloc resection rate and complete resection rate compared to ESD, though this did not reach statistical significance. However, importantly, on surveillance of hybrid ESD by the Korean specialist (n = 21) and United States novice practitioner (n = 9), there was no recurrence in either group[4]. While this study was limited by overall low numbers, it provided early suggestion that circumferential incision alone may help improve the outcomes of polyp resection compared to EMR. A major advantage of knife-assisted endoscopic resection over ESD is the relative technical simplicity; in particular, circumferential incision is a relatively safe technique that in our experience is easily taught to trainees with sufficient experience in routine colonoscopy. Over time, with increased experience and proficiency performing ESD, we expect that many endoscopists will choose ESD over knife-assisted endoscopic resection to maximize en bloc and R0 resection, but our data highlights the generally excellent long-term results of the knife-assisted technique.
While there is justifiable concern about the risk of perforation with ESD, and the 3 cases of perforation in this series were all in the ESD/knife-assisted endoscopic resection group rather than EMR, it is notable that the perforations occurred in very challenging cases where EMR was deemed not feasible, and surgery was the only other viable option. For large lesions involving greater than half the circumference of the lumen, the Japan Gastroenterological Endoscopy Society does not recommend piecemeal EMR, but rather ESD and consideration for surgery if ESD is not endoscopically feasible[8].
There were several limitations for our study. First, retrospective data from only two endoscopists were used. However, given the lack of ESD experts in the country, having 125 cases of ESD is relatively robust. In addition, while more EMR cases could have been achieved by including other endoscopists at the two hospitals included, this would potentially introduce more bias with variation in technique and approach to EMR as well as skill with polypectomy. Another concern is the limited follow-up (67.1%). A lot of the patients were referred for endoscopic removal but received follow-up with the referring provider. Despite reaching out to community providers, we only received limited response. Further, the retrospective nature of EMR and ESD studies introduce selection bias in the determination of which polyp to undergo EMR, ESD, or knife-assisted endoscopic resection. A randomized clinical trial would be ideal but is logistically challenging given the overall low frequency of these procedures.
In this multicenter study evaluating ESD, knife-assisted endoscopic resection and EMR, ESD and knife-assisted endoscopic resection were able to achieve higher rates of en bloc resection and was able to achieve significantly lower risk of recurrence compared to EMR. Given the results of this study, ESD and knife-assisted endoscopic resection should be strongly considered when possible for polyps ≥ 20 mm to improve en bloc and curative resection and decrease risk of recurrence.
Adoption of endoscopic submucosal dissection (ESD) has been slow in the United States, largely related to lack of experts, long training required and significant time for procedure compared to endoscopic mucosal resection (EMR).
In this study, we seek to evaluate our experience of ESD compared to EMR in California.
We evaluate ESD, knife-assisted endoscopic resection as well as EMR to identify factors for recurrence.
This was a retrospective comparison performed at two tertiary centers within California between 2016 and 2020. Adult patients that received colonoscopy with endoscopic removal of a polyp at least 20 mm in size were included. Primary outcome of interest was recurrence on follow-up.
ESD achieved highest en bloc resection followed by knife-assisted endoscopic resection and EMR. On follow-up, recurrence rate was lowest in knife-assisted endoscopic resection (0.0%) and ESD (1.3%), while EMR had the highest recurrence rate (12.9%, P = 0.0017).
In our study, we found that EMR had significantly higher recurrence compared to ESD or knife-assisted endoscopic resection.
We have demonstrated efficacy of ESD in a Western population.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kobayashi N, Japan; Tsou YK, Taiwan S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Gupta N, Rodríguez-Ruiz G, Siddiqui UD, Chapman CG, Donboli K, Hart J, Xiao SY, Waxman I. Endoscopic submucosal dissection for colorectal lesions: outcomes from a United States experience. Surg Endosc. 2022;36:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014;6:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 3. | Draganov PV, Aihara H, Karasik MS, Ngamruengphong S, Aadam AA, Othman MO, Sharma N, Grimm IS, Rostom A, Elmunzer BJ, Jawaid SA, Westerveld D, Perbtani YB, Hoffman BJ, Schlachterman A, Siegel A, Coman RM, Wang AY, Yang D. Endoscopic Submucosal Dissection in North America: A Large Prospective Multicenter Study. Gastroenterology. 2021;160:2317-2327.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 4. | Yang DH, Kwak MS, Park SH, Ye BD, Byeon JS, Myung SJ, Yang SK, Kim HG, Friedland S. Endoscopic Mucosal Resection with Circumferential Mucosal Incision for Colorectal Neoplasms: Comparison with Endoscopic Submucosal Dissection and between Two Endoscopists with Different Experiences. Clin Endosc. 2017;50:379-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 402] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 6. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1325] [Article Influence: 60.2] [Reference Citation Analysis (4)] |
| 7. | Inoue T, Nakagawa K, Yamasaki Y, Shichijo S, Kanesaka T, Maekawa A, Higashino K, Uedo N, Ishihara R, Takeuchi Y. Underwater endoscopic mucosal resection versus endoscopic submucosal dissection for 20-30 mm colorectal polyps. J Gastroenterol Hepatol. 2021;36:2549-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Saitoh Y, Tsuruta O, Sugihara KI, Igarashi M, Toyonaga T, Ajioka Y, Kusunoki M, Koike K, Fujimoto K, Tajiri H. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2020;32:219-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 9. | Lim XC, Nistala KRY, Ng CH, Lin SY, Tan DJH, Ho KY, Chong CS, Muthiah M. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal polyps: A meta-analysis and meta-regression with single arm analysis. World J Gastroenterol. 2021;27:3925-3939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Jacques J, Wallenhorst T, Chevaux JB, Lépilliez DV, Chaussade S, Rivory J, Legros R, Schaefer M, Leblanc S, Rostain F, Barret M, Belle A, Crepin S, Magne J, Albouys J, Preux PM, Lepetit H, Dahan M, Ponchon T, Pioche M. Endoscopic submucosal dissection (ESD) vs piece-meal endoscopic mucosal resection (PM-EMR) for large laterally spreading lesions: French randomized controlled trial RESECT-COLON (Abstract). Gastrointest Endosc. 2022;95 Suppl 6. [DOI] [Full Text] |