Published online Apr 16, 2023. doi: 10.4253/wjge.v15.i4.259
Peer-review started: December 19, 2022
First decision: February 21, 2023
Revised: February 26, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: April 16, 2023
Processing time: 115 Days and 22.6 Hours
Different traction devices that can provide a visual field and attain appropriate tension at the dissection plane during endoscopic submucosal dissection (ESD) have been developed. Clip-with-line (CWL) is a classic traction device that can offer per-oral traction toward the direction where the line is drawn. A multicenter randomized controlled trial (CONNECT-E trial) comparing the conventional ESD and CWL-assisted ESD (CWL-ESD) for large esophageal tumors was conducted in Japan. This study showed that CWL-ESD was associated with a shorter procedure time (defined as the time from initiating submucosal injection to completing tumor removal) without increasing the risk of adverse events. Multivariate analysis revealed that whole-circumferential lesion and abdominal esophageal lesion were independent risk factors for technical difficulties, which were defined as a procedure time of > 120 min, perforation, piecemeal resection, inadvertent incision (any accidental incision caused by the electrosurgical knife within the marked area), or handover to another operator. Therefore, techniques other than CWL should be considered for these lesions. Several studies have shown the usefulness of endoscopic submucosal tunnel dissection (ESTD) for such lesions. A randomized controlled trial conducted at five Chinese institutions showed that compared with the conventional ESD, ESTD had a significantly reduced median procedure time for lesions covering ≥ 1/2 of the esophageal circumference. In addition, a propensity score matching analysis conducted at a single Chinese institution showed that compared with the conventional ESD, ESTD had a shorter mean resection time for lesions at the esophagogastric junction. With the appropriate use of CWL-ESD and ESTD, esophageal ESD can be performed more efficiently and safely. Moreover, the combination of these two methods may be effective.
Core Tip: Clip-with-line (CWL) was developed to overcome the challenges faced in endoscopic submucosal dissection (ESD). A multicenter randomized controlled trial comparing the conventional ESD and CWL-assisted ESD (CWL-ESD) for large esophageal tumors was conducted in Japan. Results showed that CWL-ESD had a shorter procedure time without increasing the risk of adverse events. However, this study revealed that there were issues with the use of CWL-ESD for whole-circumferential and abdominal esophageal lesions. Endoscopic submucosal tunnel dissection may be a promising option for treating these lesions.
- Citation: Nagata M. Two traction methods that can facilitate esophageal endoscopic submucosal dissection. World J Gastrointest Endosc 2023; 15(4): 259-264
- URL: https://www.wjgnet.com/1948-5190/full/v15/i4/259.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i4.259
Endoscopic submucosal dissection (ESD) can allow the en bloc resection of superficial gastrointestinal neoplasms, thereby obtaining a reliable pathological diagnosis and decreasing the risk of recurrence. However, ESD is a challenging procedure, and a long procedure time and perforation remain an issue. Unlike surgeons, endoscopists cannot use their hands to obtain traction for lesions in the gastrointestinal tract, which results in a poor visual field and insufficient tension in the dissection plane in ESD. Clip-with-line (CWL) is among the most classical traction devices developed to address these issues. A single-center randomized controlled trial (RCT) reported the usefulness of CWL-assisted ESD (CWL-ESD) vs the conventional ESD for esophageal lesions. Meanwhile, this study had some limitations. That is, it has a small sample size and few operators, and it included patients with a small lesion[1].
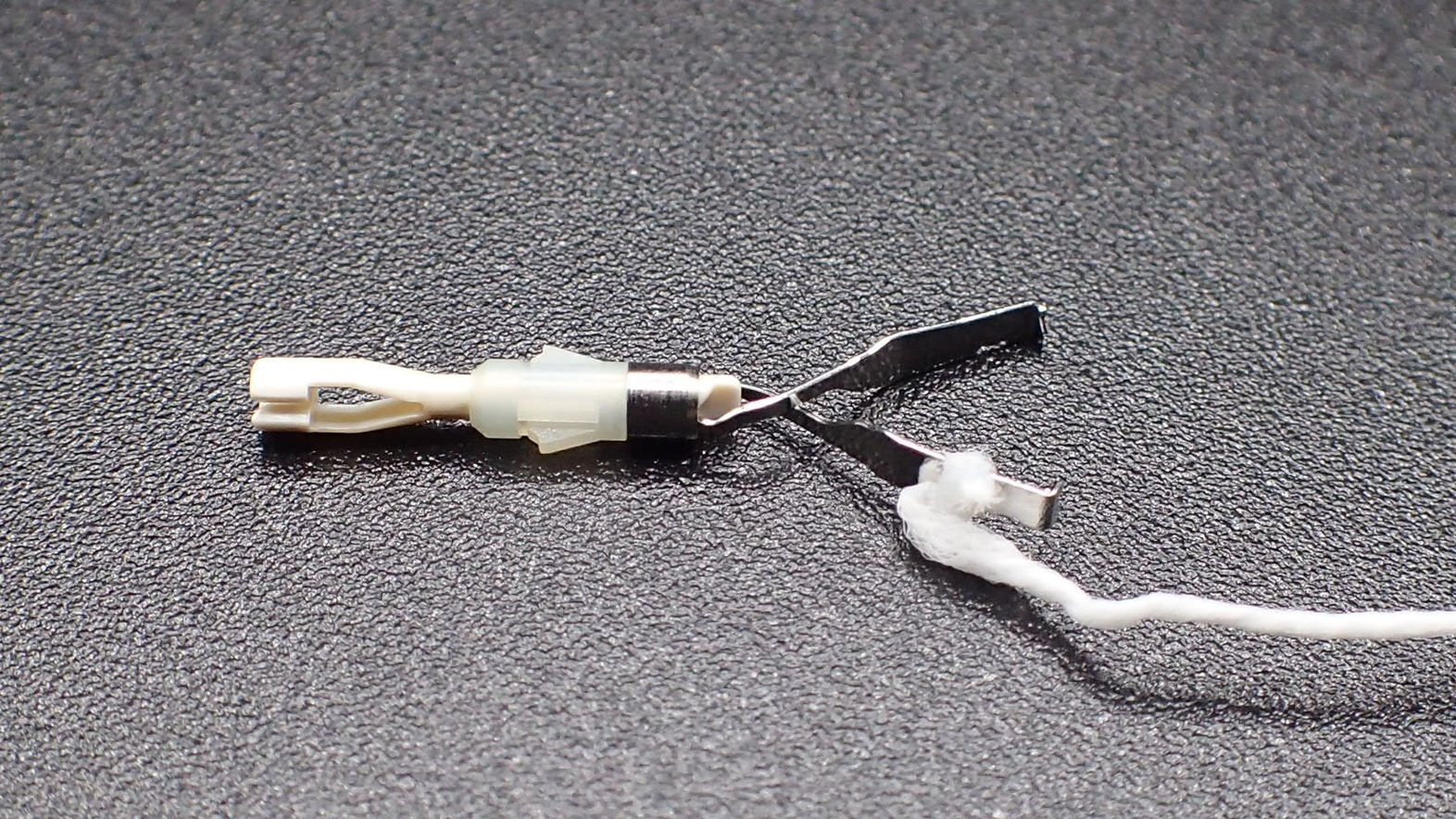
The CONNECT-E trial was the first multicenter RCT conducted at seven institutions in Japan. It aimed to compare the conventional ESD and traction-assisted ESD for treating large esophageal cancers[2]. In this study, CWL was used (Figure 1), and board-certified endoscopists who performed ≥ 40 gastric ESD procedures were included as operators. Patients endoscopically diagnosed with esophageal squamous cell carcinoma or basal cell carcinoma with a tumor diameter ≥ 20 mm and clinically diagnosed with intramucosal cancer (cT1a) or slightly invasive submucosal cancer (cT1b-SM1) were randomly assigned to the conventional ESD (n = 116) and CWL-ESD (n = 116) groups. Due to prolonged ESD (procedure time of > 120 min) or perforation, six patients in the conventional ESD group required conversion to CWL-ESD.
Although a statistical comparison of the baseline characteristics of the patients, including age, sex, tumor diameter, tumor location, and macroscopic type, was not performed, the characteristics of all patients were well balanced. The median tumor diameter was 30 mm in the conventional ESD and CWL-ESD groups. There were no significant differences in histologic depth of the tumor between the groups. The median ESD procedure time (primary endpoint; defined as the time from initiating the submucosal injection to completing tumor removal) of the CWL-ESD group was significantly shorter than that of the conventional ESD group (44.5 vs 60.5 min, P < 0.001). Although not significant, the rate of perforation was considerably lower in the CWL-ESD group (0% vs 4.3%, P = 0.060). Hence, CWL can secure the visual field and prevent blind submucosal dissection. The conventional ESD group required handover to another operator more frequently than the CWL-ESD group (6.0% vs 0.9%, P = 0.066).
In the subgroup analysis, the procedure time of the CWL-ESD group was significantly shorter than that of the conventional ESD group for lesions occupying < 50% and ≥ 50% but < 100%. However, it was not significant for lesions covering the entire circumference.
Approximately 16.4% of patients in the CWL-ESD group had CWL slip-off during ESD, and traction-related damage to the specimen was observed in 1.7% of patients. However, there were no significant differences in the rate of horizontal margin involvement (10.3% vs 6.9%, P = 0.484) and R0 resection rate (87.2% vs 91.4%, P = 0.30) between the conventional ESD and CWL-ESD groups.
Further, the CONNECT-E trial evaluated the risk factors of technical difficulties, which were defined as a procedure time of > 120 min, perforation, piecemeal resection, inadvertent incision (any accidental incision caused by the electrosurgical knife within the marked area), or handover to another operator. Multivariate analysis revealed that lesions occupying the full circumference of the esophagus and those located at the abdominal esophagus were independent risk factors for technical difficulties. Therefore, techniques other than CWL should be considered for these lesions.
Endoscopic submucosal tunnel dissection (ESTD) is a traction method that does not use a specific device[3,4], as it utilizes the mucosal tension for traction.
In ESTD, a mucosal incision is first made on the distal side and then on the proximal side of the lesion to enter into the submucosal layer. Next, the submucosal layer under the lesion is dissected from the proximal to the distal side, creating a submucosal tunnel. During submucosal dissection, the lateral position of mucosa prevents the lesion from falling distally. The endoscope inside the tunnel space thrusts the lesion, offering traction for the dissection plane and facilitating submucosal dissection. After creating a submucosal tunnel, the mucosa and submucosa around the tunnel space are dissected to attain en bloc resection. It was found to be useful for lesions covering the esophageal lumen and those located at the esophagogastric junction (EGJ). An RCT conducted at five Chinese institutions showed that compared with the conventional ESD, ESTD was significantly associated with a reduced median procedure time for lesions covering ≥ 1/2 of the esophageal circumference (85.5 vs 56.0 min, P < 0.001)[5]. In addition, muscular injury was less frequent in ESTD than in the conventional ESD (18.4% vs 38.2%, P = 0.007). In ESTD, the submucosal tunnel can hold the endoscope tip, thereby stabilizing the endoscope and facilitating a parallel approach to the muscularis layer. Moreover, the endoscope can provide sufficient tension at the dissection plane by pushing up the lesion from inside the submucosal tunnel by itself. ESTD had several advantages. That is, it may have a shorter procedure time and a lower muscle injury rate. Further, all operators in this study performed > 200 ESD procedures, and ESTD is different from the conventional ESD. Nevertheless, further studies should be performed to evaluate the feasibility of ESTD by low-experienced operators and its usefulness for whole-circumferential lesions.
A propensity score matching analysis conducted at a single Chinese institution showed that ESTD had a shorter mean resection time (71.59 vs 111.00 min; P = 0.008) for superficial neoplasms at the EGJ compared with the conventional ESD. Meanwhile, none of the patients who underwent ESTD had complications[6]. The EGJ and the abdominal esophagus are not similar. Nevertheless, ESTD may be a promising method for abdominal esophageal lesions. This study had limitations. For example, it had a small sample size (n = 17 for each group) and few operators (only two experienced endoscopists who had completed > 100 ESD procedures). Hence, a large-scale study must be performed to evaluate the feasibility of ESTD for abdominal esophageal lesions.
A recent study showed the efficacy of the combined use of traction devices and the pocket creation method in colorectal ESD[7]. The pocket creation method has the same principle as ESTD. Therefore, CWL-ESD can be combined with ESTD, which might facilitate esophageal ESD.
CWL-ESD was not significantly associated with a reduced gastric ESD procedure time in the CONNECT-G trial, unlike in the CONNECT-E trial, which aimed to compare the conventional ESD and CWL-ESD for superficial gastric neoplasms[8]. Based on this finding, the effects of CWL can differ according to the conditions where it is used. Nevertheless, it is important to discuss the causes of this difference to achieve the most out of CWL.
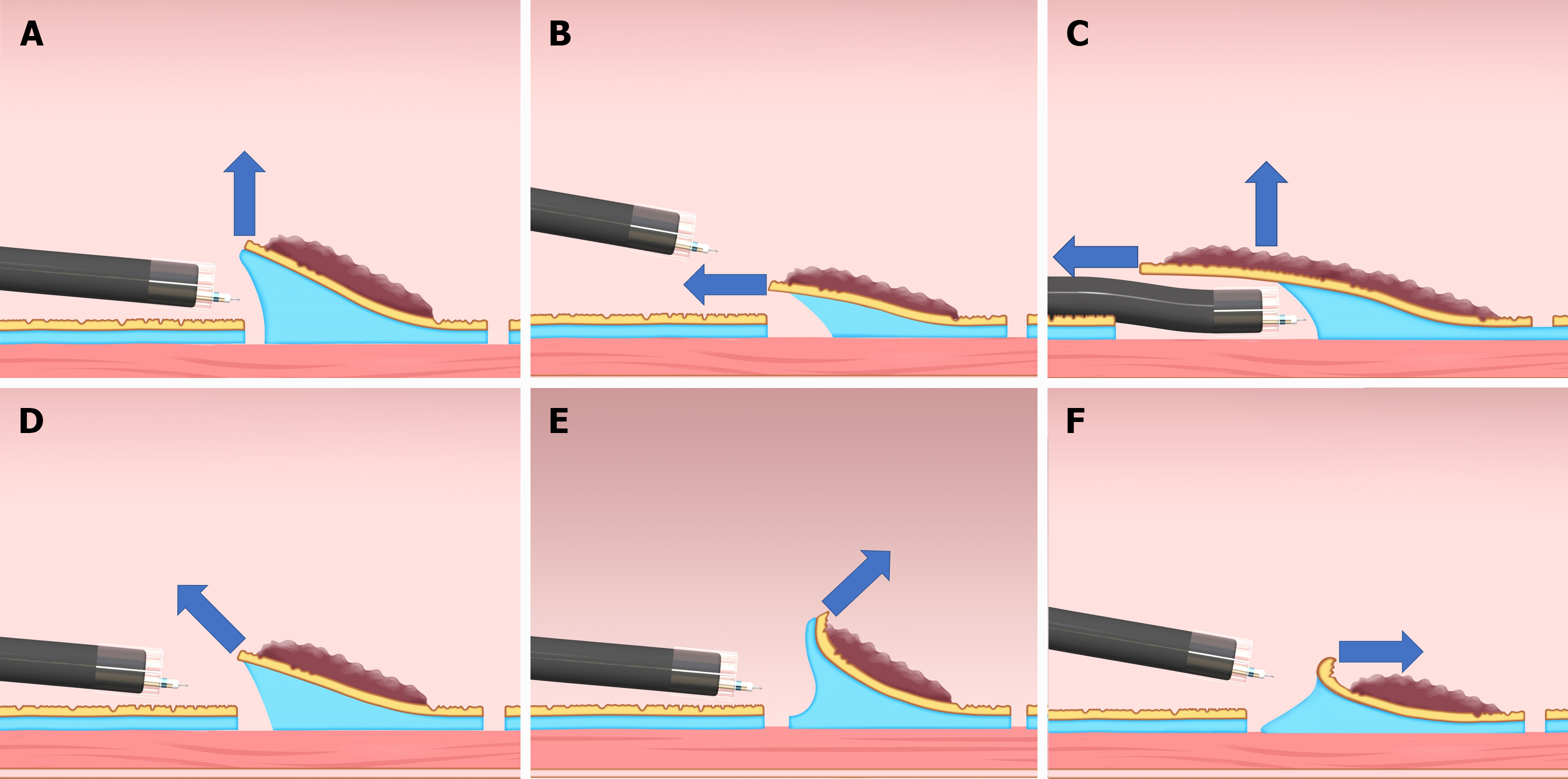
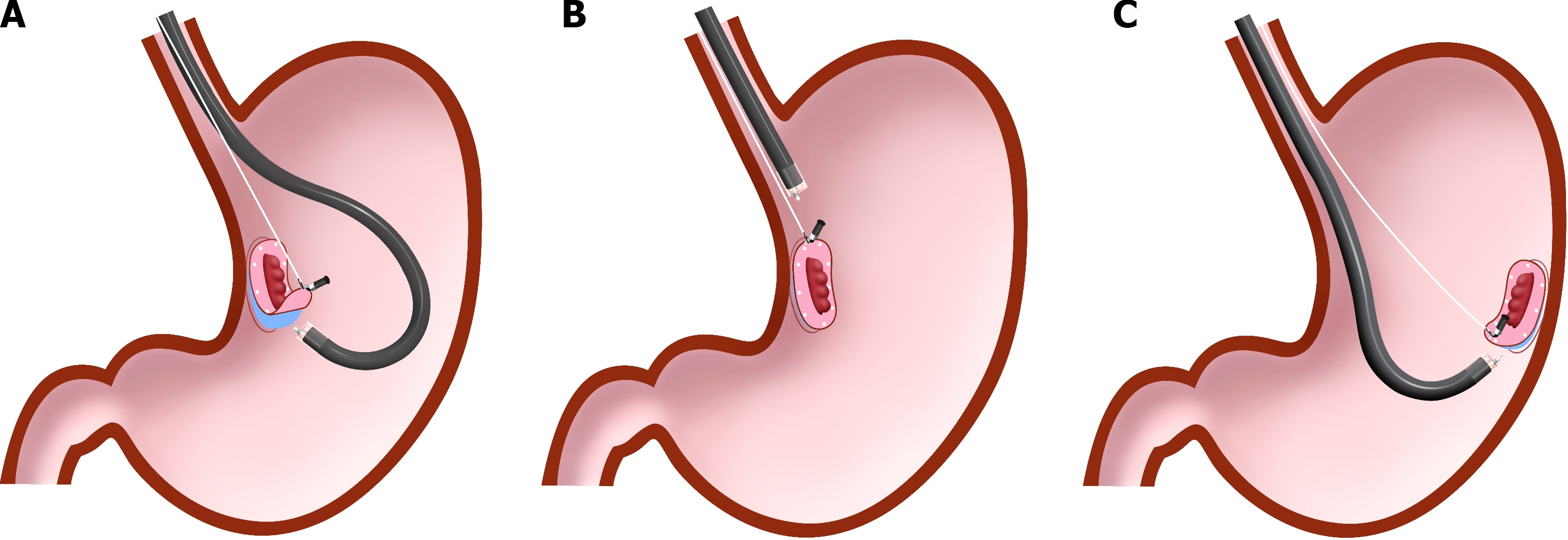
Traction direction may be the most significant factor affecting the effect of CWL in the esophagus and stomach. It can be classified into five categories (vertical, proximal, diagonally proximal, diagonally distal, and distal traction; Figure 2) based on its association with the endoscope tip and gastrointestinal wall[9]. Since the stomach lumen is large and CWL can provide the lesion with traction toward the cardia, the direction of traction of CWL is naturally restricted to the direction in which the line is drawn; therefore, the direction of traction in CWL-ESD for gastric lesions varies among the abovementioned five directions based on the lesion location (Figure 3). Among these five directions, vertical traction (Figure 2A) was found to be the most effective in the CONNECT-G trial[10]. Moreover, a single-center RCT that compared the conventional and multidirectional traction device (S–O clip; Zeon Medical, Tokyo, Japan)-assisted ESD for superficial gastric neoplasms found that vertical traction reduces the procedure time for gastric ESD[11]. Although few studies have investigated the effectiveness of traction-assisted ESD according to the traction direction, a propensity score matching analysis (42 pairs) comparing S–O clip-assisted ESD and CWL-ESD in the stomach demonstrated that the S–O clip-assisted ESD significantly could reduce the median ESD procedure time (28.3 min vs 51.0 min; P = 0.022) and accelerated the median dissection speed (24.8 mm2/min vs 17.1 mm2/min, P = 0.001)[12]. In this study, all traction directions in the S–O clip-assisted ESD were vertical whereas only 16.7% directions in the CWL-ESD were vertical, indicating that vertical traction facilitated the gastric ESD better than the other traction directions.
In contrast, the esophageal lumen is narrow and cylindrical. Therefore, endoscope position has a limited forward view, and the traction direction is naturally limited to the proximal traction (Figure 2B). Proximal traction may cause the mucosal flap to fall down toward the endoscope tip, which makes it difficult for the endoscope tip to get under the mucosal flap in some cases. However, in esophageal ESD, the tip of the endoscope can be parallel to the esophageal wall and can smoothly approach the submucosal layer, even with the proximal traction. After the endoscope tip reaches under the mucosal flap, CWL traction can be combined with the traction by the hood attached to the endoscope tip, thereby making vertical traction for the dissection plane (Figure 2C). Due to the abovementioned reasons, CWL can be effective in esophageal ESD. Meanwhile, it cannot be useful in gastric ESD in some cases. Further studies should be performed to assess the impact of traction direction in traction-assisted ESD.
Compared with the conventional method, CWL is associated with a reduced esophageal ESD procedure time, decreasing the risk of perforation. CWL slip-off is frequently observed. However, its effect on horizontal margin involvement is not significant. CWL-ESD can be the primary option for superficial esophageal neoplasms, except for lesions covering the whole circumference of the esophageal lumen and abdominal esophageal lesions. ESTD can be a promising strategy for these lesions. Moreover, combined CWL-ESD and ESTD can be feasible and facilitate esophageal ESD procedures.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan KK, India; Lim KT, Singapore; Mohamed SY, Egypt; Wang D, China S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Koike Y, Hirasawa D, Fujita N, Maeda Y, Ohira T, Harada Y, Suzuki K, Yamagata T, Tanaka M. Usefulness of the thread-traction method in esophageal endoscopic submucosal dissection: randomized controlled trial. Dig Endosc. 2015;27:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Yoshida M, Takizawa K, Nonaka S, Shichijo S, Suzuki S, Sato C, Komori H, Minagawa T, Oda I, Uedo N, Hirasawa K, Matsumoto K, Sumiyoshi T, Mori K, Gotoda T, Ono H; CONNECT-E Study Group. Conventional versus traction-assisted endoscopic submucosal dissection for large esophageal cancers: a multicenter, randomized controlled trial (with video). Gastrointest Endosc. 2020;91:55-65.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Arantes V, Albuquerque W, Freitas Dias CA, Demas Alvares Cabral MM, Yamamoto H. Standardized endoscopic submucosal tunnel dissection for management of early esophageal tumors (with video). Gastrointest Endosc. 2013;78:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Linghu E, Feng X, Wang X, Meng J, Du H, Wang H. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy. 2013;45:60-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Fan X, Wu Q, Li R, Chen W, Xie H, Zhao X, Zhu S, Fan C, Li J, Liu M, Liu Z, Han Y. Clinical benefit of tunnel endoscopic submucosal dissection for esophageal squamous cancer: a multicenter, randomized controlled trial. Gastrointest Endosc. 2022;96:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 6. | Shao BZ, Chai NL, Li LS, Wang SS, Feng XX, Wang NJ, Wang ZT, Liu SZ, Linghu EQ. Comparison between endoscopic submucosal tunnel dissection and endoscopic submucosal dissection for superficial neoplasia at esophagogastric junction: a case-matched controlled study of a single center from China. Surg Endosc. 2022;36:8371-8378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Ide D, Ohya TR, Saito S, Mitsuyoshi Y, Hatamori H, Ikenoyama Y, Suzuki K, Ishioka M, Yakabi S, Yasue C, Chino A, Igarashi M, Saruta M, Fujisaki J. Clinical utility of the pocket-creation method with a traction device for colorectal endoscopic submucosal dissection. Surg Endosc. 2021;35:2110-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Yoshida M, Takizawa K, Suzuki S, Koike Y, Nonaka S, Yamasaki Y, Minagawa T, Sato C, Takeuchi C, Watanabe K, Kanzaki H, Morimoto H, Yano T, Sudo K, Mori K, Gotoda T, Ono H; CONNECT-G Study Group. Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: a multicenter, randomized controlled trial (with video). Gastrointest Endosc. 2018;87:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 9. | Nagata M. Advances in traction methods for endoscopic submucosal dissection: What is the best traction method and traction direction? World J Gastroenterol. 2022;28:1-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 10. | Nagata M. Optimal traction direction in traction-assisted gastric endoscopic submucosal dissection. World J Gastrointest Endosc. 2022;14:667-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Nagata M, Fujikawa T, Munakata H. Comparing a conventional and a spring-and-loop with clip traction method of endoscopic submucosal dissection for superficial gastric neoplasms: a randomized controlled trial (with videos). Gastrointest Endosc. 2021;93:1097-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Nagata M, Namiki M, Fujikawa T, Munakata H. Impact of Traction Direction in Traction-Assisted Gastric Endoscopic Submucosal Dissection (with Videos). Dig Dis Sci. 2023;1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |