Published online Mar 16, 2023. doi: 10.4253/wjge.v15.i3.163
Peer-review started: November 2, 2022
First decision: November 18, 2022
Revised: December 12, 2022
Accepted: March 1, 2023
Article in press: March 1, 2023
Published online: March 16, 2023
Processing time: 133 Days and 22.8 Hours
Previous studies that compared the postoperative health-related quality of life (HRQoL) outcomes after receiving laparoscopic resection (LR) or open resection (OR) in patients with colorectal cancer (CRC) have different conclusions.
To explore the medium-term effect of postoperative HRQoL in such patients.
This study randomized 567 patients undergoing non-metastatic CRC surgery managed by one surgeon to the LR or OR groups. HRQoL was assessed during the preoperative period and 3, 6, and 12 mo postoperative using a modified version of the 36-Item Short Form (SF-36) Health Survey questionnaire, emphasizing eight specific items.
This cohort randomly assigned 541 patients to receive LR (n = 296) or OR (n = 245) surgical procedures. More episodes of postoperative urinary tract infection (P < 0.001), wound infection (P < 0.001), and pneumonia (P = 0.048) were encountered in the OR group. The results demonstrated that the LR group subjectively gained mildly better general health (P = 0.045), moderately better physical activity (P = 0.006), and significantly better social function recovery (P = 0.0001) 3 mo postoperatively. Only the aspect of social function recovery was claimed at 6 mo, with a significant advantage in the LR group (P = 0.001). No clinical difference was found in HRQoL during the 12 mo.
Our results demonstrated that LR resulted in better outcomes, including intra-operative blood loss, surgery-related complications, course of recovery, and especially some health domains of HRQoL at least within 6 mo postoperatively. Patients should undergo LR if there is no contraindication.
Core Tip: Previous randomized controlled trials that compare laparoscopic (LR) and open resection (OR) in colorectal cancer (CRC) management have led to different conclusions regarding the health-related quality of life (HRQoL). Our study analyzed the objective surgical outcomes and subjective HRQoL in 541 patients with non-metastatic CRC randomized to the LR (n = 296) or OR (n = 245) group operated by one surgeon. Better HRQoL was noticed in the LR group in general health, physical activity, and social function recovery with various degrees. These patients should consider LR to gain better HRQoL if not contraindicated because these two operative methods resulted in similar cancer-oriented outcomes and survival.
- Citation: Hung CM, Hung KC, Shi HY, Su SB, Lee HM, Hsieh MC, Tseng CH, Lin SE, Chen CC, Tseng CM, Tsai YN, Chen CZ, Tsai JF, Chiu CC. Medium-term surgical outcomes and health-related quality of life after laparoscopic vs open colorectal cancer resection: SF-36 health survey questionnaire. World J Gastrointest Endosc 2023; 15(3): 163-176
- URL: https://www.wjgnet.com/1948-5190/full/v15/i3/163.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i3.163
Colonic resection under laparoscopy was first performed in 1991, and several randomized clinical trials that compare laparoscopic resection (LR) with open resection (OR) in patients with colorectal cancer (CRC) have been performed since then[1-3]. Initial studies revealed that LR patients gained similar clinical results, along with short-term advantages, such as lesser blood loss, reduced analgesic use, and a shorter hospital stay[4].
Health-related quality of life (HRQoL) is often overlooked, emphasizing more focus on survival and oncologic outcomes[5]. However, patients’ self-assessed outcomes must reveal the effects of their health status on their physical and psychological functioning[4]. Some studies revealed LR’s superiority regarding HRQoL in managing patients with colon cancer[2], but others demonstrated the opposite results. However, most studies focused on the longer-term effects (more than one year)[3,6]. Moreover, Li et al[7] revealed improved HRQoL 1 week after laparoscopic rectal cancer surgery but not after 1 year.
Our prospective study aims to assess the HRQoL effects within 1 year after non-metastatic CRC surgery by a single surgeon since previous studies that compare postoperative HRQoL outcomes of CRC after LR and OR have come to different conclusions and lack medium-term results (from 3 mo to 1 year)[2,6,8-12].
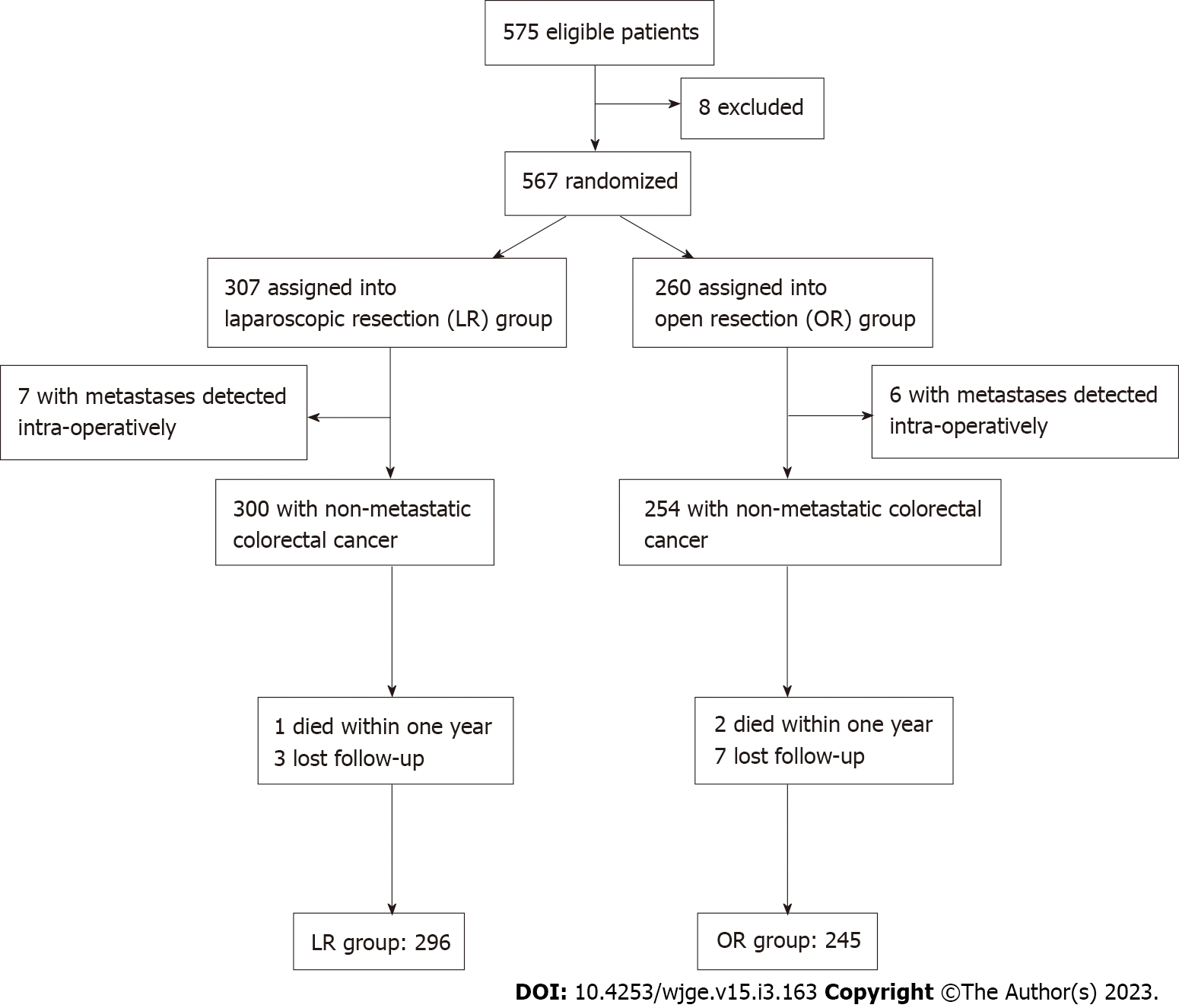
Dr. Chiu performed surgeries for 575 patients with CRC in two regional hospitals from January 2014 to October 2021 (Chi Mei Medical Center, Liouying, Tainan, and E-Da Cancer Hospital, Kaohsiung, Taiwan) (Figure 1). The guidelines of the National Comprehensive Cancer Network (NCCN) were followed. Our study included patients with non-metastatic colon or rectal cancer with no adjacent organ invasion. However, we excluded patients with colon polyposis conditions, repeated episodes of adhesion-related ileus, synchronous tumors, operative method conversion, emergent surgeries, denial of participation, loss of follow-up, refusal of subsequent postoperative management, or expiration not related to cancer within 1 year postoperatively. All patients must sign the informed consent form. This study was reviewed and accepted by the Institutional Review Board of both surgical hospitals.
Patients were divided into several groups based on different tumor locations. Patients were randomly assigned to perform LR or OR, as blindly selected by the surgeon using sealed envelopes preoperatively.
The clinical stage of oncologic status was described according to the tumor node metastasis (TNM) system, as advocated by the American Joint Committee on Cancer. Physical examination, colonoscopy with biopsy, carcinoembryonic antigen serum level, and abdominal computed tomography were performed for each patient.
We followed the standard procedures of right or left-side hemicolectomy, sigmoid colectomy, or rectal resection by performing a standard medial-to-lateral way. High ligation of related vessels was routinely performed for all patients based on the non-touch technique concept. The rule of keeping the surgical safety margin at 5 cm for all patients with colon cancer was followed. Additionally, intestinal anastomosis was done extra-corporeally for proximal lesions. An immediate intra-corporeal intestinal anastomosis with circular stapling was performed via a trans-anal approach following left-side colon or rectum lesion resections. However, a protective diversional stoma would be considered if the anastomosis dehiscence is possible, mainly for high-risk patients with ultra-low rectal cancer. Further, a preoperative endoscopic tattoo would be requested for patients with a smaller or probably non-palpable intestinal lesion to mark the location for subsequent surgical resection 1 day later. An intra-operative endoscopy examination would be requested if we could not localize the lesion by vision or palpation under laparoscopy.
Follow-up examinations would be arranged according to the NCCN guidelines[13]. All patients were expected to visit the outpatient department every 3 mo for follow-up within the first postoperative year. All patients with stage III CRC would be arranged to receive intravenous or oral adjuvant chemo
HRQoL was assessed by professionally trained members in the outpatient department preoperatively and 3, 6, and 12 mo postoperatively using a modified version of the 36-Item Short Form (SF-36) Health Survey questionnaire.
SF-36 included a multi-item scale and estimated eight health domains, including: (1) Physical activity limits related to health issues; (2) Social activity limits associated with physical or emotional issues; (3) Vitality (energy and fatigue); (4) General mental health (well-being and psychological distress); (5) Usual role activity limits caused by physical health issues; (6) Usual role activity hindered by emotional issues; (7) Physical pain, and (8) General health awareness[14]. The scores ranged from 0 to 100 in each domain, with higher scores revealing better HRQoL[3]. This study concentrated on HRQoL assessment via these self-reported domains.
Tables 1 and 2 show the baseline patient information. The difference between medians of continuous variables was investigated with the unpaired Student’s t-test. Categorical variables were compared by χ2 test with Yates’ correction. All P values were two-tailed, and those < 0.05 implied a significant statistical difference. The calculations were performed using the Statistical Package for the Social Sciences version 20.0 statistical package (IBM Co., Armonk, NY, United States).
| Variables | LR (n = 296) | OR (n = 245) | P value |
| Gender | 0.412 | ||
| Male | 162 | 135 | |
| Female | 134 | 110 | |
| Age (mean ± SD) | 67.2 ± 11.3 | 70.1 ± 8.9 | 0.19 |
| ASA class | 0.673 | ||
| I | 162 | 129 | |
| II | 111 | 99 | |
| III | 23 | 17 | |
| Pre-operative TNM stage | 0.342 | ||
| 0 | 9 | 5 | |
| I | 93 | 71 | |
| II | 99 | 80 | |
| III | 95 | 89 | |
| Tumor location | 0.452 | ||
| Cecum | 42 | 31 | |
| Ascending colon | 57 | 45 | |
| Transverse colon | 33 | 25 | |
| Descending colon | 51 | 42 | |
| Sigmoid colon | 71 | 62 | |
| Rectum | 42 | 40 | |
| Pre-surgery serum CEA level | 1.021 | ||
| < 5 ng/mL | 51 | 34 | |
| ≥ 5 ng/mL | 245 | 211 | |
| Pre-operative CCRT | 40 | 39 | 0.391 |
| Intervention | 0.729 | ||
| Right hemicolectomy | 93 | 76 | |
| Left hemicolectomy | 64 | 45 | |
| Transverse colectomy | 28 | 22 | |
| Sigmoid colectomy | 69 | 60 | |
| Proctectomy | 39 | 38 | |
| Abdominal perineal resection | 3 | 4 | |
| Protective diversional stoma | 13 | 15 | |
| Post-operative TNM stage | 0.359 | ||
| 0 | 9 | 5 | |
| I | 92 | 70 | |
| II | 96 | 79 | |
| III | 99 | 91 | |
| Histopathology | 0.637 | ||
| Well differentiated | 112 | 105 | |
| Moderate differentiated | 122 | 93 | |
| Poorly differentiated | 62 | 47 |
| Variables | LR (n = 296) | OR (n = 245) | P value |
| Lymph nodes removed | 15.2 ± 4.5 | 16.3 ± 5.5 | 0.067 |
| Hospitalization (days) | 11.3 ± 2.5 | 17.6 ± 5.3 | < 0.001c |
| Operation blood loss (mL) | 60.5 ± 21.2 | 156.2 ± 30.4 | < 0.001c |
| Operation time (min) | 182.1 ± 35.2 | 130.5 ± 21.3 | < 0.001c |
| Peri-operative complications | |||
| Total | 26 | 62 | |
| Ileus (Grade II) | 8 | 11 | 0.273 |
| Urinary tract infection (Grade II) | 3 | 14 | < 0.001c |
| Wound infection (Grade I) | 4 | 15 | < 0.001c |
| Pneumonia (Grade II) | 5 | 12 | 0.048a |
| Anastomosis leakage (Grade IIIb) | 6 | 10 | 0.14 |
| Abscess drainage, stoma diversion | 2 | 5 | 0.231 |
| Recurrence within 1 year | 0 | 2 | 0.054 |
| Long-term (> 1 year) complications | |||
| Incisional hernia (Grade I) | 4 | 7 | 0.261 |
| Ileus (Grade II) | 10 | 14 | 0.343 |
Figure 1 demonstrates our patient profile. At first, we sorted 575 patients with CRC managed by Dr. Chiu. Eight patients were excluded based on our study design (three received laparotomies several times with severe intestine adhesion noted at the beginning of the operation, three received emergent surgeries, one refused to participate study and one received conversion). We assessed 567 patients receiving curative resection, distributed as 307 LR, and 260 OR. Incidental peritoneal carcinomatosis was noticed in seven LR and six OR patients during the operation, and they were excluded. Additionally, nine patients in the OR group were excluded because two expired unrelated to cancer within 1 year postoperatively, and five patients with stage I and two with stage II did not show up at the outpatient department. Similarly, four patients in the LR group were excluded because one expired unrelated to cancer, and three with stage I lost contact. Finally, 296, and 245 patients in the LR and OR groups, respectively, were eligible, and compliant with subsequent follow-ups at the outpatient department. The median follow-up period was 69.4 mo.
Demographic and clinicopathologic variables of patients were well matched in both groups, with no statistical difference (Table 1). A slight disparity was found between the preoperative (clinical/radiologic) and postoperative (pathological) TNM stages in both groups. Moreover, nearly all patients with rectal cancer in both groups received preoperative concurrent chemoradiotherapy (CCRT) according to the guideline. Two and one patients in the LR and OR groups, respectively, received radical proctectomy directly due to partially obstructive symptoms.
Table 2 shows no significant statistical difference in the number of removed lymph nodes (15.2 ± 4.5 in LR and 16.3 ± 5.5 in OR, P = 0.067). None of the surgical specimen margins was involved with the tumor. However, we noted that patients could benefit from LR with a shorter hospitalization period (P < 0.001) and less blood loss (P < 0.001). On the contrary, the operation time was longer in the LR group than the OR group (182.1 ± 35.2 min vs 130.5 ± 21.3 min, P < 0.001).
More episodes of urinary tract infection (UTI) (P < 0.001), surgical wound infection (P < 0.001), and pneumonia (P = 0.048) were found in the OR group during the recovery course. No statistical difference was found regarding postoperative ileus (P = 0.273). Additionally, anastomosis leakage was complicated in 6 and 10 patients in the LR and OR groups, respectively, whose tumors were all located at lower rectum status postproctectomy without a protective diversional stoma. However, two and five patients in the LR and OR groups, respectively, needed re-operation for abscess drainage and stoma establishment for stool diversion. Others could recover after conservative treatment, and this complication did not reach a statistical difference (Table 2).
Moreover, any significant difference was not noticed in the complication rate of abdominal incisional hernia or ileus after a median follow-up period of 69.4 mo. Additionally, no patient in both group encountered these complications within the postoperative year (Table 2).
Regarding the oncologic outcome of tumor recurrence during the follow-up of 1 year postoperatively, only two patients with stage III in the OR group encountered tumor recurrence with peritoneal metastasis, which did not reach a statistical difference (p = 0.054) (Table 2).
All follow-up patients were compliant in answering the questionnaires within 1 year. Table 3 demonstrates that the LR group subjectively gained mildly better general health (P = 0.045), moderately better physical activity (P = 0.006), and significantly better social function recovery (P = 0.0001) 3 mo postoperatively. We noted that the LR group only mentioned a significant advantage in social function recovery at 6 mo follow-up (p = 0.001). No clinical difference was found in HRQoL between both groups at 12 mo follow-up.
| Health domains | LR group | OR group | P value |
| Physical functioning | 0.121 | ||
| Preoperative | 91.3 (20.5) | 92.7 (23.6) | 0.541 |
| 3 months after surgery | 82.3 (18.0) | 65.6 (23.4) | 0.006b |
| 6 months after surgery | 86.3 (20.4) | 84.2 (22.5) | 0.399 |
| 12 months after surgery | 88.3 (14.3) | 85.0 (23.0) | 0.081 |
| Social functioning | 0.113 | ||
| Preoperative | 88.9 (21.8) | 86.5 (18.3) | 0.213 |
| 3 months after surgery | 86.5 (20.3) | 52.1 (22.0) | 0.0001d |
| 6 months after surgery | 87.0 (21.0) | 60.3 (19.0) | 0.001c |
| 12 months after surgery | 86.5 (18.3) | 83.3 (19.2) | 0.321 |
| Vitality | 0.749 | ||
| Preoperative | 74.0 (18.5) | 74.2 (19.9) | 0.983 |
| 3 months after surgery | 70.9 (14.3) | 70.6 (23.2) | 0.97 |
| 6 months after surgery | 72.2 (14.1) | 70.3 (15.5) | 0.503 |
| 12 months after surgery | 73.1 (13.8) | 72.8 (16.8) | 0.675 |
| Mental health | 0.553 | ||
| Preoperative | 75.6 (15.1) | 78.0 (22.1) | 0.631 |
| 3 months after surgery | 84.5 (12.8) | 81.2 (18.7) | 0.724 |
| 6 months after surgery | 84.2 (11.5) | 82.6 (14.3) | 0.621 |
| 12 months after surgery | 81.6 (13.5) | 82.2 (17.1) | 0.827 |
| Role physical | 0.112 | ||
| Preoperative | 70.2 (32.1) | 68.3 (32.4) | 0.734 |
| 3 months after surgery | 49.7 (33.2) | 45.1 (39.2) | 0.346 |
| 6 months after surgery | 70.5 (28.6) | 65.6 (32.1) | 0.933 |
| 12 months after surgery | 80.2 (32.4) | 77.3 (29.4) | 0.192 |
| Role emotional | 0.922 | ||
| Preoperative | 81.0 (32.5) | 79.1 (29.1) | 0.633 |
| 3 months after surgery | 84.7 (32.4) | 81.3 (28.1) | 0.21 |
| 6 months after surgery | 89.2 (24.3) | 86.8 (29.1) | 0.191 |
| 12 months after surgery | 90.2 (22.1) | 87.6 (28.4) | 0.422 |
| Bodily pain | 0.315 | ||
| Preoperative | 52.1 (21.3) | 55.2 (29.1) | 0.937 |
| 3 months after surgery | 59.3 (22.1) | 65.4 (29.6) | 0.432 |
| 6 months after surgery | 63.2 (23.9) | 65.1 (22.6) | 0.341 |
| 12 months after surgery | 66.2 (29.2) | 62.0 (21.5) | 0.653 |
| General health | 0.253 | ||
| Preoperative | 66.4 (22.7) | 62.8 (19.3) | 0.571 |
| 3 months after surgery | 59.2 (23.1) | 49.7 (22.9) | 0.045a |
| 6 months after surgery | 70.2 (16.3) | 67.6 (23.2) | 0.31 |
| 12 months after surgery | 68.7 (23.2) | 69.2 (28.1) | 0.449 |
| PCS | 0.812 | ||
| Preoperative | 71.0 (8.5) | 69.1 (10.1) | 0.423 |
| 3 months after surgery | 54.7 (11.4) | 43.3 (13.1) | 0.021a |
| 6 months after surgery | 69.2 (7.3) | 67.8 (9.7) | 0.592 |
| 12 months after surgery | 72.2 (12.1) | 70.6 (12.4) | 0.482 |
| MCS | 0.451 | ||
| Preoperative | 69.2 (11.2) | 68.4 (8.2) | 0.621 |
| 3 months after surgery | 55.9 (12.8) | 49.4 (10.6) | 0.015a |
| 6 months after surgery | 65.2 (8.4) | 61.3 (12.6) | 0.414 |
| 12 months after surgery | 71.2 (14.2) | 69.0 (10.3) | 0.543 |
| SF-6D | 0.513 | ||
| Preoperative | 0.851 (0.142) | 0.812 (0.213) | 0.571 |
| 3 months after surgery | 0.732 (0.121) | 0.617 (0.149) | 0.045a |
| 6 months after surgery | 0.781 (0.213) | 0.722 (0.192) | 0.320 |
| 12 months after surgery | 0.807 (0.192) | 0.781 (0.122) | 0.495 |
Moreover, our results revealed that the LR group subjectively gained a better presentation of the Physical Component Summary (P = 0.021) and Mental Component Summary (P = 0.015) 3 mo postoperatively. Similarly, we noticed this phenomenon regarding the short form 6 dimensions (SF-6D) (P = 0.045).
Over 1.8 million people were diagnosed with CRC worldwide, and >880000 related patients expired in 2018, accounting for approximately one-tenth of total cancer occurrence and mortality. CRC ranks third in cancer incidence but second in mortality[15]. Many elderly are found with CRC indicated for surgical intervention as the population ages. The laparoscopic approach for CRC resection has become the mainstay of surgery in the recent two decades. Clinicians gradually alerted this evidence-based fact through more and more results of extensive and well-conducted studies and reinforced by numerous meta-analysis data[16-17].
LR is related to better short-term outcomes, including decreased postoperative pain, morbidity, minor immune impairment, faster recovery, shorter hospital stay, and better cosmetics than OR[11]. Additionally, a secure oncologic dissection could be acquired under laparoscopy, and current trials proved that LR did not adversely influence the prognosis of cancer treatment[1]. All this evidence is expected to result in a highly improved postoperative HRQoL. Moreover, HRQoL assessments further contribute to improved treatment.
Traditionally, surgical care outcomes were merely assessed by mortality, morbidity, cancer-free, and overall survival rates. Recently, additional judgment criteria have emerged and been illuminated with significant concern. The success of each therapeutic strategy is carefully explored in the management or its effects on patients’ daily lives and well-being. For example, the uncontrolled case series suggested that patients undergoing LR experience a more rapid bowel function return[2].
Generally, applying some objective parameters to assess the postoperative outcome is crucial in defining a patient’s degree of health. However, subjective patient perceptions and expectations should be factored into objective assessment to determine their real HRQoL. Thus, assessing self-reported HRQoL in surgical patients is of paramount importance. Accordingly, HRQoL measures have helped forecast the mortality and cost of health care[3].
The modified SF-36 Health Survey questionnaire is a comprehensive health status assessment tool, including an evaluation of physical functioning, social functioning, vitality, mental health, role physical, role emotional, bodily pain, and general health recovery over a specific period. Most interviewed patients could easily understand and complete the questionnaire within 10 min. In 2003, one study revealed that the concepts embodied in the SF-36 measurement model could be feasibly applied in the translated version in Taiwan[18]. Most items related to the psychometric properties were satisfactory based on the criteria of the International Quality of Life Assessment project. The rate of missing data was approximately 0%-2.7% at the item level, which was favorably compared with the original Medical Outcomes Study results in the United States[19] and other Western countries[20]. Additionally, this multitrait scaling study supported the hypothesized scale structure of the SF-36 Taiwan version and indicated the use of standard scoring algorithms score the eight SF-36 scales. All patients visiting the outpatient department in our study were compliant in answering the questionnaire within a 1-year postoperative follow-up, except two patients who expired within this period.
The surgery-related inconvenience of daily life and complications were actual events that significantly impacted the patients’ medium-term (from 3 to 12 mo after the operation) HRQoL, showing lower SF-36 scores in some domains. Table 3 shows that patients undergoing OR encountered peri-operative complications that mainly reflect burden in the social (P = 0.0001) and physical (P = 0.006) functioning items in their daily lives instead of facing significant general health deterioration (P = 0.045) 3 mo postoperatively. The reported higher HRQoL scores after LR at this period could be attributed to the essential benefit of minimally invasive surgery. Minimally invasive surgical approaches cause more minor wounds, lesser peri-operative blood loss, lesser inflammatory response, lesser postoperative pain, fewer respiratory complications, faster postoperative recovery, and enhanced postoperative mobilization[1-2]. LR could cause less surgical injury to the abdominal wall. Thus, the disparity in HRQoL is expected to be more evident within the first week postoperatively. However, these consequences may benefit patients’ well-being and report higher HRQoL scores in the medium-term period. The fascia is the most critical layer during abdomen wound healing because this tissue provides the most remarkable wound tensile strength. Patients might still feel discomfort during this period because the recovery of tensile strength could last over 70 days; even maximum strength exceeds 80%-90% of the intact fascia. However, only 15%-20% maximum strength is necessary for normal daily activities[21].
However, the decreasing negative effect of social functioning on patients undergoing OR had a mildly significant influence on the patients’ HRQoL as the inconvenience of daily life and complications improved 6 mo postoperatively (P = 0.001) (Table 3).
HRQoL outcomes of 12 mo after LR for CRC were not superior to OR (Table 3). The absence of statistical difference between the two groups in the modified SF-36 scores associated with postoperative 12-month complications might be interfered with by our small-size patient cohort. Hence, a prospective study of more significant patient numbers is undoubtedly necessary to address this issue in the future.
Most complications encountered by our patients belonged to grade I and II levels according to the Clavien-Dindo classification. The concepts regarding UTI, ileus, and incisional hernia must be clarified because postoperative HRQoL is closely related to surgical complications.
A discrepancy exists in UTI incidence among various methods of interventions in surgical patients, with a significantly higher occurrence rate after colorectal surgery than others[22]. Kang et al[23] examined a nationwide inpatient sample database for patients with CRC undergoing surgery and revealed the elderly, female gender, open approach method, and some morbidities as risk factors. They concluded that pelvic dissection surgeries were prone to a significant risk of UTI, as we noticed all UTI cases in the groups receiving radical proctectomy and abdominal perineal resection in our study. We admitted that rectal-associated surgical intervention is an independent risk factor. Pelvic dissection leads to various degrees of regional inflammation and nerve injury, which might increase the risk of urinary retention after catheter removal, thereby limiting the trial of early catheter removal[24]. We try to remove the urinary catheters as soon as possible when patients can mobilize postoperatively although published studies do not indicate the exact timing of catheter removal. Our study revealed that LR was significantly beneficial to preventing the episodes of postoperative UTI, which might be related to minor tissue injury and pain because of its minimally invasive characteristics, thereby bringing patients the benefits of earlier mobilization and catheter removal.
Ileus is defined as the presence of a dilated loop of the small intestine on abdominal imaging with the clinical presentation of abdominal pain, distension, or vomiting. The most common complication of abdominal and pelvic surgery is postoperative adhesion caused by aberrant fibrous bands connecting the tissues or organs that should be separated, usually within the abdominal cavity. Approximately 65%-75% of episodes of acute ileus are the consequences of adhesions, mainly involving the small intestine. A study of over four years revealed that colorectal surgeries lead to approximately 30% risk of adhesion-associated complications in various surgical fields. Additionally, OR for colorectal surgery has been the most common cause of adhesion-relevant readmissions[25].
The onset of adhesive ileus after CRC incredibly differs after index surgery. Some specialists have revealed that the median time of its first episode was approximately 1.3 years[26], and others stated it should be 3 years[27]. The earliest time was > 1 year in our patients who encountered postoperative adhesive ileus, with a median time of approximately 2.7 years. However, LR should lead to a much lower possibility of postoperative adhesion formation. Adhesion formation is regarded as a consequence of a stepwise failure during the repair process of peritoneal tissue. Technically, we used microsurgical instruments for LR, which brings patients the benefits of less direct surgical trauma, meticulous hemostasis, and minimal blood loss. Moreover, constant irrigation, manipulation within a smaller operative field, avoidance of bowel exposure to the environment, and clean dissection might lower the adhesion formation rate, despite no statistical difference between our patients undergoing LR and OR.
The incidence of incisional hernia ranges from 10% to 20% of patients receiving abdominal operations[28], which could influence patients’ HRQoL and body image. Factors predicting incisional hernia development after CRC surgery include dehiscence of the fascial layer, obesity, intestinal anastomosis leak, and surgical wound infection. A Denmark nationwide research studied 8489 patients with colon cancer receiving elective surgery with primary intestinal anastomosis from 2001 to 2008. It concluded that patients undergoing LR faced a relatively lower risk of this complication than those receiving OR approach[29]. However, our study revealed no significant statistical difference between the two groups.
One retrospective study, including 2983 patients undergoing OR, revealed that approximately 31.5% of incisional hernias occurred in the first 6 mo postoperatively, 54.4% in 12 mo, 74.8% in 2 years, and 88.9% in 5 years[30]. Winslow and Ng noticed that incisional hernia mainly developed in patients undergoing LR at the specimen extraction site[31-32]. Additionally, Ng et al[32] emphasized a similar incidence rate of incisional hernia at the midline extraction site in both the LR and OR groups. Moreover, no relationship was found between the incision wound length and hernia occurrence incidence. However, the burden of an incisional hernia caused by a large midline OR incision may be more severe than a small hernia at a limited specimen extraction site. The degree of challenge in hernia repair is positively related to the hernia size and is usually not amenable to minimally invasive repair techniques[31]. In our practice, we always tried to remove the specimen via the extraction wound as small as possible under a wound retractor’s protection.
The anastomotic leakage rate is not rare but challenging to surgeons (Clavien-Dindo classification Grade III). The decision of re-operative strategies is arduous and highly complex[33], which depends on the anastomosis location and the characteristics of the anastomotic dehiscence, e.g., the degree of tissue trauma during operation. Laparoscopy could provide a clear view of the pelvis, which is usually inaccessible to the naked eye during the process of OR, based on our experience. Additionally, precise dissection in a narrow male pelvis is comparatively easier to follow for the anatomic planes and completely secure hemostasis under the well-illuminated and magnified laparoscopic view. More importantly, we could reduce tissue trauma with less inadvertent handling by gently displacing the rectum and mesorectum from side to side during the LR procedure, which could lower the leakage rate. Fortunately, we successfully treated all patients after abscess drainage and stoma establishment for stool diversion. Additionally, no statistical difference was found in the complication between our LR and OR groups.
Some researchers studied the HRQoL after LR and OR but have miscellaneous conclusions. Several studies revealed no significant disparity in postoperative HRQoL[8-10], but others reported improved results in HRQoL after LR[2,6,11-12]. We have the following assumptions about this phenomenon. First, surgical techniques influenced the HRQoL, which might differ among the studies, especially with inconsistent surgeon volumes of multiple surgeons in the same survey. However, all patients in our study were treated by a single surgeon in two institutions, which could lower this bias. Second, HRQoL was not regarded as a chief outcome parameter in many studies, which probably resulted in an incompetent HRQoL analysis. Third, HRQoL might be interfered with various postoperative factors, although the baseline patient characteristics were identical at the initial preoperative evaluation. Fourth, different patients might experience other subsequent clinical courses even if they had the same pathologic TNM staging, which might affect the HRQoL. Finally, the clinical heterogeneity among various studies might be one important cause, mainly when we chose different HRQoL assessment instruments. Therefore, future ideal studies should be designed based on the standard guidelines with evidence-based consensus.
Our study on postoperative HRQoL evaluation has limitations. First, pelvic surgeries, especially rectal tumor excisions, lead to long-term and perilous consequences to male sexual function because of possible surgical trauma to the pelvic autonomic nerves[34]. However, this aspect is not included in the modified SF-36 Health Survey questionnaire. Thus, our study could not evaluate the effect on male sexual function after OR or LR. Second, two patients with stage III cancer were excluded because they expired within 6 mo after another radical surgery for cancer recurrence. Third, our study’s case number is small, and the latter two limitations may cause a bias. Therefore, more significant patient numbers in further research are necessary to certify our conclusion.
Few studies focused on the HRQoL of patients between 3 and 12 mo postoperatively, and our study discussed the LR approach with significantly better HRQoL than OR 3 mo postoperatively. Meanwhile, fewer detrimental factors (complication rates and blood loss) and similar oncologic results were found in the LR group than in the OR group. Thus, we suggest patients with non-metastatic CRC to undergo the LR approach if not contraindicated.
There are seldom studies about the medium-term effect on postoperative health-related quality of life (HRQoL) in patients undergoing colorectal cancer (CRC) surgery.
This study aimed to evaluate the medium-term effect of postoperative HRQoL in patients undergoing surgical CRC.
This study analyzed the objective outcomes and subjective HRQoL in 541 patients with non-metastatic CRC operated by one surgeon.
This study randomized 541 patients undergoing surgery for non-metastatic CRC by one surgeon to the laparoscopic resection (LR) (n = 296) or open resection (OR) (n = 245) groups. We used a modified version of the 36-Item Short Form (SF-36) Health Survey questionnaire to assess the HRQoL preoperatively and 3, 6, and 12 mo postoperatively.
The LR group reported better HRQoL in general health, physical activity, and social function recovery with various degrees and had lower complications of postoperative urinary tract infection, wound infection, and pneumonia than the OR group.
Patients with CRC should consider LR to gain better HRQoL if not contraindicated.
Seldom studies were conducted about the medium-term effect on postoperative HRQoL in patients undergoing surgery for CRC, and this study could provide vital information for reference.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Wang P, China; Zhao Y, China; Zou Y, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, Lai PB, Lau WY. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 656] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 2. | Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G; Clinical Outcomes of Surgical Therapy (COST) Study Group. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 540] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 3. | Thaler K, Dinnewitzer A, Mascha E, Arrigain S, Weiss EG, Nogueras JJ, Wexner SD. Long-term outcome and health-related quality of life after laparoscopic and open colectomy for benign disease. Surg Endosc. 2003;17:1404-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Dowson HM, Ballard K, Gage H, Jackson D, Williams P, Rockall TA. Quality of life in the first 6 wk following laparoscopic and open colorectal surgery. Value Health. 2013;16:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Andersson J, Angenete E, Gellerstedt M, Angerås U, Jess P, Rosenberg J, Fürst A, Bonjer J, Haglind E. Health-related quality of life after laparoscopic and open surgery for rectal cancer in a randomized trial. Br J Surg. 2013;100: 941-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Fujii S, Ota M, Ichikawa Y, Yamagishi S, Watanabe K, Tatsumi K, Watanabe J, Suwa H, Oshima T, Kunisaki C, Ohki S, Endo I, Shimada H. Comparison of short, long-term surgical outcomes and mid-term health-related quality of life after laparoscopic and open resection for colorectal cancer: a case-matched control study. Int J Colorectal Dis. 2010;25:1311-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Li J, Chen R, Xu YQ, Wang XC, Zheng S, Zhang SZ, Ding KF. Impact of a laparoscopic resection on the quality of life in rectal cancer patients: results of 135 patients. Surg Today. 2010;40: 917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM; MRC CLASICC trial group. Short-term endpoints of conventional vs laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2298] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 9. | King PM, Blazeby JM, Ewings P, Kennedy RH. Detailed evaluation of functional recovery following laparoscopic or open surgery for colorectal cancer within an enhanced recovery programme. Int J Colorectal Dis. 2008;23:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Polle SW, Dunker MS, Slors JF, Sprangers MA, Cuesta MA, Gouma DJ, Bemelman WA. Body image, cosmesis, quality of life, and functional outcome of hand-assisted laparoscopic vs open restorative proctocolectomy: long-term results of a randomized trial. Surg Endosc. 2007;21:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Braga M, Frasson M, Vignali A, Zuliani W, Civelli V, Di Carlo V. Laparoscopic vs. open colectomy in cancer patients: long-term complications, quality of life, and survival. Dis Colon Rectum. 2005;48:2217-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Sokolovic E, Buchmann P, Schlomowitsch F, Szucs TD. Comparison of resource utilization and long-term quality-of-life outcomes between laparoscopic and conventional colorectal surgery. Surg Endosc. 2004;18:1663-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Fakih M, Sandhu J, Wang C, Kim J, Chen YJ, Lai L, Melstrom K, Kaiser A. Evaluation of Comparative Surveillance Strategies of Circulating Tumor DNA, Imaging, and Carcinoembryonic Antigen Levels in Patients With Resected Colorectal Cancer. JAMA Netw Open. 2022;5: e221093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] |
| 15. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4898] [Article Influence: 699.7] [Reference Citation Analysis (1)] |
| 16. | Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008;2008:CD003432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | van der Pas MHGM, Deijen CL, Abis GSA, de Lange-de Klerk ESM, Haglind E, Fürst A, Lacy AM, Cuesta MA, Bonjer HJ; COLOR II study group. Conversions in laparoscopic surgery for rectal cancer. Surg Endosc. 2017;31:2263-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Tseng HM, Lu JF, Gandek B. Cultural issues in using the SF-36 Health Survey in Asia: results from Taiwan. Health Qual Life Outcomes. 2003;1:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32: 40-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3052] [Cited by in RCA: 3202] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 20. | Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, Sprangers MA, te Velde A, Verrips E. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1547] [Cited by in RCA: 1693] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 21. | Douglas DM. The healing of aponeurotic incisions. Br J Surg. 1952;40:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Regenbogen SE, Read TE, Roberts PL, Marcello PW, Schoetz DJ, Ricciardi R. Urinary tract infection after colon and rectal resections: more common than predicted by risk-adjustment models. J Am Coll Surg. 2011;213:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Kang CY, Chaudhry OO, Halabi WJ, Nguyen V, Carmichael JC, Mills S, Stamos MJ. Risk factors for postoperative urinary tract infection and urinary retention in patients undergoing surgery for colorectal cancer. Am Surg. 2012;78:1100-1104. [PubMed] |
| 24. | Sheka AC, Tevis S, Kennedy GD. Urinary tract infection after surgery for colorectal malignancy: risk factors and complications. Am J Surg. 2016;211:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Ha GW, Lee MR, Kim JH. Adhesive small bowel obstruction after laparoscopic and open colorectal surgery: a systematic review and meta-analysis. Am J Surg. 2016;212:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Parker MC, Ellis H, Moran BJ, Thompson JN, Wilson MS, Menzies D, McGuire A, Lower AM, Hawthorn RJ, O'Briena F, Buchan S, Crowe AM. Postoperative adhesions: ten-year follow-up of 12,584 patients undergoing lower abdominal surgery. Dis Colon Rectum. 2001;44:822-29; discussion 829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 258] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Taylor GW, Jayne DG, Brown SR, Thorpe H, Brown JM, Dewberry SC, Parker MC, Guillou PJ. Adhesions and incisional hernias following laparoscopic vs open surgery for colorectal cancer in the CLASICC trial. Br J Surg. 2010;97:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Kingsnorth A, LeBlanc K. Hernias: inguinal and incisional. Lancet. 2003;362:1561-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 628] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 29. | Jensen KK, Krarup PM, Scheike T, Jorgensen LN, Mynster T. Incisional hernias after open vs laparoscopic surgery for colonic cancer: a nationwide cohort study. Surg Endosc. 2016;30:4469-4479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Kössler-Ebs JB, Grummich K, Jensen K, Hüttner FJ, Müller-Stich B, Seiler CM, Knebel P, Büchler MW, Diener MK. Incisional Hernia Rates After Laparoscopic or Open Abdominal Surgery-A Systematic Review and Meta-Analysis. World J Surg. 2016;40:2319-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Winslow ER, Fleshman JW, Birnbaum EH, Brunt LM. Wound complications of laparoscopic vs open colectomy. Surg Endosc. 2002;16:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Ng SS, Lee JF, Yiu RY, Li JC, Hon SS, Mak TW, Ngo DK, Leung WW, Leung KL. Laparoscopic-assisted vs open total mesorectal excision with anal sphincter preservation for mid and low rectal cancer: a prospective, randomized trial. Surg Endosc. 2014;28:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Jin D, Chen L. Early prediction of anastomotic leakage after laparoscopic rectal surgery using creactive protein. Medicine (Baltimore) 2021; 100: e26196.. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Kin C, Rhoads KF, Jalali M, Shelton AA, Welton ML. Predictors of postoperative urinary retention after colorectal surgery. Dis Colon Rectum. 2013;56:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |